Introduction
During the Pleistocene, glacial refugia in Europe facilitated the survival of biota by providing suitable habitats during climate instability (e.g., Leroy and Arpe, Reference Leroy and Arpe2007; Zhelev, Reference Zhelev2017) and played a crucial role in vegetation dynamics during interglacial periods (e.g., Cheddadi et al., Reference Cheddadi, De Beaulieu, Jouzel, Andrieu-Ponel, Laurent, Reille, Raynaud and Bar-Hen2005; Tzedakis, Reference Tzedakis2007, Reference Tzedakis and Woodward2009). Higher latitudes experienced the most extreme glaciations (e.g., Médail and Diadema, Reference Médail and Diadema2009), leading to population crashes of the temperate flora and fauna (e.g., Hewitt, Reference Hewitt1999; Zhelev, Reference Zhelev2017). At the same time, temperate and Mediterranean biomes were confined to specific areas in moist valleys and mid-altitude sites in the mountains of the Mediterranean peninsulas, particularly in the southwestern Balkans, Italy, and Iberia (e.g., Bennett et al., Reference Bennett, Tzedakis and Willis1991; Tzedakis, Reference Tzedakis1993; Willis, Reference Willis1996; Carrión et al., Reference Carrión, Yll, Walker, Legaz, Chaín and López2003; Tzedakis et al., Reference Tzedakis, Hooghiemstra and Pälike2006; Donders et al., Reference Donders, Panagiotopoulos, Koutsodendris, Bertini, Mercuri, Masi and Combourieu-Nebout2021). All three of these regions are regarded as the main glacial refugia in Europe over multiple glacial–interglacial cycles (Petit et al., Reference Petit, Aguinagalde, De Beaulieu, Bittkau, Brewer, Cheddadi and Ennos2003; Griffiths et al., Reference Griffiths, Kryštufek and Reed2004).
The Mediterranean Basin is among the world’s major biodiversity hotspots (Médail and Myers, Reference Médail, Myers, Mittermeier and Robles Gil2004) due to its significant topographic and climatic heterogeneity. Furthermore, given its geographic position between Europe, Africa, and Asia, it is regarded as an important biogeographic crossroads for the European, Saharan, and Irano-Turanian floristic bioprovinces (Quézel, Reference Quézel and Gómez-Campo1985), with the highest taxonomic diversity existing in the Apeninno-Balkan region (Médail et al., Reference Médail, Monnet, Pavon, Nikolić, Dimopoulos, Bacchetta and Arroyo2019). The Balkan Peninsula, especially, is considered an important glacial refugium for several currently widespread tree taxa (e.g., Willis, Reference Willis1994; Donders et al., Reference Donders, Panagiotopoulos, Koutsodendris, Bertini, Mercuri, Masi and Combourieu-Nebout2021). Pollen sequences from the southwest Balkans demonstrate abrupt and high-amplitude vegetation and climate changes on multimillennial timescales during the last 1.36 Ma (e.g., Okuda et al., Reference Okuda, Yasuda and Setoguchi2001; Tzedakis et al., Reference Tzedakis, Lawson, Frogley, Hewitt and Preece2002, Reference Tzedakis, McManus, Hooghiemstra, Oppo and Wijmstra2003, Reference Tzedakis, Hooghiemstra and Pälike2006; Fletcher et al., Reference Fletcher, Müller, Koutsodendris, Christanis and Pross2013; Pross et al., Reference Pross, Koutsodendris, Christanis, Fischer, Fletcher, Hardiman and Kalaitzidis2015; Sadori et al., Reference Sadori, Koutsodendris, Panagiotopoulos, Masi, Bertini, Combourieu-Nebout and Francke2016, Reference Sadori, Koutsodendris, Panagiotopoulos, Masi, Bertini, Combourieu- Nebout and Francke2018; Koutsodendris et al., Reference Koutsodendris, Kousis, Peyron, Wagner and Pross2019; Panagiotopoulos et al., Reference Panagiotopoulos, Holtvoeth, Kouli, Marinova, Francke, Cvetkoska and Jovanovska2020; Donders et al., Reference Donders, Panagiotopoulos, Koutsodendris, Bertini, Mercuri, Masi and Combourieu-Nebout2021). Nevertheless, many temperate tree species survived those Quaternary climatic oscillations in isolated habitats under favourable microclimatic conditions (e.g., Tzedakis, Reference Tzedakis, Griffiths, Krystufek and Reed2004). The Balkan mountain ranges and islands provided enough climatic and topographic variability, promoting the survival of small, isolated plant populations during the glacial periods (Tzedakis et al., Reference Tzedakis, Lawson, Frogley, Hewitt and Preece2002; Médail and Diadema, Reference Médail and Diadema2009). These microhabitats represent multiple refugial areas (small refugia), the so-called refugia within refugia, thus indicating that the Balkan Peninsula was not a homogeneous refugium (Zhelev, Reference Zhelev2017) but sheltered multiple Pleistocene small refugia, as also suggest by phylogeographic studies of Balkan mammal species (e.g., Krystufek et al., Reference Krystufek, Buzan, Hutchinson and Hänfling2007).
In the southernmost part of the Balkans, Greece, the existence of such small refugia is evidenced by a number of long pollen sequences recovered from various sites throughout its north-to-south gradient, encompassing multiple climatic cycles of the Quaternary (e.g., Wijmstra Reference Wijmstra1969; van der Wiel and Wijmstra, Reference van der Wiel and T.A1987; Tzedakis, Reference Tzedakis1999; Okuda et al., Reference Okuda, Yasuda and Setoguchi2001, Reference Okuda, van Vugt, Nakagawa, Ikeya, Hayashida, Yasuda and Setoguchi2002; Tzedakis et al., Reference Tzedakis, Lawson, Frogley, Hewitt and Preece2002, Reference Tzedakis, Frogley, Lawson, Preece, Cacho and de Abreu2004, Reference Tzedakis, Hooghiemstra and Pälike2006; Panagiotopoulos et al., Reference Panagiotopoulos, Aufgebauer, Schäbitz and Wagner2013, Reference Panagiotopoulos, Böhm, Leng, Wagner and Schäbitz2014; Pross et al., Reference Pross, Koutsodendris, Christanis, Fischer, Fletcher, Hardiman and Kalaitzidis2015; Kafetzidou et al., Reference Kafetzidou, Fatourou, Panagiotopoulos, Marret and Kouli2023; Koutsodendris et al., Reference Koutsodendris, Dakos, Fletcher, Knipping, Kotthoff, Milner and Müller2023). Although all pollen sequences demonstrate the presence of temperate tree taxa during interglacial periods, they show different local vegetation responses to cold stages. More specifically, the palynological studies from northwestern (Lake Prespa; Panagiotopoulos et al., Reference Panagiotopoulos, Aufgebauer, Schäbitz and Wagner2013, Reference Panagiotopoulos, Böhm, Leng, Wagner and Schäbitz2014) and western Greece (Ioannina; Tzedakis et al., Reference Tzedakis, Lawson, Frogley, Hewitt and Preece2002) suggest the persistence of local temperate tree populations even under climate extremes (Fig. 1). In contrast, significant tree population crashes are documented in central (Kopais; Tzedakis, Reference Tzedakis1999; Tzedakis et al., Reference Tzedakis, Frogley, Lawson, Preece, Cacho and de Abreu2004) and northeastern Greece (Tenaghi Philippon; Tzedakis et al., Reference Tzedakis, Frogley, Lawson, Preece, Cacho and de Abreu2004). The persistence of temperate trees under cold conditions in northwestern Greece was associated with the local precipitation regime, implying that humidity was a crucial parameter for the survival of tree taxa (Tzedakis et al., Reference Tzedakis, Frogley, Lawson, Preece, Cacho and de Abreu2004). Additional evidence for the survival of other biota in Greece comes from archaeological records, which are by definition associated with the presence of hominins, raising the possibility that the region also acted as a glacial refugium for human populations. Particularly, many Middle Palaeolithic (hereafter MP) sites (generally corresponding to the late Pleistocene; Greek Mousterian period) were situated in well-watered regions such as west of the Pindus mountain range (western Greece); regions sharing similar topographic and climatic characteristics to those mentioned earlier (see Tourloukis and Harvati, Reference Tourloukis and Harvati2018). In southern Greece, lines of evidence come from the Peloponnese. Recent anthracological and faunal studies from Middle and Upper Palaeolithic sites in the region document the persistence of biota owing to the local topography and microclimate, suggesting the potential of the Peloponnese’s role as a hominin refugium (Ntinou, Reference Ntinou2020; Roditi and Starkovich, Reference Roditi and Starkovich2022).

