Introduction
Population declines in long-distance migratory birds are difficult to diagnose because the drivers of decline may be operating in any or all of the birds' breeding, migratory and wintering areas (Newton, Reference Newton2004). To demonstrate the effect of conditions in any of these areas on population declines, explicit connections need to be made between wintering and breeding populations (Webster et al., Reference Webster, Marra, Haig, Bensch and Holmes2002; Thaxter et al., Reference Thaxter, Joys, Gregory, Baillie and Noble2010). There is an urgent need to identify what is driving the decline of the relict population of Critically Endangered northern bald ibis Geronticus eremita, which breeds in the Middle East and winters in Ethiopia, as the last known wild colony in this region is now on the verge of extinction (Serra et al., Reference Serra, Peške, Abdallah, Al Qaim and Kanani2009).
Since its discovery in 2002 (Serra et al., Reference Serra, Abdallah, Assaed, Abdallah, Al Quaim and Fayed2004) this colony has declined from seven mature birds to three in 2010, despite fledging 24 young during the same period. Although adult return rates to the breeding grounds in Syria during this period were typical of large-bodied, slow-breeding and long-distance migratory species (Serra et al., Reference Serra, Peške, Abdallah, Al Qaim and Kanani2009) there was very low recruitment of immature birds into the breeding population.
Despite a long history of reports of the species throughout the region, the migratory route and the wintering site of breeding ibises were revealed only recently through satellite telemetry (Lindsell et al., Reference Lindsell, Serra, Abdallah, al Qaim and Peske2009). Their route is part of a major flyway for several threatened Afro-Palearctic migrants (BirdLife International, 2010), thus factors driving the decline of ibis in this region may also be of concern for other species. Although we did not observe any short-term threats at the wintering site in the Ethiopian highlands (Serra et al., Reference Serra, Bruschini, Peške, Kubsa, Wondafrash and Lindsell2013), no immature birds were observed in Ethiopia during four consecutive winters (2006–2010) despite their confirmed departure from the breeding site (Serra et al., Reference Serra, Peške and Wondafrash2007; Serra & Wondafrash, Reference Serra and Wondafrash2009).
The decline of the species may be attributable to the variation in behaviour between adult and immature birds and their differential return rates to the breeding colony in Syria. Therefore we used a combination of satellite telemetry, analysis of historical observations and contemporary fieldwork, including evidence from birds released from a semi-captive population, to study the post-fledging behaviour of northern bald ibises that may provide insights into the poor breeding recruitment observed in this species.
Methods
We regarded immature birds as juveniles from fledging to first moult and subadults from first moult to first breeding attempt.
Satellite telemetry
Eleven immature northern bald ibises were fitted with satellite PTT (platform transmitter terminal) tags (battery-powered Northstar Geotrack, c. 30 g; solar-powered Microwave PTT 100, c. 12 g) at the wild colony in Syria (n = 6) and the semi-captive breeding site in Turkey (n = 5) between 2007 and 2011 (Table 1). Trapping and harnessing methods followed Lindsell et al. (Reference Lindsell, Serra, Abdallah, al Qaim and Peske2009) and satellite locations were filtered following the methods of Serra et al. (Reference Serra, Bruschini, Lindsell, Peške and Kanani2011).

Fig. 1 Migration trajectories of PTT-tagged juvenile northern bald ibises Geronticus eremita from the wild breeding site at Palmyra, Syria, and from the semi-captive breeding site at Birecik, Turkey, during 2007–2011 (n = 5, Table 1). The rectangle on (a) shows the location of (b).
