Introduction
There is overwhelming evidence for effectiveness of thrombectomy for acute stroke with large vessel occlusion (LVO), Reference Goyal, Demchuk and Menon1–Reference Berkhemer, Fransen and van den Berg3 including recent trials with expanded time windows. Reference Nogueira, Jadhav and Haussen4,Reference Albers, Marks and Kemp5 A clinical tool assessing for presence of cortical signs in LVO will facilitate the accurate identification and efficient transfer of patients to comprehensive stroke centers (CSC), using eligibility criteria based on recently published guidelines. Reference Powers, Rabinstein and Ackerson6
Many tools have been explored, but these tools have important limitations. One of the most widely used tools has been the Los Angeles Motor Score (LAMS), which scores stroke severity between 0 and 10 based only on the degree of hemiparesis. LAMS has shown some degree of accuracy in predicting LVO by physicians Reference Nazliel, Starkman and Liebeskind7 and prehospital paramedics, Reference Noorian, Sanossian and Shkirkova8 usually with a cutoff score of ≥4. Among physicians, while high sensitivity, specificity, and accuracy are reported, the actual number of patients with LAMS ≥ 4 with LVO is not reported. This foregoes calculation of positive predictive value (PPV), which is arguably the most important statistic as it will most accurately represent the impact of bypass protocols. In the paramedic study, LAMS ≥ 4 was associated with a 37% chance of finding an LVO, but again the raw numbers were not reported. Perhaps the single most important shortcoming of the LAMS score is that it does not follow basic principles of clinical localization by ignoring the significance of cortically based deficits, specifically gaze preference, aphasia, and neglect, such that lacunar strokes with significant hemiparesis will have high LAMS scores with very low likelihood of LVO.
In order to address the importance of cortical features that are commonly associated with middle cerebral artery (MCA) syndromes, other scales have been explored. Teleb et al Reference Teleb, Ver Hage and Carter9 developed the Vision Aphasia Neglect (VAN) score, correctly arguing that the presence of these features should differentiate LVO strokes from lacunar syndromes. In a small pilot study of 62 patients conducted with experienced and VAN trained stroke nurses in the emergency room, the VAN tool showed a PPV of 74%, superior to National Institutes of Health (NIH) stroke score of >6. Limitations of this VAN tool include its length and complexity, differentiating between hemianopias, quadrantanopias, and subtypes of aphasia, which are challenging concepts to distribute to a large number of prehospital care providers, and add little to the likelihood of an LVO beyond the simple presence or absence of any type of aphasia, for example. This VAN tool has yet to be validated in the prehospital setting with large numbers.
The Safe Implementation of Thrombolysis in Stroke International Stroke Thrombolysis Registry (SITS ISTR) Reference Scheitz, Abdul-Rahim and MacIsaac10 retrospectively assessed which NIH items could increase specificity for LVO in patients with a positive FAST screen and found that best gaze could potentially increase the likelihood of LVO with an odds ratio of 4.5. The study only included patients who were treated with acute revascularization, indicating a problematic selection bias when calculating specificity, since hemorrhage and stroke mimics had already been excluded, such that external validity to the prehospital setting is very limited. Finally, aphasia and neglect are not assessed, which are the two primary features of LVO in dominant and non-dominant MCA syndromes, respectively, and are disabling problems that may be present without a gaze preference.
Most recently, the Ambulance clinical triage (ACT-FAST) tool was published, Reference Zhao, Pesavento and Coote11 which used the presence of hemiparesis to guide next steps in assessment of aphasia (with right hemiparesis) or neglect (with left hemiparesis), followed by a screen for eligibility or stroke mimics. An elaborate validation algorithm based on retrospective chart review of NIHSS items most likely to be seen with LVO led to selection of the above- described features. The results among 60 patients who underwent assessment by paramedics using the full ACT-FAST algorithm were sensitivity of 86%, specificity of 94%, and PPV of 80% for identification of M1 occlusion on CTA. In prospective assessment with the ACT-FAST examination steps (N = 104), PPV was 56%. The ACT FAST algorithm is therefore accurate with acceptable specificity and sensitivity, although there remains a 15% false negative rate. Additional limitations include the small number of prospectively validated results, a variable exam based on initial findings, potentially inaccurate language screen with no requirement to name objects, exclusion of isolated aphasias which are disabling, and exclusion of gaze preference in patients with right hemiparesis. Finally, the eligibility criteria are extensive at eight items, and the 6-hour time window excludes patients who have been shown to benefit based on DAWN and DEFUSE-3 criteria Reference Nogueira, Jadhav and Haussen4,Reference Albers, Marks and Kemp5 and recommendations in AHA guidelines. Reference Powers, Rabinstein and Ackerson6
A useful clinical tool should be rapid, easily learned, follow basic principles of localization, and avoid the requirement to calculate scores. Reference Michel12 Based on these criteria and limitations of existing tools, we developed the FAST VAN tool as a brief and easily implemented modification of the original VAN screen. Reference Teleb, Ver Hage and Carter9 Currently, most acute stroke patients are screened by paramedics on scene with the existing Heart and Stroke Foundation FAST tool (Face, Arm, Speech, Time), which identifies patients likely to be suffering a stroke on the basis of hemiparesis and/or change in speech. The VAN screen is then applied to identify features associated with LVO.
