Bowel disease represents a wide spectrum of pathologies affecting the small intestine, colon and rectum. It includes not only minor conditions such as irritable bowel syndrome (IBS) but also colorectal cancers and various types of inflammatory disorders. The latter are represented by some autoimmune disorders without clearly defined causes such as Crohn's disease (CD) and ulcerative colitis (UC) or others specifically triggered by food components such as coeliac and non-coeliac disease.
IBS produces often similar symptoms to those of inflammatory bowel disease (IBD), which includes CD and UC, but as they are not the same condition they involve very different treatments. IBS is a functional gastrointestinal disorder (and does not cause inflammation), which results in chronic abdominal pain or discomfort and diarrhoea, constipation or alternating bouts of the two. However, people with UC or CD are also more likely to have other functional disorders such as fibromyalgia, chronic fatigue syndrome, chronic pelvic pain and temporomandibular joint disorder. Furthermore, UC and CD cause destructive chronic inflammation to the intestines that could lead to permanent damage of the intestines and harmful complications, and might require surgery and/or pharmacological treatment with steroids or immunosupressives.
UC affects only the colon while CD can affect any part of the digestive tract. In addition to the clinical evaluation for their diagnosis using imaging and endoscopic procedures, the measurement of biomarkers such as Perinuclear Anti-Neutrophil Cytoplasmic Antibodies for UC and Anti-Saccharomyces Cerevisiae Antibody for CD, along with C-reactive protein, calprotectin and lactoferrin, can help differentiate between both inflammatory disorders. However, these tests are not conclusive. In some cases, patients have none of those antibodies, or both, and further tests for other antibodies (anti-OmpC, anti-CBir1, etc.) can be performed to help differentiate between those disorders.
Bowel cancer, for which environmental factors such as diet play a critical role( 1 ), is the third most common cancer worldwide after lung and breast( 2 ). A recent systematic review on prospective observational studies evaluating the associations between dietary fibre, whole grains and risk of colorectal cancer indicates that a high intake of dietary fibre, in particular, cereal fibre and whole grains, is associated with a reduced risk of colorectal cancer: relative risk 0·90 (95 % CI 0·83, 0·97) for each 10 g/d intake of cereal fibre, and relative risk 0·83 (95 % CI 0·78, 0·89) for an increment of three servings daily of whole grains( Reference Aune, Chan and Lau 3 ). Whole grains are a major source of several vitamins, minerals and phytochemicals that have anticancer properties and could possibly influence the risk of colorectal cancer by various potential mechanisms( Reference Slavin, Martini and Jacobs 4 ). However, the chemical composition of whole-grain foods varies greatly and could differentially influence the activity and composition of the gut microbiota (DJ Rose, in this supplement), and their potential protective effects against cancer and other chronic diseases. Therefore, it is possible that particular whole-grain foods such as oats affect various health outcomes, particularly with regard to bowel-related disorders.
This is also relevant for inflammatory bowel disorders. Europe has the highest annual incidence of IBD, with 24·3 cases per 100 000 person-years and 12·7 cases per 100 000 person-years for UC and CD, respectively( Reference Molodecky, Soon and Rabi 5 ). The link between diet and IBD is not clear, but butyrate, a SCFA produced from the colonic fermentation of dietary fibres, has been shown to be effective in the reduction of UC( Reference Vernia 6 ) and CD( Reference Di Sabatino, Cazzola and Ciccocioppo 7 ), which suggests potential benefits for dietary interventions aiming to increase the production of butyrate and/or other SCFA from the gut microflora.
Coeliac disease is common, occurring in about 1 % of the population worldwide( Reference Reilly and Green 8 , Reference Verdu, Armstrong and Murray 9 ). It is traditionally characterised by chronic inflammation of the proximal small intestine that can develop in genetically susceptible people exposed to gluten. The symptoms associated with coeliac disease can affect any area of the body, and differ greatly between individuals, but usually include headaches, diarrhoea, stomach pains and lethargy, associated with sudden or unexpected weight loss and unexplained Fe-deficiency anaemia, or other unspecified anaemia. Some patients can also develop dermatitis herpetiformis, which is a chronic blistering skin condition. Coeliac disease is diagnosed by blood test (for the presence of endomysial antibodies and tissue transglutaminase antibodies) and biopsy of the small intestine. The disease induces morphological changes of the small intestine lining showing atrophy of the villi with crypt hyperplasia, and lymphocyte infiltration, leading to nutrient malabsorption.
The treatment of coeliac disease requires life-long adherence to a gluten-free diet. Oats, compared with wheat, barley and rye, contain low levels of prolamins, the components of gluten responsible for the toxicity in susceptible individuals, and could therefore represent a good source of fibre in a gluten-free diet. Importantly, several coeliac disease organisations support, and clinical studies suggest, that oats may be consumed by adults and children with coeliac disease without adverse events( 10 – Reference Janatuinen, Kemppainen and Julkunen 13 ).