Figure 1. Location of the studied site Marathousa 1 (MAR-1). (A) Overview map of the Mediterranean region (by Ocean Data View) showing key pollen records and the MAR-1 middle Pleistocene archaeological site in Megalopolis, Peloponnese (white square). (B) A 3D image of SW-central Peloponnese showing the location of the coring site MAR-1 (red dot) within the Megalopolis Basin (grey square) and a previous pollen record by Okuda et al. (Reference Okuda, van Vugt, Nakagawa, Ikeya, Hayashida, Yasuda and Setoguchi2002) (white dot).
Archaeological evidence for earlier human presence in Greece is scarce (see Tourloukis and Harvati, Reference Tourloukis and Harvati2018). Marathousa 1 (hereafter MAR-1), discovered in 2013 by our team in Megalopolis (Peloponnese; Panagopoulou et al., Reference Panagopoulou, Tourloukis, Thompson, Athanassiou, Tsartsidou, Konidaris and Harvati2015, Reference Blackwell, Sakhrani, Singh, Gopalkrishna, Tourloukis, Panagopoulou and Karkanas2021; Thompson et al., Reference Thompson, Tourloukis, Panagopoulou and Harvati2018), constitutes the only open-air site in Greece with a primary context, preserving lithics, and faunal and palaeobotanical remains in stratigraphic association (Doukas et al., Reference Doukas, van Kolfschoten, Papayianni, Panagopoulou and Harvati2018; Field et al., Reference Field, Ntinou, Tsartsidou, van Berge Henegouwen, Risberg, Tourloukis, Thompson, Karkanas, Panagopoulou and Harvati2018; Giusti et al., Reference Giusti, Tourloukis, Konidaris, Thompson, Karkanas, Panagopoulou and Harvati2018; Harvati et al., Reference Harvati, Konidaris and Tourloukis2018; Karkanas et al., Reference Karkanas, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018; Konidaris et al., Reference Konidaris, Athanassiou, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018, Reference Konidaris, Athanassiou, Panagopoulou and Harvati2022; Michailidis et al., Reference Michailidis, Konidaris, Athanassiou, Panagopoulou and Harvati2018; Tourloukis et al., Reference Tourloukis, Muttoni, Karkanas, Monesi, Scardia, Panagopoulou and Harvati2018a, Reference Tourloukis, Thompson, Panagopoulou, Giusti, Konidaris, Karkanas and Harvati2018b; Guibert-Cardin et al., Reference Guibert-Cardin, Tourloukis, Thompson, Panagopoulou, Harvati, Nicoud and Beyries2022). It dates to the glacial period of the middle Pleistocene, corresponding to the Marine Isotope Stage (MIS) 12 (Blackwell et al., Reference Blackwell, Sakhrani, Singh, Gopalkrishna, Tourloukis, Panagopoulou and Karkanas2021; Jacobs et al., Reference Jacobs, Li, Karkanas, Tourloukis, Thompson, Panagopoulou and Harvati2018; Tourloukis et al., Reference Tourloukis, Muttoni, Karkanas, Monesi, Scardia, Panagopoulou and Harvati2018a). Recent palaeoenvironmental studies conducted on MAR-1 sediments (Karkanas et al., Reference Karkanas, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018; Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021) and faunal remains (Roditi et al., Reference Roditi, Bocherens, Konidaris, Athanassiou, Tourloukis, Karkanas, Panagopoulou and Harvati2024) support the hypothesis that the site and the Megalopolis Basin in general served as “a refugium within a refugium” during MIS 12.
The MAR-1 sequence reveals a wetland situated on the margins of a lake in the low-lying area of the basin. Wetlands located in low-altitude areas, notably in locally moist and mild sites, are considered refugia for mesophilous trees (see Médail and Diadema, Reference Médail and Diadema2009). Lying between terrestrial and aquatic ecosystems (riparian zone), wetlands are among the world’s temporally and spatially most heterogeneous and dynamic habitats (Naiman and Décamps, Reference Naiman and Décamps1997) and can be perceived as mosaics of habitat patches within which soil moisture and nutrient properties vary (Stromberg, Reference Stromberg2001). Local environmental parameters can overrule the effects of climate on wetland vegetation communities (e.g., Sieben, Reference Sieben2019), as the hydrologic regime is the primary factor influencing the growth and performance of wetland plants (Tan et al., Reference Tan, Zhang, Li, Li, Xu and Jiang2016). Wetland vegetation, in turn, is a vital source of nourishment for semi-aquatic and terrestrial fauna and provides nesting habitats and shelter to some animals (see Mishra et al., Reference Mishra, Tripathi, Tripathi, Chauhan, Azooz and Ahmad2016). So far, the limited evidence concerning the MAR-1’s wetland vegetation dynamics (Field et al., Reference Field, Ntinou, Tsartsidou, van Berge Henegouwen, Risberg, Tourloukis, Thompson, Karkanas, Panagopoulou and Harvati2018; Reference Kyrikou, Marinova, Bludau, Karkanas, Panagopoulou, Tourloukis, Junginger and Harvatiin press) constrains our knowledge of the palaeoenvironmental setting that sustained the Megalopolis ecosystem during the course of MIS 12, one of the most severe glaciations of the entire Quaternary (e.g., Naafs et al., Reference Naafs, Hefter and Stein2014; Koutsodendris et al., Reference Koutsodendris, Kousis, Peyron, Wagner and Pross2019) and highly impactful for the middle Pleistocene of Greece (Hughes et al., Reference Hughes, Woodward and Gibbard2006; Leontaritis et al., Reference Leontaritis, Kouli and Pavlopoulos2020). While evidence for the presence of wetland taxa in the Megalopolis Basin has become available from palynological datasets of low temporal resolution spanning the middle Pleistocene (Nickel et al., Reference Nickel, Riegel, Schönherr and Velitzelos1996; Okuda et al., Reference Okuda, van Vugt, Nakagawa, Ikeya, Hayashida, Yasuda and Setoguchi2002), a detailed analysis of wetland vegetation dynamics through high-resolution palynological records is still lacking. Therefore, to contribute to a better understanding of the local conditions at MAR-1, we performed a detailed palynological analysis—including pollen, spores, non-pollen palynomorphs (NPPs), and pollen-slide charcoal—on a sediment sequence approximately spanning the period from ∼485 to ∼412 ka. The outcome of this analysis was interpreted in terms of (1) reconstructing the wetland vegetation responses to environmental and climatic changes in conjunction with various NPP types and the signal from the upland vegetation, (2) tracking particularly herbivore presence/absence in the area using coprophilous (and semi-coprophilous) fungal spores (hereafter CFS), and (3) detecting the major trends of the Megalopolis palaeofire regime based on pollen-slide charcoal and examining its potential linkages with the climate and/or local ecosystem feedbacks (including fauna and hominin presence). Our goal is to provide detailed insights into the local palaeoenvironmental conditions that shaped the Megalopolis ecosystem and to discuss the contribution of the palynological evidence for a better understanding of MAR-1’s role as a refugium for middle Pleistocene hominins during the harsh conditions of MIS 12.
Study area
Located in the central Peloponnese, southern Greece, the Megalopolis Basin is a half-graben, intermontane basin, covering ∼250 km2 and a maximum extension of 18 km at an altitude of 330–450 m above sea level (m asl) (Fig. 1). It is surrounded by Mt. Mainalo to the east, (1981 m asl), Mt. Lykaion to the west (1421 m asl), and the Taygetos range to the south (2407 m asl), and is drained by the Alfeios river system to the north.
MAR-1 is situated in the northwestern part of the Marathousa coal mine (37°24′31.6″N, 22°05′29.01″E at 350 m asl; Thompson et al., Reference Thompson, Tourloukis, Panagopoulou and Harvati2018; Fig. 1). Excavation was conducted in two areas, A and B, which are 60 m apart (Panagopoulou et al. Reference Panagopoulou, Tourloukis, Thompson, Athanassiou, Tsartsidou, Konidaris and Harvati2015, Reference Panagopoulou, Tourloukis, Thompson, Karkanas and Harvati2018). Situated 3 m north of Area B, the profile studied here is 7 m in height and subdivided into 10 lithological units (hereafter UB) 10 to 1 (Fig. 2). The sequence is bounded by the lignite seam (L) IIb (UB10) at the bottom and LIIIa (UB1) at its top, while the layers from UB9 to UB2 consist of lacustrine and fluviolacustrine clastic deposits (silts, sands, clays, and lignitic clays) (Supplementary Fig. 1). The find-bearing archaeological horizon, which contains >2000 lithic and organic artefacts (Tourloukis et al., Reference Tourloukis, Thompson, Panagopoulou, Giusti, Konidaris, Karkanas and Harvati2018b) as well as extensive faunal remains, is situated at the lower part of UB4 (close to the UB5–UB4 contact). A major erosional contact was identified in the middle part of the sequence, separating the profile into two parts (lower: UB10–UB6; upper: UB5–UB1), with UB6 being completely eroded laterally at some areas of the site. The sedimentological analysis strongly suggests a localised character to this erosional event (Karkanas et al., Reference Karkanas, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018).