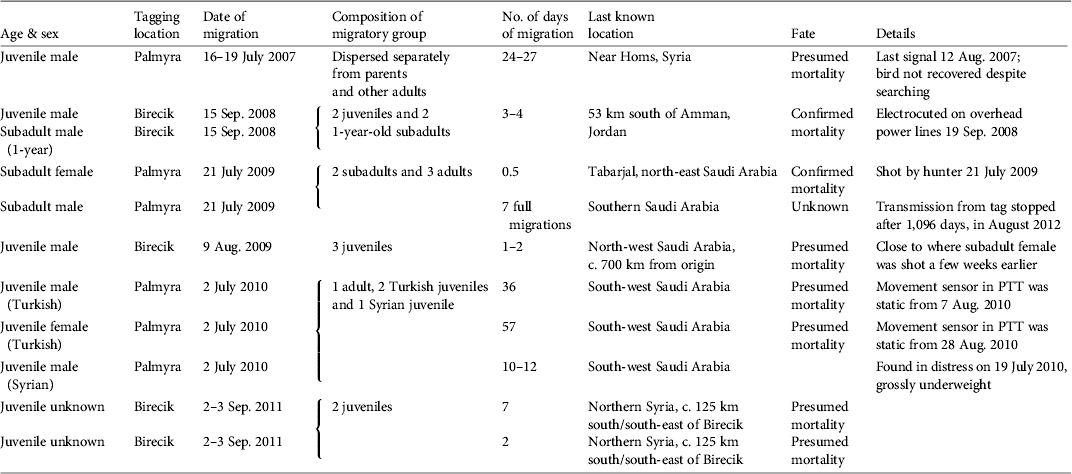
Table 1 Data on northern bald ibises Geronticus eremita tagged with satellite PTTs and released at Birecik, Turkey, and Palmyra, Syria, including age & sex, tagging location, date of migration, composition of migratory group, no. of days of migration, last known location, fate, and other details. All birds released at Birecik were from the semi-captive colony at that site, which have been prevented from migrating since 1989. Birds released at Palmyra were all wild-born in Syria with the exception of two juveniles supplemented from the semi-captive Turkish colony.
Field observations
Tracking of PTT-tagged birds enabled the observation of accompanying untagged birds in Yemen in 2006 and in Ethiopia in 2011.
We updated a detailed historical database of ibis sightings, covering the period 1877–1997 (n = 181; Welch & Welch, Reference Welch and Welch2004), with new reports for 2007–2008 (C. Rohde, pers. obs., 2007; H. Lind, pers. obs., 2008). We removed records that were unintelligible or unquantified, potentially related to experimental releases (Mendelssohn, Reference Mendelssohn1994) or to known breeding sites in Syria, Turkey and surrounding areas. Multiple records from the same area (radius ⩽ 300 km) within the same month were condensed into one. The remaining 74 records were divided into (1) those prior to March 1977, when a fully functional migratory eastern population comprised c. 20–40 breeding pairs from Birecik, Turkey, and dozens of pairs from Syria (Serra et al., Reference Serra, Abdallah, Assaed, Abdallah, Al Quaim and Fayed2004) and (2) those after March 1977, when the eastern population declined and fragmented (Akcakaya, Reference Akcakaya1990) and after 1989–1991 was presumed to comprise only Syrian birds. We also considered the recorded population dynamics of the colony breeding in Birecik, experimental releases undertaken at this site during 1976–1990 (Arihan, Reference Arihan1998; Pegoraro, Reference Pegoraro2003), experimental releases in Israel during 1983–1987 (Mendelssohn, Reference Mendelssohn1994) and the reported abundance of ibises in the Syrian desert until the 1980s (Serra et al., Reference Serra, Abdallah, Assaed, Abdallah, Al Quaim and Fayed2004). The difference in frequency of observations of ibises over the latitudinal range before and after March 1977 was tested non-parametrically (χ 2 test with Yates correction and an alpha level of 0.05; Siegel & Castellan, Reference Siegel and Castellan1989).