The purpose of the present study was to systematically review the literature describing intervention studies that had investigated the effect of long-term consumption of whole-grain oat-based products (including oat bran) on risk factors for bowel disease. The objectives of the study were (1) to summarise the large body of literature on the subject, (2) to describe the relative strengths and weaknesses of the studies and (3) to evaluate the need for large intervention trials.
Methods
Literature search
Embase, Medline and the Cochrane library (Cochrane Central Register of Controlled Trials) were searched for articles describing intervention studies with oat-based products published before 26 November 2012. The search was limited to full-text English language articles carried out in human subjects aged 18 years or over, and excluded editorials, reviews and meta-analyses. Table 1 shows the search terms used, limits applied and number of articles identified in Embase. Similar searches were carried out in Medline and the Cochrane library databases. Additional articles were identified by searching for ‘oat(s)’ in the title of references in relevant articles obtained from the database search.
Table 1 Embase Classic+Embase search strategy (1947 to 26 November 2012)

* mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword.
Study selection
The three databases identified a combined total of 1174 articles, of which 520 were duplicates (Fig. 1). Titles and abstracts of the remaining 654 articles were reviewed independently by two reviewers to identify articles describing studies that assessed the effect of oat consumption on the risk of bowel diseases. Articles were excluded if the studies were conducted in children or animals, if the intervention involved a cereal that was not oats, if the study was investigating (non-) oat cell carcinoma or if the article used OAT as an abbreviation, e.g. for oral anticoagulant therapy, occluded artery trial, oligoasthenoterato-zoospermia or organic anion transporter. The two reviewers agreed that the full text should be obtained for 244 articles.

Fig. 1 Flow diagram of article selection.
Of these 244 articles, a further thirty-two articles were excluded because the full text was not in English, the article did not describe an intervention study, the intervention did not involve oats or OAT was used as an abbreviation. We excluded conference abstracts, articles describing studies in which the intervention was less than 7 d (2- or 3-d interventions (n 3) and test meals (n 25)), and articles in which the effect of oats alone could not be determined or the outcomes were not relevant. Articles describing the effect of oat-bran concentrate (n 8), oat extracts with β-glucans (n 16), oat fibre from oat husks/hulls (n 7), oat gum (n 2), avenanthramide-enriched mixtures (n 1) and fermented oat products (n 1) were excluded. The exclusion of each article was agreed by two reviewers.
Data extraction
Data were extracted by one reviewer into pre-prepared tables and a 10 % random sample of the data extraction was checked and agreed by a second reviewer. The data extracted included the country of study, participant characteristics (number, sex, age and health status), intervention characteristics (length, design and diet), variables adjusted for in the analysis and relevant outcomes. The primary outcomes of interest included characteristics of coeliac disease and UC; risk markers for colorectal cancer; stool weight, transit time and frequency; and faecal SCFA.
Quality of reporting
The Jadad scale for reporting randomised controlled trials (RCT)( Reference Halpern and Douglas 14 ) was used with a slight modification to score the quality of reporting of each article. Each article received one point (1) if randomisation of participants to treatment was mentioned, (2) if the method of randomisation was appropriate, (3) if blinding of the researchers/lab technicians to the intervention was mentioned, (4) if the method of blinding was appropriate and (5) if the number of all patients who started and completed the trial was clear, with reasons stated if no data were given. One point was also deducted if either the method of randomisation or blinding was considered inappropriate. Possible scores could range from 0 to 5. Studies were categorised as having a low or high quality of reporting if the total score was between 0 and 2, or 3 and 5, respectively. We also recorded the presence or absence of a sample size or power calculation for each article.
Reporting preferences
Studies were classified as RCT (1) if subjects were randomised to treatments groups, (2) if there was an appropriate control group and (3) if responses were compared between the oat and control groups. For studies that showed a statistically significant (P< 0·05) effect of oats consumption, the percentage change from baseline in the oat intervention group relative to the control group was the preferred outcome of interest to present. If this was not available in the article, it was calculated from the results given, and such values are indicated in the tables.
Results
We identified thirty-eight articles( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Kashtan, Stern and Jenkins 15 – Reference Payler, Pomare and Heaton 51 ) describing twenty-nine studies that assessed the effect of oat consumption on bowel disease (Fig. 1). Two studies were each carried out in participants with a history of colorectal adenomas( Reference Kashtan, Stern and Jenkins 15 , Reference Macrae, Kilias and Selbie 16 ) (Table 2) or UC( Reference Hallert, Bjorck and Nyman 17 , Reference Zhang, Hallmans and Andersson 18 ) (Table 3), eleven studies (described in seventeen articles( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Baker and Read 19 – Reference Størsrud, Hulthen and Lenner 34 )) were carried out in participants with coeliac disease (Table 4), and fourteen studies( Reference Abrahamsson, Goranzon and Karlstrom 35 – Reference Valle-Jones 48 ) were carried out in participants without a history of colorectal adenomas, UC or coeliac disease (Table 5).