Figure 2. Panoramic view of excavation areas A and B showing the section profile at Marathousa 1 (MAR-1) between the two lignite seams LIIb and LIIIa. The white box indicates the sampling site and the distribution of the lithological units UB1 to UB10 (modified from Bludau et al. Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021). See people for scale.
Falling within the temperate continental climate zone of the Northern Hemisphere, Greece is characterised by typical Mediterranean climate conditions. Specifically, southern Greece, which extends deeply into the Mediterranean region, is influenced by the maritime Mediterranean climate (http://climatlas.hnms.gr/sdi/?lang=EN). According to the Köppen-Geiger classification system and meteorological data obtained between 1971 and 2000 for the Megalopolis region (http://climatlas.hnms.gr/sdi/?lang=EN), the climate is classified as temperate, marked by hot and dry summers and mild winters (Csa; Kottek et al. Reference Kottek, Grieser, Beck, Rudolf and Rubel2006). The data yielded mean annual precipitation of 680 mm and mean annual air temperature of ∼15°C. During summers, the highest mean annual air temperature of 25.4°C was recorded in August, while in winters, the coldest month (January) reached a mean annual temperature of 6°C.
The topography and climate of the Peloponnese, comprising rugged limestone highlands fringed by sandstone foothills and a narrow coastal plain alongside its southern geographic position and isolation from the mainland, have contributed to the evolution of its unique present-day flora (∼3000 species) and high endemism (Thorogood, Reference Thorogood2019). The vegetation surrounding Megalopolis at the low and mid-altitudes (0–800 m) comprises Mediterranean vegetation, with Pinus halepensis forming pure stands near the sea. In river valleys and on flood plains, riparian woodlands are dominated by Platanus orientalis, accompanied by Salix spp., Alnus glutinosa, Populus nigra L., and Populus alba L.(Polunin, Reference Polunin1980). Further inland, the vegetation is characterised by maquis ecosystems, which mainly consist of evergreen shrubs and trees (such as Arbutus unedo, Olea europaea, Ceratonia, Juniperus phoenicea, Quercus coccifera, Q. ilex, Phillyrea latifolia, Pistacia terebinthus, Pistacia lentiscus, Calicotome villosa, Myrtus communis) and mixed deciduous forests (e.g., Carpinus orientalis, Ostrya carpinifolia, Quercus pubescens, Celtis australis, Acer spp., Sorbus domestica, Fraxinus ornus) situated at higher altitudes within the zone (Polunin, Reference Polunin1980). At higher elevations (>∼900–1800 m), the dominant Greek silver fir (Abies cephalonica) forms montane forests, while the lower slopes (∼1300–1600 m) are covered by dense forests of black pine (Pinus nigra subsp. pallasiana) (Polunin, Reference Polunin1980; Thanogianni, Reference Thanogianni2012). Above the timberline, at ∼1800 m elevation, subalpine grasslands develop along with shrubs (e.g., Juniperus communis, Prunus prostrata) (Polunin, Reference Polunin1980).
Materials and methods
Sampling, sample processing, and quantification
Pollen and charcoal samples were collected from a 7 m profile from MAR-1 Area B, with the study interval spanning from 25 to 700 cm. Pollen and charcoal analyses were performed on 61 samples, with sampling intervals ranging between 5 and 20 cm, except for the uppermost part (at the UB2–UB1 boundary), where the sampling resolution is 75 cm. Selected depths (240–340 cm/UB5–UB4) of particular interest—layers containing the archaeological horizon—were sampled at a resolution of 5 cm, aiming to reach the highest resolution analysis. Based on the revised age model for MAR-1 (see Age model section ), the temporal resolution of our pollen record spans between ∼350 (archaeological horizon) and 4500 (UB2–UB1 boundary) yr, with a mean of ∼700 yr.
For the palynological study, 3 g of dry sediment were processed for each sample. The processing was carried out using the standard pollen analytical methods modified by Fægri and Iversen (Reference Fægri, Iversen, Fægri, Kaland and Krzywinski1989). The steps included adding of two tablets of Lycopodium spores (Batch No.1031 [20,484 spores/tablet] and No. 10032 [14,285 spores/tablet] in each sample, chemical treatment with cold HCl [10%], KOH [10%], and cold HF [40%]), acetolysis, and sieving through 250 and 10 μm sieves. Residues were stored and mounted in glycerin jelly. For the charcoal study, we followed the pollen-slide method (Whitlock and Larsen, Reference Whitlock, Larsen, Smol, Birks and Last2001), which meets the standard pollen preparation methods described later. Microscopic analysis was performed using an Olympus light microscope under 400× magnification (for further details concerning identification of pollen, NPPs, and charcoal see Supplementary Material).
A minimum sum of 320 terrestrial-pollen grains was recorded per sample (mean: 400), while charcoal particles were counted until the total pollen sum in each sample was reached (Burjachs and Expósito, Reference Burjachs and Expósito2015). The calculation of terrestrial-pollen percentages was based on a pollen sum (PS) excluding Pinus pollen (due to long-distance transport) as well as spores and aquatic-pollen grains (as local elements). Furthermore, because we aim to reconstruct the wetland environment particularly, the local pollen sum (LPS) was introduced for calculating aquatic-pollen percentages. LPS includes all pollen taxa originating from wetland vegetation (helophytes + hydrophytes) and some herbaceous taxa coming from plants growing near wet areas (wet meadow group) (Supplementary Table 1). Spore percentages were calculated based on the LPS plus their total counts (monolete + trilete). Charcoal and palynomorph concentrations (number of palynomorphs/charcoal per gram) were calculated using a known quantity of Lycopodium spore markers; to ensure reliable concentration estimates, a minimum of 100 Lycopodium spores/sample was reached (Finsinger and Tinner, Reference Finsinger and Tinner2005). Influx rates of pollen (grains/g cm/ka), NPPs (microfossils/g cm/ka) and charcoal (particles/g cm/ka) were calculated by dividing the concentrations by the estimated depositional time (ka/cm) as inferred by MAR-1’s age–depth model.
Palaeoenvironmental reconstruction
We assigned pollen taxa into ecological groups to perceive and explain spatial and temporal vegetation dynamics in the Megalopolis catchment concerning hydrological, depositional, and climatic regimes. Our approach was based on considering relationships between plant taxa (taxa sharing common ecological/functional traits) and their corresponding habitats (e.g., wetland, upland area) (see Supplementary Table 1). Given that our study focuses on the highly local vegetation development at MAR-1, we present in detail the pollen taxa originating from obligate wetland vegetation (Fig. 3) to analyse the wetland ecosystem dynamics. Furthermore, all groups associated with the wetland vegetation are correlated with selected geochemical proxies from Bludau et al. (Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021) (Fig. 4) to infer local vegetation responses to palaeoenvironment and climate. Ecological groups showing the upland vegetation are also presented (Fig. 5) to infer persistence (or absence) of temperate trees to test the refugium hypothesis for Megalopolis and climate conditions throughout the study period. A detailed description of the upland vegetation on a taxon/species level and closer comparisons with regional climate-proxy records will be published subsequently.

Figure 3. Selected pollen and spore percentage diagram for the Marathousa 1 (MAR-1) sequence plotted against chronology. Substages of Marine Isotope Stage (MIS) 13 and MIS 12 are after Railsback et al. (Reference Railsback, Gibbard, Head, Voarintsoa and Toucanne2015); MIS 11c after Tzedakis et al. (Reference Tzedakis, Hodell, Nehrbass-Ahles, Mitsui and Wolff2022). Exaggeration factor (×10) on selected curves. Palynological zones (MARPZ), lithological units (UB), and dendrogram made by CONISS. The light-pink band shows the archaeological layer. The slash symbols on the y-axis and the blank space across the figure show the hiatus in the MAR-1 sequence.

Figure 4. Composite percentage diagram of selected ecological groups and selected geochemical proxies from the Marathousa 1 (MAR-1) sequence. Plotted against chronology. Exaggeration factor (×10) on selected curves. The slash symbols on the y-axis and the blank space across the figure show the hiatus in the MAR-1 sequence.

Figure 5. Pollen percentage diagram of selected ecological groups from the Marathousa 1 (MAR-1) sequence plotted against chronology (for detailed description of pollen taxa included in each group see Supplementary Table 1). Exaggeration factor (×10) on selected curves. The slash symbols on the y-axis and the blank space across the figure show the hiatus in the MAR-1 sequence.