Components of the VAN screen were conceived and validated based on principles of localization and designed to maintain simplicity and speed without sacrificing accuracy (Table 1). We hypothesized that any single positive VAN finding would predict LVO with high accuracy, such that there is no requirement to calculate a score. The vision assessment is simply the presence or absence of a gaze preference away from the hemiparesis. Gaze preference can be seen toward hemiparesis in the setting of thalamic or pontine strokes and therefore remains valid in the setting of posterior circulation strokes, although the primary target is MCA syndromes. Language is tested with naming objects. Since all types of aphasias share the common feature of impaired naming, this suffices as a screen for aphasia (name a pen, watch, or other available high frequency items), supported by other basic assessments during acquisition of the clinical history. Any errors in naming of high frequency words, in the absence of confounds such as delirium, represent a positive screen for aphasia. Neglect is screened with double simultaneous tactile stimulation as per usual practice. Visual field defects are considered technically challenging to teach and execute in the field, and more often relate to PCA syndromes in the absence of other FAST VAN signs, and they were therefore excluded from the tool. Hemiparesis has already been screened as either present or absent via the FAST tool, and since degree of hemiparesis does not reliably distinguish between large and small vessel syndromes, further quantification was excluded from the VAN tool.
Table 1: FAST VAN components

The FAST VAN tool was studied to determine its accuracy in identifying LVOs in real-world prehospital settings. We hypothesize that the FAST VAN tool will show high sensitivity, acceptable specificity, and PPV superior to other tools and be easily learned and implemented.
Methods
Consecutive acute strokes were analyzed between April 2017 and Jan 2021. The presence or absence of VAN signs was recorded, as determined by emergency medical services (EMS) providers who initiated acute stroke protocols. These findings were compared to the presence or absence of LVO on CT angiography (CTA) performed at the time of presentation. The presence of LVO was defined as a cervical or intracranial anterior circulation occlusion or near occlusion that would potentially be amenable to mechanical thrombectomy. Practically, this includes acute symptomatic cervical or intracranial carotid occlusion, or intracranial occlusion of MCA in either M1 or proximal M2 segments. Patients were excluded from the study if no CTA was performed, and the rest of the imaging was normal. Patients with abnormal imaging including hemorrhages, neoplasms, etc. with VAN signs were included as false positives. Patients with migrainous aphasia, delirium, or other non-stroke diagnoses were included as false positives if CTA was performed. EMS training consisted of a locally developed 10-minute video explaining the rationale and examination technique for the FAST VAN tool.
False positives were categorized as appropriate for CSC if urgent assessment by neurology or neurosurgery was ultimately required for other indications, since these would not be considered futile transfers. False positives were considered inappropriate for CSC if the final diagnosis was deemed VAN-positive stroke without LVO that would be amenable to treatment at the PSC, other relatively benign stroke mimics, or no stroke related diagnosis. Qualitative feedback was sought from EMS providers regarding time required for training, ease of use, and perceived utility.
Results
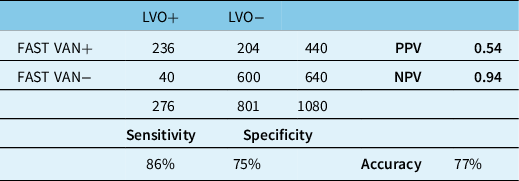
Comprehensive data from 1080 consecutive acute stroke patients were analyzed (Figure 1). Four-hundred forty patients were screened as having VAN signs or symptoms by EMS providers, among whom 236 (54%) demonstrated a symptomatic LVO that was considered potentially amenable to thrombectomy (Table 2). Sensitivity was 86% and specificity was 75%. Overall accuracy was 77%. Among 204 false-positive cases, 143 (70%) were considered appropriate for assessment at the CSC based on final diagnosis.