Table 2 Characteristics of studies in participants with a history of colorectal adenomas
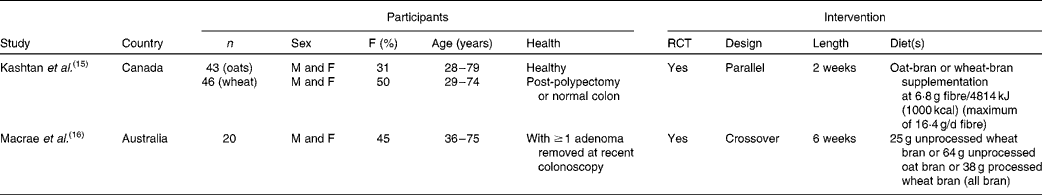
F, female; RCT, randomised controlled trials; M, male.
Table 3 Characteristics of studies in participants with ulcerative colitis

F, female; RCT, randomised controlled trials; M, male.
Table 4 Characteristics of studies in participants with coeliac disease

F, female; RCT, randomised controlled trials; M, male; SC, self-controlled; DH, dermatitis herpetiformis; IR, in remission; ND, newly diagnosed.
* Not available.
† Means and standard deviations (shown in parentheses).
Table 5 Characteristics of studies in participants without a history of colorectal adenomas, ulcerative colitis or coeliac disease

F, female; RCT, randomised controlled trials; M, male; HC, hypercholesterolaemic; SC, self-controlled; NC, normocholesterolaemic.
* Mean and standard deviation (shown in parentheses).
Colorectal cancer risk
Two studies( Reference Kashtan, Stern and Jenkins 15 , Reference Macrae, Kilias and Selbie 16 ) were carried out in participants with a history of colorectal adenomas (Table 2). A 2-week oat-bran intervention had no significant effect on putative risk measures for colorectal cancer (colonic mucosal labelling index, mucosal labelling pattern and micronuclei per crypt) in post-polypectomy and non-polyp Canadian patients( Reference Kashtan, Stern and Jenkins 15 ). Similarly, 64 g/d of oat-bran for 6 weeks had no significant effect on measures of rectal epithelial cell proliferation (number of crypts, number of labelled cells/crypt column, number of cells/crypt column, total labelling index and percentage of labelled cells within compartments) in twenty Australian patients with recent adenomas( Reference Macrae, Kilias and Selbie 16 ). Both articles were categorised as having a low quality of reporting.
Ulcerative colitis
Two Swedish studies( Reference Hallert, Bjorck and Nyman 17 , Reference Zhang, Hallmans and Andersson 18 ) assessed the effect of oat-bran consumption in participants with UC (Table 3). Hallert et al. ( Reference Hallert, Bjorck and Nyman 17 ) found no signs or symptoms of colitis relapse in twenty-two patients adding 60 g/d of oat bran to their daily diet for 12 weeks. In fact, those who reported any gastrointestinal complaint at entry had a significant reduction in abdominal pain and reflux by 24 and 55 %, respectively, after consuming oat bran( Reference Hallert, Bjorck and Nyman 17 ). Zhang et al. ( Reference Zhang, Hallmans and Andersson 18 ) found that the addition of oat-bran bread to the diet for 3 weeks in nine participants procotocolectomised for UC significantly increased the wet and dry weights of ileostomy effluent by 73 and 88 %, respectively, in comparison with wheat flour bread. The quality of reporting for both articles was considered low.
Coeliac disease
Table 4 shows the characteristics of the eleven studies carried out in patients with coeliac disease (described in seventeen articles( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Baker and Read 19 – Reference Størsrud, Hulthen and Lenner 34 )). Three studies were carried out in the UK( Reference Baker and Read 19 , Reference Dissanayake, Truelove and Whitehead 20 , Reference Hardman, Garioch and Leonard 26 ), five in Finland( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Kemppainen, Heikkinen and Ristikankare 21 – Reference Kemppainen, Heikkinen and Ristikankare 23 , Reference Reunala, Collin and Holm 25 , Reference Janatuinen, Pikkarainen and Kemppainen 27 – Reference Peraaho, Kaukinen and Mustalahti 30 ), and one each in Norway( Reference Lundin, Nilsen and Scott 24 ), Ireland( Reference Srinivasan, Leonard and Jones 31 , Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 ) and Sweden( Reference Størsrud, Olsson and Arvidsson Lenner 33 , Reference Størsrud, Hulthen and Lenner 34 ). The number of subjects in each intervention group ranged from four to thirty-five, and the interventions involved consuming between 34 and 100 g/d of oats for between 14 d to 5 years. The quality of reporting for the seventeen articles( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Baker and Read 19 – Reference Størsrud, Hulthen and Lenner 34 ) was considered low (modified Jadad score between 0 and 2) for ten articles( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Baker and Read 19 – Reference Reunala, Collin and Holm 25 , Reference Srinivasan, Leonard and Jones 31 , Reference Størsrud, Hulthen and Lenner 34 ) and high (modified Jadad score between 3 and 5) for seven articles( Reference Hardman, Garioch and Leonard 26 – Reference Peraaho, Kaukinen and Mustalahti 30 , Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 , Reference Størsrud, Olsson and Arvidsson Lenner 33 ).