To obtain further palaeoenvironmental information, we performed detailed analysis of various NPP types (all identified NPPs, their preferred trophic conditions, water environment, and substrate are listed in Supplementary Table 2). Selected algae and fungi were analysed (Fig. 6) to reconstruct water-level trends and trophic status of MAR-1’s aquatic environment. Analysis of the (semi-)CFS was performed to assess the presence of herbivores at MAR-1. Investigating the megafaunal presence through the sequence, along with the wetland landscape, climate, and fire regime, further enhances our high-resolution palaeoenvironmental reconstruction of the site and the Megalopolis Basin in general. Finally, to further evaluate woody vegetation development in the catchment, wood-decay and freshwater fungi are correlated with the influx rates of tree-pollen taxa (Supplementary Fig. 2).

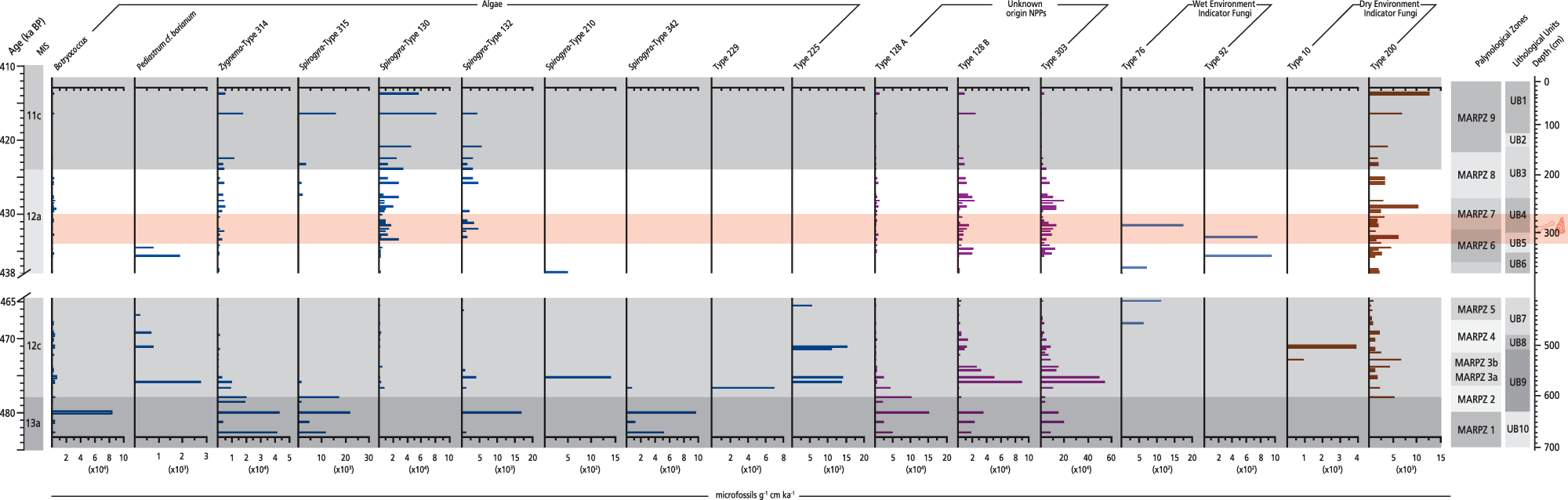
Figure 6. Non-pollen palynomorph (NPP) influx rate diagram plotted against chronology for the Marathousa 1 (MAR-1) sequence. Selected algae and fungi related to the aquatic environment are shown. The slash symbols on the y-axis and the blank space across the figure show the hiatus in the MAR-1 sequence.
Charcoal source area and taphonomic processes
Charcoal classification in size groups can aid in clarifying the source area from which the charcoal originates. More specifically, the fire source might be local (within the watershed), extralocal (close to the waterside but not within), and regional (distant areas) (Whitlock and Larsen, Reference Whitlock, Larsen, Smol, Birks and Last2001). According to the same authors, the pollen-slide charcoal represents regional source areas in general and interprets fire occurrence over broad temporal and spatial scales (e.g., subcontinental; Pan et al., Reference Pan, Mu, Gao, Behling, Liu and Wu2023). Particularly, microscopic (<50 µm) and mesoscopic (50–125 µm) particles are considered background noise, as they might be transported aloft many kilometres away from the fire site (e.g., Scott and Damblon, Reference Scott and Damblon2010), thus indicating regional to extralocal fires. However, when following the pollen-slide technique, the data should be considered with caution, given that chemical processing during the sample preparation can cause fragmentation of the larger particles (>125 µm)—which cannot be evaluated—thereby leading to artificially high abundance of microscopic charcoals (Whitlock and Larsen, Reference Whitlock, Larsen, Smol, Birks and Last2001) and underrepresentation of the local fire signal (Sadori and Giardini, Reference Sadori and Giardini2007). In contrast, the larger, heavier particles (>125 µm) tend to remain close to their source (<500 m from the lake shore; Lynch et al., Reference Lynch, Clark and Stocks2004) and incorporate into the soils, reflecting local fire occurrence (e.g., Sadori and Giardini, Reference Sadori and Giardini2007).
Therefore, we hypothesise that high abundance of microcharcoals in MAR-1’s sediments should represent either a distant fire source or the secondary fragmentation of larger particles from the basin, considering the deposition environment of the corresponding UB of the sequence (see “Discussion”). Nevertheless, the abundance of micro- to mesoscopic charcoals (particularly of <100 µm) can increase during local fire episodes, but other proxies (i.e., macrocharcoal and sedimentology) are required to support a local fire origin (see Whitlock and Larsen, Reference Whitlock, Larsen, Smol, Birks and Last2001). The macroscopic and (partly) a portion of the mesoscopic charcoals ((possibly products of macrocharcoal fragmentation) should reflect a local source, albeit the distance from the burned area cannot be assessed. Considering the basin’s topography, MAR-1—which lies at the west margin of the former palaeolake and is surrounded by mountain ranges—was isolated from the mainland of the Peloponnese (Fig. 1) as well as the main drainage system that developed in the southern and eastern part of the basin. Only small streams drained the western part, transporting relatively little input to the site (Karkanas et al., Reference Karkanas, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018) during the study period, and we therefore propose that macrocharcoal data should reflect fires that occurred within or close to the site.
Furthermore, biomass availability is also crucial for the amount and size of charcoal produced, so we compare charcoal data with pollen ones (Fig. 7) to reinforce our inferences regarding fire origins.

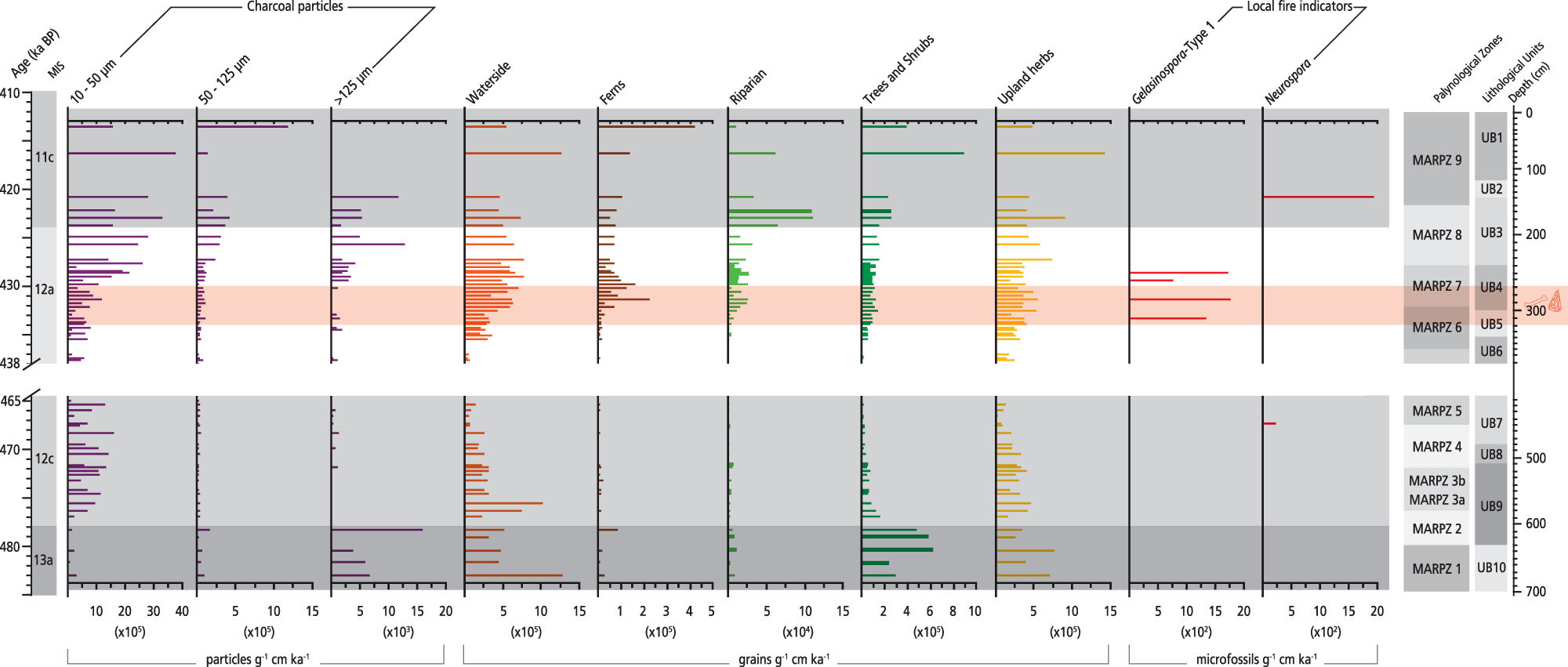
Figure 7. Influx rate diagram of charcoal, selected ecological groups, and spores from the Marathousa 1 (MAR-1) sequence plotted against chronology. The slash symbols on the y-axis and the blank space across the figure show the hiatus in the MAR-1 sequence.