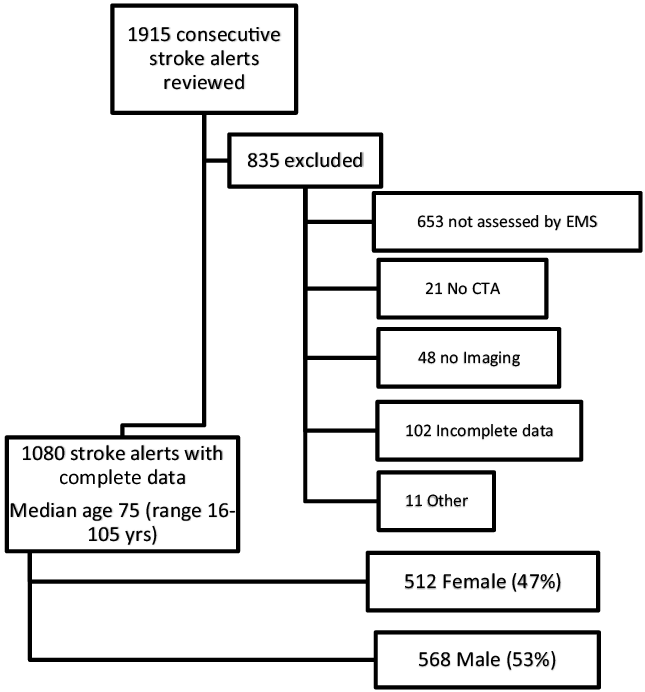
Figure 1: Diagram summarizing the data from the 1080 consecutive acute stroke patients included in the study.
Table 2: FAST VAN results
False positives were primarily related to intracranial hemorrhages or ischemic stroke without proximal LVO. Delirium and seizures were other common causes of false-positive FAST VAN screening by EMS (Table 3).
Table 3: False positives by etiology (N = 204)

Among 34 patients with isolated aphasia (no hemiparesis) as their primary complaint, 10 (29%) had a symptomatic LVO considered amenable to thrombectomy. Two of these 10 patients underwent mechanical thrombectomy.
We received qualitative data from 44 EMS providers. In response to the question “how confident are you that you can perform the examination correctly? (1 = not confident, 10 = very confident)”, 73% responded between 8 and 10, with a median of 8. On a separate 10-point scale, 77% reported the ease of learning the examination between 1 and 3 (lower numbers indicating easier) out of 10. Eighty-nine percent understood the purpose of the FAST VAN tool in the identification of LVO, and 96% of providers had used the tool in patient contact since receiving training.
Discussion
The FAST VAN tool for identification of LVO has shown high sensitivity, specificity, and accuracy in large number of patients and follows basic principles of neurologic localization. Based on our findings, this tool has been adopted for clinical practice and triage of stroke patients in Saskatchewan and British Columbia. In comparison to previously published tools, the FAST VAN offers simplicity, speed, ease of use, and exclusion of mandatory scoring, without sacrificing accuracy. Web-based training has been efficient and well-received by care providers and has facilitated interprovincial collaboration. Qualitative responses from EMS support ease of implementation and good understanding of the tool. Most false-positive cases were considered appropriate for assessment at the PSC based on final diagnosis, indicating very low overall rate of futile transfers.
Challenges in identifying posterior circulation strokes remain; fortunately, these are relatively rare diagnoses compared to anterior circulation strokes. However, a gaze preference may be seen in both thalamic and pontine strokes, although they can be toward the hemiparesis (so called “wrong-way eyes”). It seems likely that a gaze preference will still be noted by providers, and in the setting of a hemiparesis still permit appropriate escalation of care based on the FAST VAN tool.
This is the largest real-world study of LVO screening tools in the prehospital setting to our knowledge. The main limitation of this tool is the potential for false positives in the setting of isolated aphasia, which could meet criteria of both FAST and VAN with a low NIHSS score. One source of error is the finding that non-aphasic patients are occasionally called aphasic, when in fact the patient may simply be delirious, or have dysarthria, which may be seen in lacunar or large vessel syndromes. However, aphasia is considered highly disabling, and excluding isolated aphasia due to absence of hemiparesis could lead to missed opportunities for improved outcomes if there is a reasonable incidence of LVO in this population. We found that almost 30% of patients with isolated aphasia demonstrated an LVO, suggesting that this finding should be included in the target population for triggering LVO stroke protocols. Most false-positive cases in this series were considered appropriate for transfer to CSC based on final diagnosis. We recommend that all stroke systems should employ a screening tool for identifying LVO in the prehospital setting.
Acknowledgments
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosures
The following authors have no conflicts of interests or disclosures: Sanchea Wasyliw, Ruth Whelan, Kim Davy, Layla Gould, and Gary Hunter. Brett Graham and Mike Kelly are board members for the Heart and Stroke Provincial Board. Mike Kelly is also the Saskatchewan Clinical Stroke Research Chair and reported a consulting fee for Imperative Care for an unrelated medical device.
Statement of Authorship
SW contributed to study design and manuscript review. RW contributed to the study design, data collection, data analysis, and manuscript preparation. KD carried out the data analysis and manuscript review. MK and BG reviewed the manuscript and contributed to the draft of the manuscript. LG contributed to the manuscript review, manuscript submission, and performed the journal administration duties. GH contributed to the study design, data collection, data analysis, and manuscript preparation.