Analyses of intestinal biopsy specimens have mostly shown either no change or a slight improvement in various measures including ratios of surface to volume( Reference Dissanayake, Truelove and Whitehead 20 ) and villous height to crypt depth( Reference Reunala, Collin and Holm 25 , Reference Hardman, Garioch and Leonard 26 , Reference Peraaho, Kaukinen and Mustalahti 30 ), enterocyte height( Reference Hardman, Garioch and Leonard 26 , Reference Srinivasan, Leonard and Jones 31 , Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 ), villous atrophy( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Kemppainen, Heikkinen and Ristikankare 21 , Reference Kemppainen, Heikkinen and Ristikankare 23 , Reference Janatuinen, Pikkarainen and Kemppainen 27 ), villous architecture( Reference Hardman, Garioch and Leonard 26 , Reference Størsrud, Olsson and Arvidsson Lenner 33 ), histomorphometric index( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Janatuinen, Pikkarainen and Kemppainen 27 ), morphological damage( Reference Srinivasan, Leonard and Jones 31 , Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 ), mononuclear-cell infiltration( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Janatuinen, Pikkarainen and Kemppainen 27 ), Marsh score( Reference Lundin, Nilsen and Scott 24 ), mucosal inflammation( Reference Kemppainen, Heikkinen and Ristikankare 21 , Reference Kemppainen, Heikkinen and Ristikankare 23 ), grade of inflammation( Reference Størsrud, Olsson and Arvidsson Lenner 33 ), mucosal human leucocyte antigen DR (HLA-DR) expression( Reference Reunala, Collin and Holm 25 , Reference Kemppainen, Janatuinen and Holm 29 , Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 ), enterocyte lactase expression( Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 ), CD25 positive cells/1 mm2( Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 ), intercellular adhesion molecule 1 (ICAM-1) (CD54) staining( Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 ) and lamina propria blood vessel size( Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 ). In the Norwegian study( Reference Lundin, Nilsen and Scott 24 ) however, one patient was found to be intolerant to oats and developed mucosal changes and dermatitis.
Intraepithelial lymphocyte counts were also reported to have remained within normal limits( Reference Hardman, Garioch and Leonard 26 ), or not changed significantly in five studies( Reference Reunala, Collin and Holm 25 , Reference Janatuinen, Kemppainen and Pikkarainen 28 , Reference Kemppainen, Janatuinen and Holm 29 , Reference Srinivasan, Leonard and Jones 31 , Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 ). While a Finnish study( Reference Peraaho, Kaukinen and Mustalahti 30 ) found a slight but significant increase in CD3+ and γδ intraepithelial lymphocyte densities, the authors stated that the density was relatively mild when compared with patients treated for coeliac disease in general.
Evidence from nine studies suggests that oats consumption has little impact on serum levels of antibodies to gliadin( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Lundin, Nilsen and Scott 24 – Reference Hardman, Garioch and Leonard 26 , Reference Janatuinen, Kemppainen and Pikkarainen 28 , Reference Srinivasan, Leonard and Jones 31 – Reference Størsrud, Olsson and Arvidsson Lenner 33 ), reticulin( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Hardman, Garioch and Leonard 26 , Reference Janatuinen, Kemppainen and Pikkarainen 28 ) or endomysium( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Kemppainen, Heikkinen and Ristikankare 21 – Reference Hardman, Garioch and Leonard 26 , Reference Peraaho, Kaukinen and Mustalahti 30 – Reference Størsrud, Olsson and Arvidsson Lenner 33 ) following the consumption of oats.
The addition of oats to a gluten-free diet appears to have no negative effects on nutritional status, as indicated by little change in BMI( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Kemppainen, Heikkinen and Ristikankare 22 , Reference Janatuinen, Pikkarainen and Kemppainen 27 , Reference Størsrud, Olsson and Arvidsson Lenner 33 , Reference Størsrud, Hulthen and Lenner 34 ), weight( Reference Kemppainen, Heikkinen and Ristikankare 22 ) or blood concentrations of vitamin A, D or E( Reference Kemppainen, Heikkinen and Ristikankare 22 ); folate or vitamin B12 ( Reference Dissanayake, Truelove and Whitehead 20 , Reference Janatuinen, Pikkarainen and Kemppainen 27 , Reference Peraaho, Kaukinen and Mustalahti 30 , Reference Størsrud, Olsson and Arvidsson Lenner 33 , Reference Størsrud, Hulthen and Lenner 34 ) ; Hb, ferritin or Fe( Reference Janatuinen, Pikkarainen and Kemppainen 27 , Reference Peraaho, Kaukinen and Mustalahti 30 , Reference Størsrud, Olsson and Arvidsson Lenner 33 , Reference Størsrud, Hulthen and Lenner 34 ); Ca( Reference Kemppainen, Heikkinen and Ristikankare 22 , Reference Janatuinen, Pikkarainen and Kemppainen 27 ); Mg( Reference Kemppainen, Heikkinen and Ristikankare 22 ); albumin( Reference Janatuinen, Pikkarainen and Kemppainen 27 , Reference Størsrud, Olsson and Arvidsson Lenner 33 , Reference Størsrud, Hulthen and Lenner 34 ) or alkaline phosphatase( Reference Størsrud, Olsson and Arvidsson Lenner 33 , Reference Størsrud, Hulthen and Lenner 34 ). However, Kemppainen et al. ( Reference Kemppainen, Heikkinen and Ristikankare 22 ) found a significant reduction in serum vitamin B12 after the consumption of kilned or unkilned oats.