Apart from the particle size, charcoal accumulations also reflect various processes associated with the fire history of a site. Particularly, the number of charcoal particles incorporated annually per unit area of substrate (influx rates) in lakes depends upon (1) charcoal production, which is based on fire characteristics (i.e., intensity, duration and temperature); (2) the type of material being burnt (i.e., fuel); and (3) other taphonomic processes (i.e., charcoal dispersal, deposition, and burial in the sediments) (e.g., Patterson et al., Reference Patterson, Edwards and Maguire1987). Thus, charcoal particles found in pollen slides represent material introduced in sediments during or shortly after a fire event, the so-called primary charcoal, and during non-fire periods, secondary charcoal (i.e., allochthonous input), owing to surface runoff or lake-sediment mixing (see Whitlock and Larsen, Reference Whitlock, Larsen, Smol, Birks and Last2001).
Therefore, high charcoal accumulations recorded in MAR-1’s sediments—particularly within the stratigraphic levels with abundant macrocharcoals (peaks), which are inferred to be evidence of local fires (e.g., Whitlock and Larsen, Reference Whitlock, Larsen, Smol, Birks and Last2001)—are considered cautiously and in conjunction with the depositional regime and sedimentation rates. These peaks may comprise both primary and secondary charcoal, reflecting either more than one palaeofire episode or even artificial peak values representing allochthonous charcoal input introduced in the sediments due to a runoff or erosion event.
Age model
Bludau et al. (Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021) created a Bayesian age model for the MAR-1 sequence based on its chronostratigraphic attribution to MIS 12 (Tourloukis et al., Reference Tourloukis, Thompson, Panagopoulou, Giusti, Konidaris, Karkanas and Harvati2018), as well as optical luminescence (OL) dates produced by the multiple-aliquot pre-dose multiple elevated temperature post-infrared infrared stimulated luminescence (pMET-pIRIR) dating method (Jacobs et al., Reference Jacobs, Li, Karkanas, Tourloukis, Thompson, Panagopoulou and Harvati2018). However, that model did not consider a major hiatus observed in the middle of the MAR-1 sequence, in UB6 (Karkanas et al., Reference Karkanas, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018). A major soil disturbance and erosion event identified close to the UB7–UB6 contact in the present study (see “Discussion”) further supports the sedimentary and stratigraphic evidence. Therefore, a new age model is developed here using the age modelling approach originally presented by Trauth (Reference Trauth2014) utilizing the software MATLAB, which considers possible hiatuses, thereby refining the previous model. The temporal starting and ending points of the sequence are 480 ± 10 ka and 420 ± 10 ka, respectively, agreeing with those used by Bludau et al. (Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021). Also, similar input parameters reflecting the depositional properties of the sedimentary units are applied (see Supplementary Tables 3 and 4). The code computes two different preliminary models, one based on the input sediment parameters and another statistically independent model based on the input ages. Both of them are considered to be equally valid. In the final model, both preliminary ones are merged. This newly computed age model places the MAR-1 sequence between 483 ± 17 ka and 410 ± 17 ka and, hence, into the glacial stage of MIS 12. The approximate ages for the respective units are shown in Supplementary Figure 3.
The newly computed age model now covers, in addition to the glacial stage of MIS 12, the end of MIS 13 and the beginning of MIS 11 interglacial periods . This corresponds better with the sampled sequence that includes parts of the overlying and underlying lignite layers (Fig. 2), which are generally accepted as representing warm (interglacial) periods (Nickel et al., Reference Nickel, Riegel, Schönherr and Velitzelos1996; van Vugt et al., Reference van Vugt, de Bruijn, van Kolfschoten and Langereis2000; Okuda et al., Reference Okuda, van Vugt, Nakagawa, Ikeya, Hayashida, Yasuda and Setoguchi2002). The exact ages used in the “Discussion” are primarily intended to ease references to the figures and should not be considered absolute ages.
Results
Palynological and charcoal assemblage zones
Nine palynological zones (Table 1) are defined based on quantitative and qualitative shifts of the dominant terrestrial- and aquatic-pollen taxa, as well as shifts in the lithology. The zonation is confirmed by stratigraphically constrained cluster analysis performed with CONISS software (Grimm, Reference Grimm1987). The zones are labelled with the prefix MARPZ (MARathousa Palynological Zone); the charcoal record follows the same zonation. The results are presented in Table 1 along with the inferred palaeoenvironment following the zonation, and the complete dataset is provided in the Supplementary Material.
Pollen record
Concerning the terrestrial pollen, the percentages of tree-pollen taxa increase from ∼30% at ∼483 ka to a maximum of 70% at ∼478 ka during MIS 13a; mesophilous taxa account for the highest abundances (40.8% max.) (Fig. 5). In the following interval (478–464.5 ka), the tree-pollen values drop by ∼32% on average in MIS 12c compared with MIS 13a. The mesophilous taxa exhibit mean values of 13%. In contrast to tree pollen, the abundances of steppic taxa increase by 11% on average compared with MIS 13a (Fig. 5, Table 1). While there are no palynological data for the interval between ∼464.5 and 438 ka due to a hiatus (see “Study Area”), evidence becomes available at ∼438 ka with the surge of disturbed ground taxa, of which Polygonum aviculare reaches its maximal abundances (71.5%) in the entire sequence (Fig. 5). From ∼436.5 to 428 ka, the period encompassing the archaeological horizon, pollen assemblages (MARPZ6–MARPZ7) document enhanced abundances of tree-pollen taxa. Specifically, the percentages of tree-pollen show two short-term increases of ∼30.6% each at ∼433 ka and 429.7 ka, respectively (Fig. 5). The duration of these temporal increases is 1.1 ka and 1.2 ka, respectively, and is defined as the time between the onset of increasing tree-pollen minima values and the tree-pollen maxima ones (peak). The values of mesophilous taxa increase by 3.5% on average, whereas those of steppic taxa decrease by ∼9 %. From ∼426 ka onwards, the tree-pollen percentages further increase by 11.3% on average, with the abundances of mesophilous taxa increasing by 5.3% (mean), followed by the rise of Mediterranean and pioneer taxa in MIS 11c (Fig. 5).
Table 1. Overview of the major characteristics of the palynological zones (MARPZ) from the Marathousa 1 (MAR-1) MAR-1 sequence:. Ppollen, non-pollen palynomorph (NPP)NPP, and charcoal data in context with selected geochemical proxies, inferred palaeoenvironment [(including lakeshore reconstructions from Bludau et al. ([Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021])], and fire sources.

Regarding aquatic pollen, the interval from ∼485 to ∼475 ka (MARPZ1–MARPZ3a) is marked by high abundances of Cyperaceae exhibiting ∼48% on average. The pollen spectra of Nymphaea and Myriophyllum spp. illustrate pronounced fluctuations in their values, peaking at 59.4% and ∼29%, respectively (Fig. 3, Table 1). Between ∼475 and 464.5 ka (MARPZ3b– MARPZ5), the abundance of Phragmites australis increases (35.5% mean), whereas Cyperaceae taxa are reduced by 14.5% on average. The percentages of submerged and floating-leaved taxa decrease during MIS 12c, exhibiting intermediate values (e.g., Nymphaea holds ∼8% on average). In contrast to MIS 12c, Nymphaea percentages temporarily increase by ∼3% during MIS 12a, particularly the interval corresponding to the deposition of the archaeological horizon (MARPZ6–MARPZ7; Fig. 3). From the MIS 12/11 transition onwards (∼426–413 ka; MARPZ8–MARPZ9), P. australis dominates (52% mean) the pollen assemblages, which are marked by the sharp increase of ferns (39% max.) at ∼413.5 ka.