Survival and threat assessment
Adult survival rates for Syrian ibises were assessed yearly by Serra et al. (Reference Serra, Peške, Abdallah, Al Qaim and Kanani2009), based on annual return rates to the Syrian colony; survival rates for immature birds were calculated for the entire dispersal period (3–4 years, according to Pegoraro, Reference Pegoraro1996). Causes of mortality and threats to the ibises were assessed by autopsy of corpses, by veterinarians, interpretation of satellite signal characteristics (L. Peske, unpubl. data) and field surveys. Two extended surveys were carried out in western Saudi Arabia, in March and July 2010 (Serra, Reference Serra2010). The first, carried out soon after the ibises had completed their spring migration, included surveying 10 sites identified from 2006–2010 satellite locations as roosts or staging sites during more than one migration. The second survey involved tracking on the ground six satellite-tagged ibises (three juveniles, one subadult and two adults) during their autumn migration. A total of nine roosts and staging sites were surveyed. At all sites we recorded the occurrence of potential threats to the birds (direct disturbance, hunting, overhead power lines, pesticide use), availability of foraging habitat and roost sites, and any interaction between birds and humans.
Results
Contemporary movements of wild and released birds
Of three wild, immature Syrian ibises that were tracked, one juvenile ranged within western Syria for several weeks, making one short excursion south into Jordan (Fig. 1). A second juvenile migrated south in the company of two released juveniles from Turkey and a wild adult from Syria and reached the south-western coastal plains of Saudi Arabia, not far from the first staging site of the adult migratory flyway. These three juveniles then moved further south independently for c. 240 km in the following weeks (Fig. 1). A subadult male was tracked throughout the winters of 2009–2010, 2010–2011 and 2011–2012 (Fig. 2). In all three periods he wintered in the arid lowlands and highlands of eastern Ethiopia, c. 400–500 km from the adults’ wintering site in the central highlands, and returned to Syria unaccompanied in the spring.

Fig. 2 Migration trajectories of PTT-tagged subadult northern bald ibises from Palmyra, Syria, during 2006–2012 (n = 2, Table 1).
After three independent experimental releases from the semi-captive population at Birecik (Table 1) immature birds reached sites 125–700 km south–south-west and south–south-east of Birecik (Fig. 1). Two tagged Turkish juveniles released at the Syrian colony in 2010 followed a tagged Syrian adult southwards for c. 1,650 km over 6 days (Fig. 1) before being abandoned, as noted above.
Three untagged subadults departed together with a tagged Syrian adult in July 2006. Two of them were sighted with the adult at a staging site in western Yemen (Bajil) a month later (A.-R. al-Eryani, pers. obs.). Another month later two subadults were seen at the same site (J. Judas, pers. obs.), when all the tagged adults were already at the wintering site in Ethiopia. Two subadults returned to Syria together the following March unaccompanied by any adults. Two subadults were observed roosting on a cliff in the Jordan Valley on the border between Jordan and Israel in December 2007 (Fig. 2; C. Rohde, pers. obs.). In early January 2008 what was probably one of three untagged Syrian wild juveniles observed in spring 2007 was photographed alone in coastal Djibouti (Fig. 1; H. Lind, pers. obs.). Two untagged subadults were sighted together with two ringed adult females at the main wintering site in the Ethiopian highlands in January 2011 (Fig. 2; Dellelegn, Reference Dellelegn2011).
Historical changes in winter and summer ranges
Historical records of northern bald ibises from outside their known breeding grounds in Turkey and Syria show a peak in observations during the 1980s and 1990s (Fig. 3), with a cluster of observations around Taiz, south-west Yemen (Fig. 4). A peak in flock size is evident in east Africa between the 1920s and the 1950s (Fig. 4). Winter observations of immature and adult birds peak in east Africa prior to 1977, whereas after 1977 they are concentrated in western Arabia (Fig. 5a,b; χ 2 = 17.62, P < 0.005). Prior to 1977 sightings of immature ibises outside the known breeding grounds during the breeding season were recorded in western Arabia and Egypt (n = 4), with just one record from the East African Plateau (Fig. 5a). After 1977 such summer records came only from north-western Arabia (n = 12; Fig. 5b). This difference is not statistically significant (χ 2 = 3.3, P > 0.1). Winter sightings of confirmed immature ibises prior to 1977 were recorded only on the East African Plateau (n = 2), whereas after 1977 they were recorded only in north-western Arabia and the East African Rift Valley (n = 6; Fig. 5a,b).