One study( Reference Kemppainen, Heikkinen and Ristikankare 22 ) found no significant change in serum total- or HDL-cholesterol concentrations in response to the consumption of kilned or unkilned oats. However, after 6 months of consuming a diet with unkilned oats, serum TAG concentrations decreased significantly by 24 % but returned to near-baseline level after a further 6 months using kilned oats.
There were two studies( Reference Reunala, Collin and Holm 25 , Reference Hardman, Garioch and Leonard 26 ) of patients with dermatitis herpetiformis. In a UK study( Reference Hardman, Garioch and Leonard 26 ), dermal IgA showed no significant changes following oat consumption and there were no reports of pruritis or rash. In a Finnish study( Reference Reunala, Collin and Holm 25 ), IgA fluorescence on the skin increased in one patient only, two patients developed a transient rash and one patient withdrew due to the appearance of a persistent but mild rash, in comparison with three of the control patients who developed a transient rash and had IgA deposits on the skin. Both studies concluded that patients with dermatitis herpetiformis can include moderate amounts of oats in the diet and that the rash in these patients is not activated by eating oats.
In patients with coeliac disease, the effect of oats on gastrointestinal symptoms was varied. Symptoms included diarrhoea and flatulence( Reference Baker and Read 19 , Reference Lundin, Nilsen and Scott 24 , Reference Peraaho, Kaukinen and Mustalahti 30 , Reference Størsrud, Olsson and Arvidsson Lenner 33 , Reference Størsrud, Hulthen and Lenner 34 ), nausea( Reference Baker and Read 19 ), bloating or abdominal distension( Reference Kemppainen, Heikkinen and Ristikankare 22 , Reference Lundin, Nilsen and Scott 24 ), constipation( Reference Peraaho, Kaukinen and Mustalahti 30 ) and anorexia and irritability( Reference Baker and Read 19 ). In some studies, patients remained symptom free, had no adverse effects or there was no significant difference in symptoms compared with the control group( Reference Dissanayake, Truelove and Whitehead 20 , Reference Kemppainen, Heikkinen and Ristikankare 21 , Reference Hardman, Garioch and Leonard 26 , Reference Janatuinen, Kemppainen and Pikkarainen 28 , Reference Srinivasan, Leonard and Jones 31 , Reference Feighery, Srinivasan, Carolan and Lohiniemi 32 ). Some studies showed improvements in symptoms, including a reduction in indigestion( Reference Peraaho, Kaukinen and Mustalahti 30 ), improved bowel function( Reference Størsrud, Hulthen and Lenner 34 ) and an increase in the percentage of patients without symptoms from 50 % at baseline to 67 % at 24 months( Reference Størsrud, Olsson and Arvidsson Lenner 33 ).
Reasons for withdrawal of patients with coeliac disease from studies included gastrointestinal symptoms such as distension and flatulence( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Janatuinen, Kemppainen and Pikkarainen 28 , Reference Peraaho, Kaukinen and Mustalahti 30 , Reference Størsrud, Olsson and Arvidsson Lenner 33 , Reference Størsrud, Hulthen and Lenner 34 ) or pain( Reference Lundin, Nilsen and Scott 24 , Reference Peraaho, Kaukinen and Mustalahti 30 ), vomiting( Reference Kemppainen, Heikkinen and Ristikankare 23 ), rash( Reference Janatuinen, Kemppainen and Julkunen 13 ) and worsening of itching without signs of dermatitis (in patients with dermatitis herpetiformis)( Reference Janatuinen, Pikkarainen and Kemppainen 27 , Reference Janatuinen, Kemppainen and Pikkarainen 28 ). In one study( Reference Janatuinen, Kemppainen and Julkunen 13 ), the main reason given for withdrawing was uncertainty of the safety of oats.
Other studies in participants without coeliac disease reported increased flatulence( Reference Abrahamsson, Goranzon and Karlstrom 35 ) or no significant side effects( Reference Hallert, Bjorck and Nyman 17 , Reference Kirby, Anderson and Sieling 39 , Reference Valle-Jones 48 ) associated with oats consumption.