Charcoal record
High influx rates of macrocharcoal particles are recorded in MIS13a (MARPZ1–MARPZ2) exhibiting 15,056 particles/g cm/ka (max.) at ∼478 ka, whereas they decrease in the following interval (MIS 12c; MARPZ3–MARPZ5; Fig. 7). In contrast, the highest influx rates of all particle size classes are documented between MARPZ7 and MARPZ9 (∼430–413 ka); macrocharcoals reach their maxima influx (12,772 particles/g cm/ka) in the entire sequence at ∼426 ka (MARPZ8).
NPP record
This is the first NPP record also encompassing the first multispecies CFS record of herbivore activity ever analysed from a middle Pleistocene site in Greece.
The highest influx rates of algal remains in the entire sequence are recorded in MIS 13a (∼485–478 ka; MARPZ1), where Botryococcus and Zygnema-type 314 reach 83,246 and 43,410 microfossils/g cm/ka, respectively (Fig. 6). In MIS 12 (∼477–428 ka; MARPZ2–MARPZ7) the NPP spectra display discontinuous presence of various algae and fungi related to the water environment (Fig. 6). A striking feature in this interval is the increased influx of Glomus-type and Sphaerodes-type exhibiting 4267 and 1349 microfossils/g cm/ka, respectively, at ∼438 ka (Fig. 8). The influx rates of Xylomyces-type increase during MIS 12a (Supplementary Fig. 2), as well as Gelasinospora-type 1 at ∼429 ka (max. 1706 microfossils/g cm/ka; MARPZ7; Fig. 7). The MIS 11c interval is marked by rising accumulation rates of green algae (e.g., Spirogyra-type 130 shows 81,528 microfossils/g cm/ka) as well as wood decay and fire indicators (e.g., Brachysporium-type and Neurospora show maxima of 19,819 and 1920 microfossils/g cm/ka, respectively) (MARPZ9; Fig. 7, Supplementary Fig. 2).

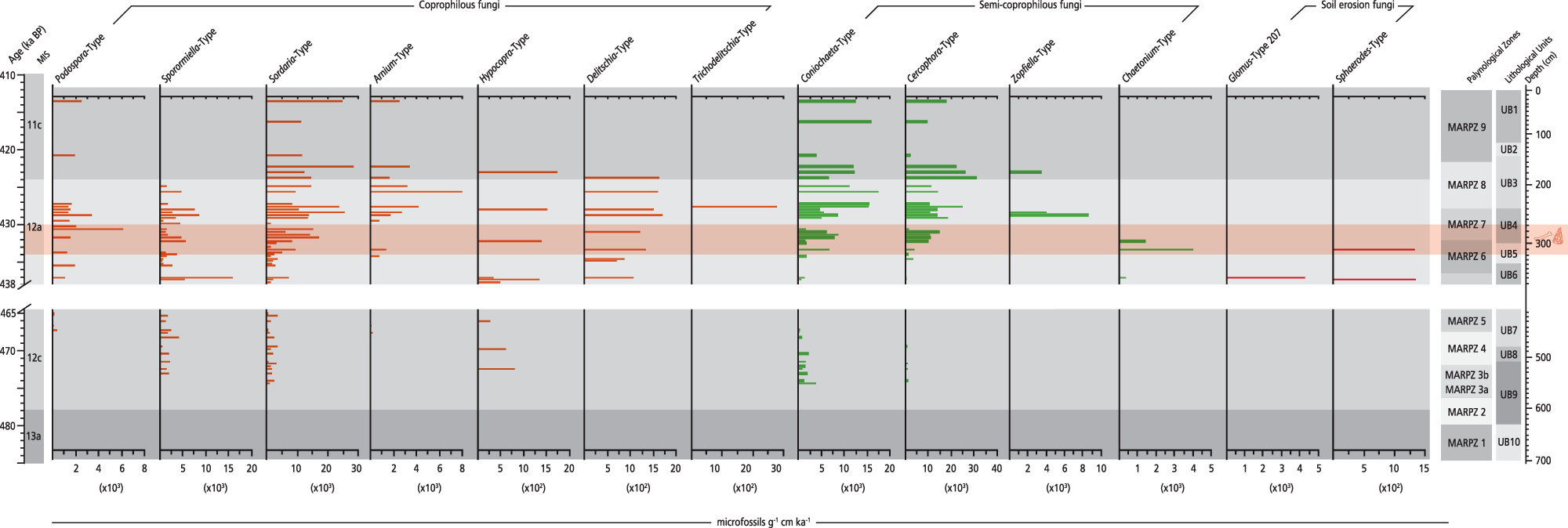
Figure 8. CFS influx rate diagram plotted against chronology for the Marathousa 1 (MAR-1) sequence. Selected coprophilous and semi-coprophilous fungi and soil erosion indicator fungi are shown. The slash symbols on the y-axis and the blank space across the figure show the hiatus in the MAR-1 sequence.
CFS record
The first CFS are documented in MIS 12c, with Sporormiella-type (1358 microfossils/g cm/ka on average) being the most common, followed by Hypocopra and Sordaria-type between ∼473 and 464.5 ka (MARPZ3b–MARPZ5; Fig. 8). The influx of all CFS increases during MIS 12a; especially within the archaeological horizon, Podospora-type and Delitschia-type exhibit their maximal influx in the entire sequence, that is, 6106 and 1706 microfossils/g cm/ka (∼435–428 ka; MARPZ6–MARPZ7). In contrast, the CFS influx values decline in MIS 11c; only Sordaria-type and a few semi-CFS are present (MARPZ9).
Discussion
Palaeoenvironmental interpretation
Descriptions of the preferred habitats for vegetation and NPP types and references are provided as Supplementary Tables 1 and 2.
The high abundance of Cyperaceae (sedges) pollen in the assemblages could have various palaeoenvironmental interpretations, given that Cyperaceae is a large family with a wide ecological range reaching from arctic tundra to tropical forests and seasonally wet grasslands (Simpson et al., Reference Simpson, Yesson, Culham, Couch, Muasya, Hodkinson, Jones, Waldren and Parnell2011). We assume that most Cyperaceae pollen reflects wetland environments, considering the overall MAR-1 pollen flora composition and palaeobotanical evidence retrieved from previous studies (Mädler, Reference Mädler1971; Nickel et al., Reference Nickel, Riegel, Schönherr and Velitzelos1996; Field et al., Reference Field, Ntinou, Tsartsidou, van Berge Henegouwen, Risberg, Tourloukis, Thompson, Karkanas, Panagopoulou and Harvati2018). Notably, plant macrofossil analysis on the Early Pleistocene lignite from Megalopolis (Mädler, Reference Mädler1971) identified various Cyperaceae wetland taxa such as Cyperus, Eriophorum, Scirpus, Eleocharis spp., and Rhynchospora. At MAR-1, Carex spp., Eleocharis palustris, Cladium mariscus, and Scirpus lacustris have been documented (Field et al., Reference Field, Ntinou, Tsartsidou, van Berge Henegouwen, Risberg, Tourloukis, Thompson, Karkanas, Panagopoulou and Harvati2018). Furthermore, high abundances of Cyperaceae—and Typha spp. (cattails) to a lesser extent—imply telmatic conditions (i.e., low water levels), given that typical wetland sedges and Typha latifolia prefer high soil moisture habitats, rich in nutrients (e.g., Uria-Diez et al., Reference Uria-Diez, Gazol and Ibáñez2014; Rasran et al., Reference Rasran, Hacker, Tumpold and Bernhardt2021). Accordingly, the predominance of sedges in the lower deposits of the sequence (UB10; MARPZ1; Fig. 3) indicates the development of fen (mire) peatlands during the formation of the lignite LII in MIS 13a. The peat originated primarily from the decomposition of Cyperaceae. Green algae might have also contributed to the formation of gyttja accumulations encountered in UB10 (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021), given that they can produce such accumulations when occurring in high concentrations (Shumilovskikh et al., Reference Shumilovskikh, O’Keefe, Marret, Marret, O’Keefe, Osterloff, Pound and Shumilovskikh2021).
Shifts in the abundances (high/low) of floating-leaved and submerged taxa indicate trends in the water column, reflecting the raising/lowering of water levels. Therefore, their fluctuating values between MARPZ1 and MARPZ2 suggest a transition from shallow wetlands where peat accumulations occurred in MAR-1 to temporarily open-water environments likely reaching up to 2 m depth (Dimopoulos et al., Reference Dimopoulos, Sýkora, Gilissen, Wiecherink and Georgiadis2005). NPP data reinforce this hypothesis; the decreasing green algae influx implies rising water levels at ∼478 ka (Fig. 6, Supplementary Table 2). This evidence suggests a variable environment at MAR-1, at the MIS 13/12 transition (interglacial–glacial).