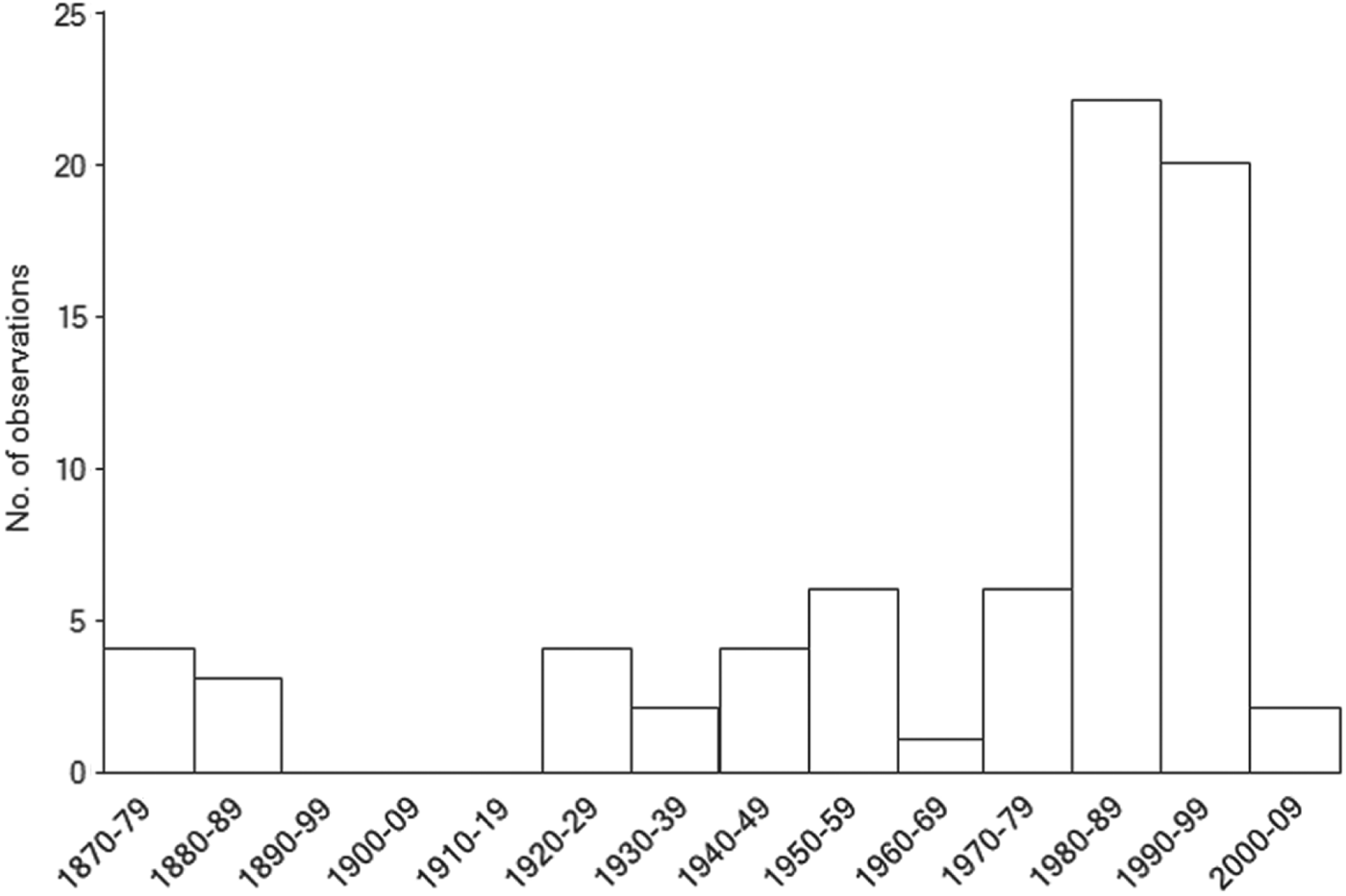
Fig. 3 Temporal distribution of opportunistic observations of northern bald ibises across the western Arabian Peninsula and east Africa, outside the known breeding grounds, during 1877–2008 (n = 74).