Bowel function
Table 5 describes the fourteen studies (seventeen articles( Reference Abrahamsson, Goranzon and Karlstrom 35 – Reference Payler, Pomare and Heaton 51 )) that were carried out in participants without a history of colorectal adenomas, UC or coeliac disease. Five studies were carried out in the USA( Reference Anderson, Story and Sieling 36 , Reference Hosig, Shinnick and Johnson 37 , Reference Kirby, Anderson and Sieling 39 – Reference Calloway and Kretsch 41 , Reference Marlett, Hosig and Vollendorf 43 ), three in the UK( Reference Judd and Truswell 38 , Reference Valle-Jones 48 , Reference Payler, Pomare and Heaton 51 ), two in Denmark( Reference Kristensen and Bugel 42 , Reference Arffmann, Hojgaard and Giese 49 ), two in Australia( Reference Noakes, Clifton and Nestel 44 , Reference Kestin, Moss and Clifton 50 ) and one each in Austria( Reference Sturtzel and Elmadfa 45 – Reference Sturtzel, Dietrich and Wagner 47 ) and Sweden( Reference Abrahamsson, Goranzon and Karlstrom 35 ). The number of subjects in each intervention group ranged from six to fifty (with only one study containing more than twenty-four subjects per group) and the interventions lasted between at least 10 d and 12 weeks. The quality of reporting for the seventeen articles was considered low for fourteen articles( Reference Abrahamsson, Goranzon and Karlstrom 35 – Reference Valle-Jones 48 ), and high for three articles( Reference Arffmann, Hojgaard and Giese 49 – Reference Payler, Pomare and Heaton 51 ).
Oat consumption significantly increased wet and dry stool weight in six out of nine studies (from 15 to 88 % increase) and five out of six studies (from 15 to 101 % increase), respectively (Table 6). Stool frequency did not change significantly in five studies, improved in two studies and reduced in one study relative to wheat-bran and rice-bran interventions. Transit time decreased significantly by 17 % in only one( Reference Kirby, Anderson and Sieling 39 ) out of four studies (Table 6). In addition, an American study( Reference Kretsch, Crawford and Calloway 40 ) found no effect of toasted or untoasted oat bran on retention time of a dye marker (the period until it was no longer detectable).
Table 6 Effect of oat consumption on stool weight, frequency and transit time

M, male; f, female.
* Not reported.
† Percentage change calculated from information in original article.
Two studies in older participants with constipation have both found beneficial effects of oat bran. A UK study( Reference Valle-Jones 48 ) found that consumption of two oat-bran biscuits per d in individuals aged 60–80 years improved bowel frequency (Table 6), increased the proportion of participants with normal stool consistency (16–84 %) and reduced the proportion who had pain on defecation (20–6 %). In addition, an Austrian study( Reference Sturtzel and Elmadfa 45 – Reference Sturtzel, Dietrich and Wagner 47 ) of frail nursing home residents found that adding 7–8 g/d of oat bran to the diet significantly reduced laxative use by 59 % in comparison with a non-significant increase of 8 % in the control group. However, a study( Reference Abrahamsson, Goranzon and Karlstrom 35 ) in younger women reported no significant effect of oat bran on stool consistency.
Faecal SCFA
There is a lack of evidence to support an effect of oats on faecal SCFA excretion( Reference Kashtan, Stern and Jenkins 15 , Reference Hallert, Bjorck and Nyman 17 , Reference Noakes, Clifton and Nestel 44 ). Kashtan et al. ( Reference Kashtan, Stern and Jenkins 15 ) found no change in faecal SCFA or butyric acid after a 2-week oat-bran RCT in participants with a history of colorectal adenomas. Similarly, Hallert et al. ( Reference Hallert, Bjorck and Nyman 17 ) found no significant change in the faecal excretion of a variety of SCFA or in the sum of SCFA after their 12-week oat-bran intervention in participants with UC, although butyric acid was increased significantly by 36 % at the fourth week and hepatonic acid was increased significantly by 100 % at the eighth week. While Noakes et al. ( Reference Noakes, Clifton and Nestel 44 )'s RCT found no change in the faecal excretion of acetate or propionate, the excretion of butyrate was significantly lower after 4 weeks of oat bran compared with a high-amylose diet.
Statistical analysis
Only one( Reference Kristensen and Bugel 42 ) of the thirty-eight( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Kashtan, Stern and Jenkins 15 – Reference Payler, Pomare and Heaton 51 ) articles described adjusting for any variables (body weight, sex, baseline values and period) in their analyses. Only two articles described carrying out a sample size or power calculation.
Quality of reporting
For the thirty-eight articles( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Kashtan, Stern and Jenkins 15 – Reference Payler, Pomare and Heaton 51 ) reviewed, the total modified Jadad score ranged from 0 (13 % of articles) to 4 (3 % of articles). The most common total score was 1 (32 %), followed by 2 (29 %) and 3 (24 %). Nineteen articles (50 %) mentioned randomisation of participants to an intervention group, and the method of randomisation was reported and judged to be appropriate for one article. Blinding of researchers to treatment was mentioned in ten articles (26 %), and the method of blinding was reported and judged to be appropriate in seven of these. The number of participants who started and completed the trial was clearly described, with the reason being stated if no data, for twenty-eight articles (74 %).