In the early MIS 12, the MAR-1 site was characterised by marshy wetlands dominated by reed beds around the lakeshore area and shallow-water environments, as deduced from low abundances of wetland taxa and increased influx of dry environment fungal indicators (Figs. 3 and 6). However, in MIS 12a, the pollen assemblages—including those corresponding to the archaeological horizon—document a diverse, mosaic landscape. Thick stands of P. australis grew on periodically flooded soils, forming marshes on the littoral zone of the seasonal lake/pond near MAR-1. Sedges and cattails grew closer to the pond’s edges, as they require higher soil moisture. Furthermore, enhanced abundances of hydrophytes imply rising water levels (Fig. 3). The riparian zone was occupied by patches of riparian trees, which expanded between ∼424 and 422 ka, indicating the establishment of alluvial woodlands at MAR-1. At the same time, the increased influx of wood-decay and freshwater fungi further attests to the presence of shallow, freshwater habitats and woodlands at MAR-1 (Supplementary Fig. 2).
During MIS 11c, the previous mosaic structure of MAR-1’s wetland became simplified. The alluvial woodland at MAR-1 turned into an open dry marshland where thick stands of P. australis thrived around the pond/lake. Low abundances of Cyperaceae, Typha spp., and riparian trees suggest lower soil moisture. The rich content of green algae in MARPZ9 indicates a shallow, eutrophic water environment (Fig. 6). From 420 ka onwards, the surge of ferns implies the transition from a marsh to a fen/bog habitat where peat accumulations occurred during the UB1 formation. Identification of fern spores on a family/genus level was not feasible, albeit due to the correspondence of MARPZ9 with UB1 (Supplementary Fig. 1), fern presence is strongly associated with the wetland type. Therefore, the formation of the lignite LIII occurred in a shallow, stagnant waterbody where peat accumulations were derived from the decomposition of pteridophytes, helophytes, and other hydrophytes. The coeval presence of wood-decay and type 200 fungi (Fig. 6, Supplementary Fig. 2) suggests that temporal desiccation of the fen possibly activated these decomposers to decay organic material.
Vegetation and fire dynamics in response to palaeoenvironment and climate changes
The interval spanning from ∼485 to 478 ka (MIS 13a; MARPZ1–MARPZ2), the high abundances of mesophilous and montane tree taxa and the low amount of steppe elements in the catchment area of Megalopolis suggest a climate regime with relatively high temperatures and sufficient moisture availability for tree growth towards the termination of the MIS 13 interglacial period. Geochemical data from MAR-1’s sediments further reinforce this palynological evidence for wetter conditions. Particularly, the Rb/Sr ratios—which are considered a precipitation proxy (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021)—gradually increase through MARPZ2 (Fig. 4), attesting to a humid climate.
On a broader regional scale, our findings and climate inferences for this interval agree well with other pollen archives from the Mediterranean region. Pollen records from Tenaghi Philippon (northeast Greece; e.g., Tzedakis et al., Reference Tzedakis, Hooghiemstra and Pälike2006) and Vallo di Diano (southern Italy; Ermolli and Cheddadi, Reference Ermolli and Cheddadi1997) (Fig. 1) exhibit a similar vegetation character marked by mesophilous trees (mainly Quercus species), implying warm and wet conditions during MIS 13.
Regarding the local environmental conditions, low abundances of wetland taxa (i.e., submerged and floating-leaved taxa; Fig. 4) and the synchronous high influx of green algae until ∼479 ka point to eutrophic conditions in MAR-1’s waterbody at the lowlands of the basin during MIS 13a. Indeed, in shallow lakes, eutrophication is marked by the dominance of phytoplankton and the loss of submerged vegetation (Dong et al., Reference Dong, Yang, Li, Li and Song2014). Rising temperatures, shallow waters, light intensity. and increased nutrient concentrations promote eutrophication in aquatic environments (Nazari-Sharabian et al., Reference Nazari-Sharabian, Ahmad and Karakouzian2018). Therefore, this interval of excessive algal blooms at MAR-1 and the coincident increased temperatures at Megalopolis suggest that the local environment was influenced by climate change towards the MIS 13/12 transition.
Between ∼479 and 477 ka, a significant rise in wetland taxa (Fig. 4)—except a short-term drop in their abundances at ∼478 ka (see below)—indicates freshwater environments with still (to slow-flowing) waters at MAR-1. Specifically, the submerged vegetation in shallow lakes, like the one found at MAR-1, is usually associated with high water transparency (e.g., Moss et al., Reference Moss, Madgwick and Phillips1996; Zhang et al., Reference Zhang, Liu, Qin, Shi, Deng and Zhou2016), because its growth and survival is directly related to light availability in the water column (Dennison et al., Reference Dennison, Orth, Moore, Stevenson, Carter, Kollar, Bergstrom and Batiuk1993). The coeval decrease of green algae implies mesotrophic conditions and improved water oxygenation (Fig. 6). The presence of sponges and gastropods in the corresponding sediments further attests to favourable conditions in the waterbody at that period (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021). Possibly, the dense sedge stands near the palaeolake and the enhanced forest cover in the surroundings of the basin played a crucial role in improving MAR-1’s aquatic environment by eliminating potential sediment input from inflowing streams, thereby preventing sediment resuspension inside the lake and the subsequent deterioration of the underwater light regime. This hypothesis is confirmed by shoreline reconstructions at MAR-1, suggesting that the fine-clay composition of the corresponding deposits (UB10–UB9) and the absence of large sediment particles are associated with the presence of a thick vegetation belt at the lake’s margins (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021).
The transition from a floating-leaved to submerged-dominated vegetation after ∼478 ka is likely associated with a shift in ecological conditions inside the water environment. Specifically, higher oxygen concentration and light availability in the water column due to Nymphaea’s decline possibly led to the Myriophyllum spp. expansion. Floating-leaved plants in high abundances can substantially reduce oxygen and underwater light, affecting the growth of submerged plants (Caraco et al., Reference Caraco, Cole, Findlay and Wigand2006; Abarike et al., Reference Abarike, Amenyogbe, Ampofo-Yeboah, Abobi, Atindana, Alhassan and Akongyuure2015). Increasing Mn/Fe ratios (Fig. 4) corroborate the notion of higher oxygenation in the waterbody.
At the same time, high influx rates of macrocharcoal particles and pollen taxa (Fig. 7) suggest local fires in the Megalopolis Basin during MIS 13a. High values of terrestrial and waterside taxa and wood-decay fungi (Supplementary Fig. 2) point to sufficient living and dead plant material accumulations for burning in the catchment. The first peak of large-sized particles at ∼482 ka (MARPZ1) represents primary charcoal, given that the corresponding clayey sediments (UB10) were deposited in a stable waterbody protected by sediment resuspension. The low influx of micro- and mesoscopic charcoals further supports the hypothesis for a low-energy deposition environment and local fire signal. Likewise, the second macrocharcoal peak at ∼478 ka (MARPZ2) constitutes mainly primary charcoal, given the site’s high build-up of vegetation biomass. The notion of a local fire signal is strengthened by a short-lived event of local dry conditions documented at ∼478 ka; the temporal degradation of wetland taxa (Fig. 4) could have partially been a consequence of the disturbance caused by local fire activity in MAR-1. Nevertheless, secondary charcoal representing more than one fire episode cannot be excluded, considering the water-level fluctuations in MAR-1’s waterbody throughout MARPZ2 and the subsequent sediment mixing. The rise of mesoscopic charcoals might (partly) result from the secondary fragmentation of macrocharcoals due to sediment mixing.
A substantial change in the floristic composition of the Megalopolis Basin characterises the following period spanning the MIS 12 glaciation. More specifically, the palynological evidence indicates a significantly reduced forest cover in the catchment area from ∼478 ka upwards, with steppe elements dominating the basin and scattered patches of tree stands being restricted to the lowlands during MIS 12c (∼478.5–464.5 ka; Fig. 5). This apparent landscape opening reflects cold and dry conditions during MIS 12c. The sporadic presence of thermophilous Mediterranean taxa suggests that they reached close to their tolerance threshold for survival in this interval, thus further supporting the inferred low temperatures and moisture deficit. Geochemical data (decreasing Rb/Sr ratios; Fig. 4) from the corresponding deposits also corroborate the notion of increased aridity during MIS 12c.