Fig. 4 Geographical distribution of opportunistic observations of northern bald ibises across the western Arabian Peninsula and east Africa, outside the known breeding grounds, during 1877–2008 (n = 74). Each circle represents an independent observation.
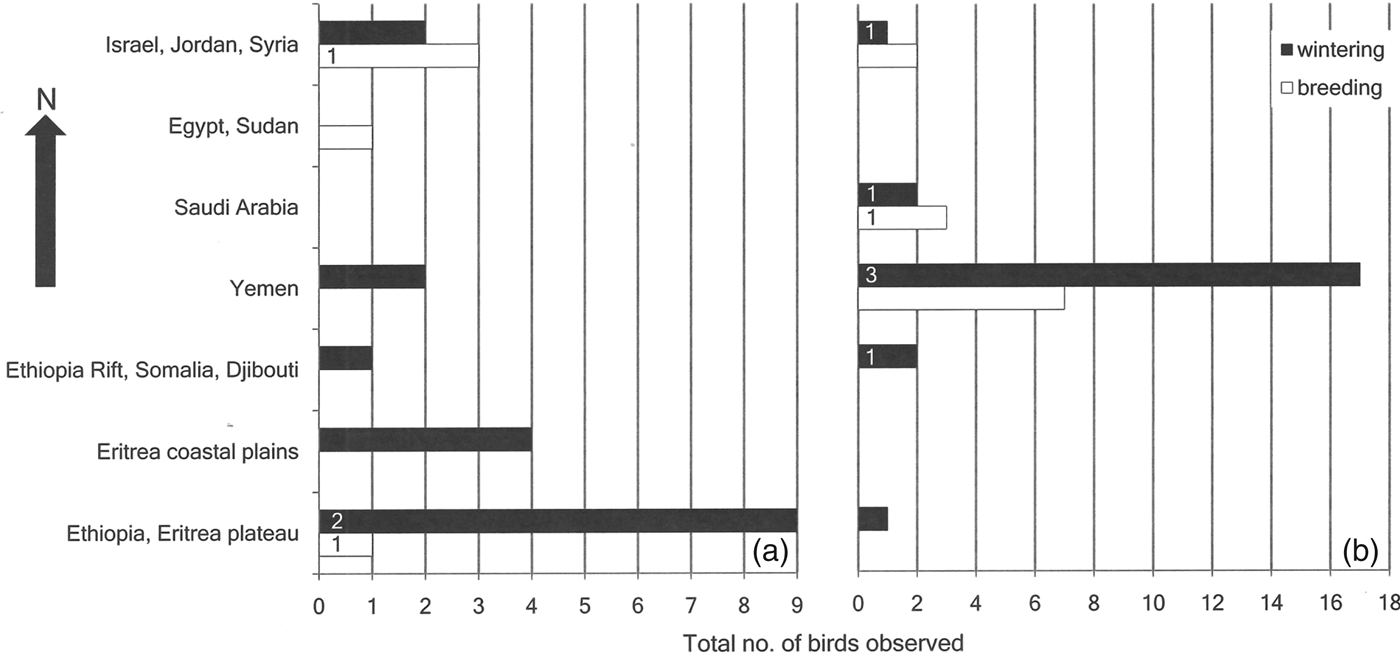
Fig. 5 Latitudinal distribution of opportunistic observations of northern bald ibises, recorded across western Arabia and east Africa during (a) 1877–March 1977 (n = 29) and (b) April 1977–2008 (n = 45). Numbers on bars denote confirmed immature birds.
Survival and threat assessment
The annual adult survival rate calculated from migratory returns to Palmyra during 2002–2010 was 81% (n = 12; Serra et al., Reference Serra, Peške, Abdallah, Al Qaim and Kanani2009). By contrast, the survival rate of immature ibises for the entire dispersal period was 20% (n = 5).