Discussion
Strength and limitations of review
To our knowledge, the present study is the first systematic review to assess the effects of long-term interventions with oat products on a range of bowel functions/diseases/risk markers. Of the thirty-eight articles( Reference Janatuinen, Kemppainen and Julkunen 13 , Reference Kashtan, Stern and Jenkins 15 – Reference Payler, Pomare and Heaton 51 ) included in the present review, eleven were identified from the reference lists rather than from the database searches, highlighting the importance of using multiple sources to identify relevant articles. It is possible that the database search strategy missed these studies because the journals were not covered by the databases, or the terms used to identify intervention studies alone were too restrictive. It is likely that publication bias had an impact on the findings of the present review: articles showing significant results are more likely to be submitted, published or published more quickly than studies without such characteristics, and articles published in languages other than English were excluded.
The present review is limited by the small number of studies in participants with a history of colorectal adenomas or UC, and the lack of studies identified in patients with CD. The majority of studies had a small sample size and many studies lacked an appropriate control group. The studies were relatively heterogeneous in terms of the intervention diet (amount and type of oat product), the control diet used and the clinical markers reported for each condition. However, it is difficult to pinpoint specific markers for each disorder, considering the variability and complexity of symptoms and biomarkers associated with bowel disease. Therefore, future studies are required to assess the effect of different types of oat products and their dose–response effect for various bowel conditions, and such studies need to be adequately powered, contain an appropriate control group and target primary outcome measures that can be compared across studies.
Colorectal cancer
The hypothesis linking fibre intake with colorectal cancer risk was originally proposed by Burkitt( Reference Burkitt 52 ) nearly 40 years ago. Increased stool bulk and dilution of carcinogens in the colonic lumen, reduced transit time and bacterial fermentation of fibre to SCFA have been suggested as possible mechanisms of action( Reference Lipkin, Reddy and Newmark 53 ). Recent evidence highlighted the protective role of whole-grain foods against colorectal cancer( Reference Aune, Chan and Lau 3 , Reference Schatzkin, Mouw and Park 54 ). However, despite the observational epidemiological evidence, few intervention studies have been conducted in order to confirm the association between whole-grain consumption and decreased risk of colorectal cancer, as well as to assess the effects of specific whole-grain foods. As described in the present review, only two intervention studies with oats have been carried out in participants with a history of colorectal adenomas, and neither showed any significant effects on putative and indirect risk factors for colorectal cancer. Furthermore, these studies included only a small number of patients and had a short follow-up. It is therefore impossible to draw any conclusion with regard to the results obtained, and further research including comprehensive and well-designed trials is warranted.
Inflammatory bowel disease
Our literature search identified only two long-term intervention studies with oats in patients with UC and none in patients with CD. However, only one of these studies was relevant in terms of improvement in the patients' conditions, with a reduction in relapse and decrease in abdominal pain and reflux( Reference Hallert, Bjorck and Nyman 17 ).
Experiments with animal models suggested that a loss of tolerance to gut microbiota could be central to the pathogenesis of IBD( Reference MacDonald 55 ); however, this has not been confirmed in patients with IBD. Antibiotic therapy has no beneficial effect on UC while it provides some clinical benefits in luminal CD( Reference Prantera 56 ). Defects in innate or humoral and adaptive immune response to host microbiota occur in CD( Reference Parkes, Barrett and Prescott 57 , Reference Strober and Fuss 58 ) and UC( Reference Fuss, Heller and Boirivant 59 , Reference Heller, Florian and Bojarski 60 ), respectively. Drug therapy for IBD includes the use of various anti-inflammatory drugs such as aminosalicylates (PPARγ agonist) or corticosteroids. However, probiotic therapy seems effective in inducing and maintaining remission in UC patients( Reference Kruis, Fric and Pokrotnieks 61 , Reference Tursi, Brandimarte and Papa 62 ), which underlines the potential benefit of using the prebiotic properties of oats as an adjuvant to probiotic/pharmaceutical treatment for UC patients.
Coeliac disease
Coeliac disease presents a wide spectrum of clinical manifestations, characterised by diarrhoea, abdominal distension and failure to thrive in young children( Reference Vivas, Ruiz de Morales and Fernandez 63 ), while older children and adolescents more often present atypical gastrointestinal complaints including pain, vomiting, or constipation as well as other symptoms such as arthritis, neurological symptoms, anaemia or asymptomatic silent disease( Reference Ludvigsson, Ansved and Fälth-Magnusson 64 ). In adults, the main feature of the disease is diarrhoea( Reference Rampertab, Pooran and Brar 65 ), but anaemia( Reference Bergamaschi, Markopoulos and Albertini 66 ) and osteoporosis( Reference Meyer, Stavropolous and Diamond 67 ) are also prominent. Other manifestations include villous atrophy, dermatitis herpetiformis, IBS, bloating, and chronic fatigue as well as various neurological presentations( Reference Chin, Latov and Green 68 ).