Although five satellite tags failed, for unknown reasons, we conclude that all nine tagged juveniles died during their first dispersal and migration, one in western Syria and the rest to the south in Jordan and Saudi Arabia (Table 1). Autopsies confirmed that the cause of death was electrocution by overhead power lines in one case and exhaustion in another. In two other cases mortality was inferred from transmissions of a static movement sensor in the tag but the corpses have not been recovered. Another juvenile was observed using power lines for roosting in the days before its tag failed and the bird was not relocated. In total the fate of seven subadults was monitored (three tagged birds and four untagged birds released in their company). Four mortality events were recorded, including one electrocution (corpse retrieved near power lines and cause confirmed by autopsy) and one hunting fatality (tag retrieved from the hunter along with a photograph of the bird). A third subadult disappeared after departing from Syria and did not arrive with its flock in Yemen.
Field surveys showed that above-ground roost site availability in western Saudi Arabia was confined to trees around oases and villages, and electricity pylons and power lines. Power lines were particularly concentrated on the Tihama plain, mainly running parallel to the coast in the direction of migratory movement. Hunting was particularly prevalent on the north-western desert plateau and was also identified as a threat within the northern section of the Tihama plain, especially around villages and urban centres, and particularly along the coast. Pesticide use was suspected to occur, although this was not clearly established.
Discussion
Current range and behaviour
In three of four cases migrating immature ibises departed the breeding grounds in the company of adults and remained with the adults as far as staging areas in south-west Saudi Arabia and western Yemen. This initial migration southward is fast, which may account for the mortality of one juvenile that could not keep up with the adults’ sustained migratory flight. When they are abandoned by the adults the immature birds can move further southward independently, as indicated by the three juveniles tracked from Syria in 2010, and may reach east Africa by mid winter, as shown by a juvenile observed on the Djibouti coast, where the open sea crossing is minimized at Bab El Mandeb (Bruderer & Jenni, Reference Bruderer, Jenni and Gwinner1990). As has been observed in black storks Ciconia nigra (Bobek et al., Reference Bobek, Pojer, Peske and Simek2003, Reference Bobek, Hampl, Peške, Pojer, Šimek and Bures2008), ibis siblings may move together and are likely to spend their first years outside the breeding range together, with others.
Of six groups of immature ibises that were either tracked or opportunistically observed in mid winter (December–January), five were found to overwinter away from the adults and probably spent the entire winter along the western Arabian Peninsula (Saudi Arabia and Yemen) or in the lowlands of Djibouti and Ethiopia. This area comprises the northernmost 70–80% of the autumn migration route of adult ibises and is the most likely current range of immature ibises during their first 1–4 years. The area overlaps with a flyway shared by a number of other threatened waterbirds and soaring birds, including sociable lapwing Vanellus gregarius, slender-billed curlew Numenius tenuirostris, greater spotted eagle Aquila clanga, eastern imperial eagle Aquila heliaca, lesser kestrel Falco naumanni and pallid harrier Circus macrourus, and by several declining soaring birds, including Demoiselle crane Grus virgo, common crane Grus grus, Steppe eagle Aquila nipalensis and white stork Ciconia ciconia (Meyburg, Reference Meyburg, del Hoyo, Elliott and Sargatal1994; Heredia, Reference Heredia, Heredia, Rose and Painter1996; Van den Bossche, Reference Van den Bossche2002; Meyburg et al., Reference Meyburg, Paillat and Meyburg2003; Terraube, Reference Terraube2009; BirdLife International, 2010).
Historical distribution and change
Satellite tracking of wild and captive-born juveniles suggests that inexperienced birds inherit the knowledge of migratory orientation during their first migration southward (Berthold, Reference Berthold2001). Their behaviour is similar to that of both white and black storks. During their first autumn migration the juveniles of these species follow the adults (not necessarily their parents) along an established migratory route (Alerstam, Reference Alerstam and Berthold1991; Berthold, Reference Berthold1996; Hake et al., Reference Hake, Kjellen and Alerstam2003; Chernetsov et al., Reference Chernetsov, Berthold and Querner2004; Bobek et al., Reference Bobek, Hampl, Peške, Pojer, Šimek and Bures2008). This social learning process underlies current experiments with human-led migration for captive-born northern bald ibises in Europe (Fritz et al., unpubl. data).
The use of traditional staging sites in western Saudi Arabia and Yemen (G. Serra, unpubl. data; Berthold, Reference Berthold2001) would allow juveniles to feed and rest for longer than adult birds and still be able to join newly arriving adults for onward movement (Alerstam, Reference Alerstam and Berthold1991). In the past, when there was still a large Turkish and Syrian breeding population of northern bald ibis (until c. 1977), most immature ibises would have been able to follow this strategy to reach the traditional wintering grounds in Ethiopia and Eritrea. We consider this to be the traditional and main wintering area of the eastern population, as it hosts the largest recorded flocks of wintering ibises. Only a small proportion of immature birds, perhaps the weakest, would over winter in the Arabian Peninsula. Similar variation in dispersal strategy during the first 1–3 years of life has been observed in immature birds from sedentary-resident colonies of northern bald ibis in Morocco (C. Bowden, pers. obs.). The sharp decline in the eastern population since 1977 would have resulted in a reduction in the number of groups of migrating adults that a staging juvenile could join and therefore fewer immature birds would be able to reach the wintering grounds in Ethiopia and Eritrea. As a result, most immature ibises now spend their first years of life in the western Arabian Peninsula.
Survival and threat assessment
The extinction of the Birecik colony in 1989 followed a steady decline in the number of birds returning from spring migration during the previous decade (Akcakaya, Reference Akcakaya1990). Our observations of Syrian birds, belonging to the same eastern population, demonstrate that 20–30 years later the poor survival of northern bald ibises during migration and at wintering grounds remains the greatest threat to the survival of this population.
The estimated survival rate of immature northern bald ibises (juveniles and subadults) in western Arabia (21.5%, n = 16, based on satellite telemetry during 2007–2011) differed little from the rate of birds returning to the natal site (20%, n = 5, 2002–2010). This survival rate is substantially lower than that of storks at a similar stage in life (26–48%) and of other large birds with comparable life history traits (Schaub et al., Reference Schaub, Kania and Köppen2005; Pistorius et al., Reference Pistorius, Follestad and Taylor2006; Gauthier et al., Reference Gauthier, Milot and Weimerskirch2010) and very much lower than for adult ibises. Although this is at least partly attributable to variations in their distribution away from Syria, it also suggests the role of experience in survival.
Three causes of mortality of immature ibises were confirmed: hunting, electrocution by power lines, and exhaustion. The latter was probably a consequence of the juvenile being in poor condition before departure from Syria (it was abandoned by its parents soon after fledging). The threats of hunting and electrocution were corroborated by field surveys in 2010. We assume that the eight instances of sudden halt of signal transmission or of movement of tagged immature birds during 2007–2011 were most likely a result of hunting or electrocution.
Hunting is a major threat for migrating birds (Guillemain et al., Reference Guillemain, Bertout, Christensen, Pöysa, Väänänen and Triplet2010) and there is clear evidence of this on the Arabian Peninsula (Combreau et al., Reference Combreau, Launay and Lawrence2001). Despite restrictions on hunting in Saudi Arabia it remains a threat, particularly in the north-west of the country. Serra (Reference Serra2010) found evidence of hunting along the western Saudi Arabia flyway during spring migration, with further evidence of the killing of a tagged ibis on the first day of autumn migration suggesting that the threat of hunting exists year round.
Migratory birds often congregate in cultivated fields and oases adjacent to villages and towns in north-west Saudi Arabia, in areas typically surrounded by hundreds of kilometres of desert. It is known that staging sites for migratory birds, especially gregarious ones, are especially vulnerable to hunters (Kirby, Reference Kirby2010); this was confirmed by evidence for Demoiselle cranes in north-west Saudi Arabia (M. Shobrak, pers. obs.).
Electrocution on power lines was confirmed in one case (two birds). Furthermore a juvenile in western Syria was observed roosting directly on an electricity cable, as was a juvenile in Saudi Arabia. By contrast, older birds have been observed using the pylons as roosts (Berthold, Reference Berthold2001; Van den Bossche, Reference Van den Bossche2002; Serra, Reference Serra2010) and it is possible that these are routinely used at one key staging site in Yemen. The concentration of power lines at certain places along the migratory route is notable, and large numbers of soaring birds can be killed when flocks collide with the lines (Shobrak, Reference Shobrak2012).