The results of the present review are in agreement with Thompson's review( Reference Thompson 69 ) of articles published between 1995 and 2003, and Haboubi et al. ( Reference Haboubi, Taylor and Jones 70 )'s systematic review of articles published up to 2005, which show that including oats in the diet of patients with coeliac disease has mostly no detrimental effects on the intestinal mucosa morphology and inflammation. Serum concentration of antibodies against gliadin also remained unchanged after oat consumption. Furthermore, symptoms generated by dermatis hepetiformis were not worsened after oat consumption. The results of the present study suggest that the majority of patients with coeliac disease can tolerate up to 100 g/d of uncontaminated oats. Relieving the restrictions on oats for patients with coeliac disease could increase the acceptability of, and adherence to, a gluten-free diet.
However, it is often difficult to avoid the contamination of oats by traces of other gluten-containing cereals such as wheat cereals. Commercial oats may be processed in facilities that also process gluten-containing cereals. Contamination can also happen in the field, or during the transport of the grains, so conventionally grown, and therefore the level of contamination in processed oats can vary. It is therefore probably not appropriate to suggest that oats can be safely consumed by coeliac patients who are very sensitive to gluten. Some coeliac disease patients may also be intolerant to oats( Reference Lundin, Nilsen and Scott 24 , Reference Arentz-Hansen, Fleckenstein and Molberg 71 ), and there is currently no accurate estimate of the prevalence of oats intolerance in coeliac disease.
Bowel function
Dysfunctional bowel function is a common feature of many intestinal disorders such as IBS and includes abdominal pain relieved after defecation, changes in stool frequency/consistency as well as flatulence and bloating( Reference Thompson, Longstreth and Drossman 72 ). Only a limited number of studies examined the effects of oats on markers of bowel function. The majority of the studies described in the present review showed no significant effect of oats on stool frequency or transit time. However, oats or oat bran significantly increased stool weight (dry and wet) and decreased constipation, which would suggest that increasing oat consumption could benefit people suffering from IBS. However, these effects do not seem to be specific to oats when comparisons with wheat or rice bran are considered( Reference Hosig, Shinnick and Johnson 37 , Reference Kestin, Moss and Clifton 50 , Reference Payler, Pomare and Heaton 51 ).
Gut motility may be one of the mechanisms linking dietary factors and physical activity with colorectal cancer risk( Reference Burkitt 52 ). However, results of epidemiological studies are inconclusive( Reference Kojima, Wakai and Tokudome 73 – Reference Simons, Schouten and Weijenberg 76 ), and appropriately powered, well-designed RCT are required to properly assess the effects of oats on bowel function. Considering bowel function as a marker for colorectal cancer risk is currently inadequate considering the discrepancy of results obtained from epidemiological studies.
SCFA
SCFA, such as butyrate, are the product of bacterial fermentation of dietary fibre in the colon. Many studies using cell culture and animal models have assessed the effects of butyrate on colon cancer, with conflicting results( Reference Sengupta, Muir and Gibson 77 ). Butyrate has been shown to modulate cellular proliferation( Reference Lupton 78 ), apoptosis( Reference McBain, Eastman and Nobel 79 , Reference Rouet-Benzineb, Aparicio and Guilmeau 80 ) and cell differentiation( Reference Andoh, Tsujikawa and Fujiyama 81 ). The few studies that examined the effects of oat consumption on SCFA production do not support a positive effect on SCFA. All studies used faecal SCFA concentration as a surrogate marker for SCFA production. However, such measurement does not represent the actual epithelial exposure. Inter- and intra-individual SCFA productions are also highly variable due to many confounders such as recent dietary intake, colonic transit and hydration status. Therefore, the interpretation of such data is greatly limited.
Conclusion
Long-term dietary intake of oats or oat bran could present some benefits for patients with IBS and UC. A protective effect on colorectal adenoma and cancer is plausible but it has not been convincingly shown. Relieving the restrictions on oats for patients with coeliac disease could increase the acceptability of, and adherence to, a gluten-free diet. However, further research including comprehensive and well-designed trials is required to assess the efficacy of increased oat consumption against bowel disorders.
Acknowledgements
The authors thank M. Mowett for sourcing the majority of the articles. F. T. reviewed articles for inclusion, and drafted the paper. L. F. M. carried out the literature search, extracted the data and contributed to writing the paper; P. B. and P. K.-E. contributed to writing the paper.
F. T., P. K.-E. and P. B. received an honorarium from Quaker Oats Company (a subsidiary of PepsiCo, Inc.) for attending the workshop in May 2012 to discuss the content of the supplement, and the University of Aberdeen received an unrestricted grant from Quaker Oats Company. L. F. M. has no conflict of interest to report.
This paper was published as part of a supplement to British Journal of Nutrition, publication of which was supported by an unrestricted educational grant from Quaker Oats Co. (a subsidiary of PepsiCo Inc.). The papers included in this supplement were invited by the Guest Editor and have undergone the standard journal formal review process. They may be cited.
The Guest Editor to this supplement is Roger Clemens. The Guest Editor declares no conflict of interest.