In summary, the observed difference in survival rates between immature and adult northern bald ibises can be accounted for by their use of different wintering areas and the relative threats in these areas. Since immature ibises spend more time than adults in western Arabia during the year they are exposed to greater threats to their survival for a longer period compared with the adults wintering in Ethiopia (Weimerskirch et al., Reference Weimerskirch, Kesson and Pinaud2006; Serra & Wondafrash, Reference Serra and Wondafrash2009; Guillemain et al., Reference Guillemain, Bertout, Christensen, Pöysa, Väänänen and Triplet2010; Serra et al., Reference Serra, Bruschini, Peške, Kubsa, Wondafrash and Lindsell2013); the relative inexperience of immature birds increases their vulnerability to these threats (Francis et al., Reference Francis, Richards, Cooke and Rockwell1992; Van den Bossche, Reference Van den Bossche2002; Menu et al., Reference Menu, Gauthier and Reed2005).
Recommendations
We recommend that measures be taken to raise awareness of the protected status of the northern bald ibis and other threatened species and prevent hunters from accidentally or otherwise targeting these species. Patrolling and enforcement are recommended across the migratory range but particularly at the known roosting sites and staging areas of the northern bald ibis in Saudi Arabia (Serra, Reference Serra2010). Preventing extinction of the last known northern bald ibis colony in the Middle East will also require reiterated supplementation of the wild colony of Palmyra with captive-born juveniles from Birecik (Fritz & Riedler, Reference Fritz and Riedler2010). Power line and pylon designs that eliminate the risk of electrocution of birds (Tinto et al., Reference Tinto, Real and Manosa2010; Kaluga et al., Reference Kaluga, Sparks and Tryjanowski2011) may be introduced most effectively through legislation and through coordination of similar initiatives. As satellite tracking has indicated that ibis staging areas are relatively consistent through the years, potential mitigation measures could be tested initially in those areas only. In the meantime, methods to deter birds from danger and the provision of safe alternative roosts in key areas should be investigated and tested (Jenkins et al., Reference Jenkins, Smallie and Diamond2010).
Although it is possible that other secondary threats may emerge on more detailed investigation at the known staging areas, in Saudi Arabia and Yemen, the use of pesticides in these areas is currently of most concern as these are likely to pose significant threats to the species (Hirsch, Reference Hirsch and Temple1978; Akcakaya, Reference Akcakaya1990). This issue should become a priority once actions are underway to address the major threats of hunting and electrocution.
Acknowledgements
Funds were made available by the Italian Development Cooperation Programme (DGCS), the National Geographic Society, the Finnish and Dutch embassies in Damascus, and the RSPB. Support and cooperation from the Saudi Wildlife Commission, the Syria Desert Commission and IUCN West Asia were instrumental. We thank Professor Mohamad Shobrak (Taif University) and Zafar Al Islam (National Wildlife Research Center) for valuable advice during field surveys.
Biographical sketches
Gianluca Serra is interested in the establishment of protected areas for the conservation of threatened species. Jeremy A. Lindsell researches threatened species and ecosystem services. Lubomir Peške is involved in international conservation projects involving raptors, storks and bustards. Johannes Fritz founded and leads the Waldrappteam project, with a major focus on northern bald ibises in Europe. Chris Bowden is International Species Recovery Officer for the Royal Society for the Protection of Birds (RSPB) and chairs the international advisory group for northern bald ibis. Claudia Bruschini's research focus is on animal physiology and behaviour, and she performs GIS ecological spatial analysis and monitoring. Geoff Welch is the International Management Plans Adviser at RSPB. Jose Tavares is the RSPB Country Programme Officer for Portugal, Greece, Cyprus and Turkey. Mengistu Wondafrash manages conservation projects throughout Ethiopia, particularly for threatened species such as the liben lark and white-winged flufftail.