Such climate conditions inferred by our pollen record for MIS 12c are also documented by other pollen archives from the south Balkans for the same period. Pollen data from Tenaghi Philippon (northeast Greece; e.g., van der Wiel and Wijmstra, Reference van der Wiel and T.A1987; Tzedakis et al., Reference Tzedakis, Hooghiemstra and Pälike2006; Pross et al., Reference Pross, Koutsodendris, Christanis, Fischer, Fletcher, Hardiman and Kalaitzidis2015) and Lake Ohrid (southwestern Balkans; Koutsodendris et al., Reference Koutsodendris, Kousis, Peyron, Wagner and Pross2019) (Fig. 1) show that the most severe tree-population contractions of the past 500 ka generally occurred during MIS 12 . Especially for the early part of MIS 12 (i.e., ∼478–457 ka; Railsback et al., Reference Railsback, Gibbard, Head, Voarintsoa and Toucanne2015), pollen data from Lake Ohrid show an average of 29.9% for arboreal pollen (AP) of which mesophilous taxa constitute 15.8% (Koutsodendris et al., Reference Koutsodendris, Kousis, Peyron, Wagner and Pross2019). For the Megalopolis catchment, the data exhibit more intense tree-pollen reductions; AP taxa account for 15.9% on average (mesophilous taxa 13% mean) for the interval between ∼478 and ∼464.5 ka (while the late part of MIS 12a is missing due to the lithological hiatus). Interestingly, pollen-based climate estimates for Lake Ohrid indicate mean temperatures of 17.6°C for the warmest month (Koutsodendris et al., Reference Koutsodendris, Kousis, Peyron, Wagner and Pross2019), which are similar to those inferred by the ostracod assemblages from MAR-1 for the same period (MARPZ4–MARPZ5/UB7), that is, summer water temperatures below 18°C (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021). Therefore, these lines of evidence imply that the moisture deficit was possibly the limiting factor for tree growth in Megalopolis rather than the temperature, reflecting more pronounced dry conditions at the southernmost latitudes of the south Balkans during MIS 12c.
While the terrestrial flora within the Megalopolis watershed was influenced by climate change, the wetland ecosystem was also affected by other environmental factors, such as changes in the hydrologic regime, limited underwater light, and eutrophication (e.g., Zhang et al., Reference Zhang, Liu, Qin, Shi, Deng and Zhou2016). In MAR-1, the degradation of wetland taxa (Fig. 4) reflects the dynamic character of the depositional environment between ∼476 and 464.5 ka. Micromorphological analysis of the sequence indicates that the deposition of its lower part (i.e., UB9–UB7) was occasionally affected by seasonal storm events, which in turn triggered hyperconcentrated flows reaching MAR-1’s aquatic environment (Karkanas et al., Reference Karkanas, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018). Furthermore, a recent palaeoenvironmental study for the corresponding sediments implies a fluctuating water table owing to the unstable riverine-deltaic environment that prevailed in-between UB8–UB7 as deduced by low total inorganic carbon TIC values and ostracod taphonomy (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021). The reduced arboreal vegetation in the catchment could have also facilitated the input of external sediment load and dissolved nutrients in the water during MIS 12c. Interestingly, the waterside taxa (Fig. 4) remained unaffected by this unstable environment; P. australis thrives among emergent taxa, as it is a strong competitor in wetland environments and can persist in wave action and currents, protecting other emergent plants (Packer et al., Reference Packer, Meyerson, Skálová, Pyšek and Kueffer2017).
Short-lived increases of riparian trees and wet meadow herbs (Fig. 3) in MIS 12c suggest temporarily higher soil moisture in the riparian zone. At the same time, however, from a catchment-scale perspective, the tree-pollen taxa exhibit extremely low abundances, and geochemical proxies show low precipitation (Rb/Sr ratios) and increasing alkalinity (Mg/Ti ratios) (Fig. 4), reflecting overall dry conditions and low evaporation. A plausible scenario for temporarily moist soils in MAR-1 might have been the higher availability of near-surface groundwater. The presence of small streams (possibly developed from melting of mountain snow covers) in the western part of the basin and the proximity of MAR-1 to their inputs—as inferred by the sedimentological analysis of the clastic sequence (UB9 to UB2; Karkanas et al., Reference Karkanas, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018)—could have allowed a more or less continuous recharge of the groundwater table in the low-lying areas of the catchment. Specifically, the sandy deposits of the sequence (UB8–UB6) would have facilitated the infiltration from water flows. Moreover, under the prevailing glacial conditions in Megalopolis, lower evapotranspiration rates might have also facilitated the rise of the groundwater table in the lowlands. Hence, temporarily damp soils can explain the vegetation development in the riparian zone under cold and particularly dry conditions. The same hypothesis was made for the formation of wetlands in the Po Plain (NE Italy) during the last glacial maximum by Miola et al. (Reference Miola, Bondesan, Corain, Favaretto, Mozzi, Piovan and Sostizzo2006), who inferred a higher groundwater table owing to the lower evapotranspiration.
While no environmental information for MAR-1 exists for the late MIS 12c and MIS 12b due to the hiatus, pollen data become available again in MIS 12a (∼438 ka). Particularly, the surge of the nitrophilous pioneer species P. aviculare—which shows substantial plasticity in response to disturbed areas (e.g., Meerts, Reference Meerts1995)—and of soil erosion fungi at ∼438 ka (Figs. 5 and 8) attest to an erosional event at the site. This evidence agrees with geochemical data and shoreline reconstructions for MAR-1; towards the UB7–UB6 (top MARPZ5) transition, all elemental and biological components show a sharp drop in their values, reflecting a change in the depositional regime, as argued by Bludau et al. (Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021). According to these latter authors, this event is accompanied by the surge of dust input in MAR-1’s sediments and is attributed to a period of warmer conditions that caused significant flooding; presumably, large volumes of meltwater from retreating glaciers—which had developed on the Peloponnese mountains during MIS 12 (Leontaritis et al., Reference Leontaritis, Kouli and Pavlopoulos2020)—eroded, transported, and deposited sediments into the basin. All these lines of evidence confirm that an erosional event caused these abrupt changes.
Regarding the Megalopolis’s fire regime, charcoal data suggest regional fire signal during MIS 12c (Fig. 7). The hypothesis for a distant fire source is further supported by low influx rates of pollen taxa, suggesting low biomass availability for burning in the catchment. Another plausible scenario could be that the increased microcharcoal influx reflects allochthonous input from the Megalopolis catchment, introduced in the sediments due to the unstable depositional environment. Patterson et al. (Reference Patterson, Edwards and Maguire1987) report increased microcharcoal values a few decades after a local fire event because of burned fallen down vegetation alongside the lake’s margin, while surface runoff following the fire event also transported secondary charcoal the following years. Considering the prevailing glacial conditions in the broader area of the Peloponnese during MIS 12 (Leontaritis et al., Reference Leontaritis, Kouli and Pavlopoulos2020), the latter scenario seems more realistic. Minor peaks of macrocharcoals are considered secondary and associated with MAR-1’s depositional history, given the deficit of plant fuels and local warm conditions—which favour fires—at Megalopolis at that time.
During the late part of MIS 12, the persistence of open vegetation (mainly steppe) in the catchment suggests the prevalence of a dry and cold climate at Megalopolis (Fig. 5). Such conditions are also documented by geochemical and biological proxies from the corresponding deposits (UB4–UB3) between ∼433 and 424 ka. Specifically, the ostracod assemblage indicates cold water temperatures (10–15°C), and decreasing Rb/Sr ratios imply low precipitation during this period (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021; Fig. 4). Nonetheless, superimposed on this long-term vegetation pattern during MIS 12, open mixed mesophilous woodlands temporarily developed between ∼433 and ∼430 ka, the interval encompassing the archaeological horizon (Fig. 5). The low, yet constant, presence of the thermophilous Mediterranean elements might be attributed to their local persistence in protected microhabitats at MAR-1’s wetland area, suggesting that the cooling was milder at the low-lying areas of the basin. Moreover, the coeval rise of the floating-leaved Nymphaea alba (Fig. 3) points to a temporal increase in moisture availability within the catchment in-between ∼434–433 ka, given that Nymphaea spp. habitats are regarded as precipitation dependent (Nzei et al., Reference Nzei, Mwanzia, Ngarega, Musili, Wang, Chen and Li2022). This hypothesis is reinforced by a synchronous short-lived moist episode (high Rb/Sr ratios; Fig. 4). Therefore, despite the overall glacial conditions, temporarily increased temperatures and sufficient moisture availability favoured the tree growth within the basin during MIS 12a.
The hypothesis of temperate conditions is further supported by palaeobotanical data from the corresponding sediments (i.e., UB4–UB3). Wood macroremains of mesophilous trees (i.e., Quercus spp. and Ulmus/Zelkova) and the presence of cf. Palmae in charcoal and phytolith assemblages attest to a relatively warm temperate climate between ∼433 and 424 ka (Field et al., Reference Field, Ntinou, Tsartsidou, van Berge Henegouwen, Risberg, Tourloukis, Thompson, Karkanas, Panagopoulou and Harvati2018). The present palynological analysis also documents the presence of identical mesophilous tree taxa in the same interval (MARPZ6–MARPZ8) (Kyrikou, S., unpublished data). According to Birks and Birks (Reference Birks and Birks2000), the presence of plant macrofossil taxa in an assemblage confirms that the respective pollen taxa identified in this assemblage originate from the catchment. From ∼428 ka onwards, the dry steppe biome gradually became replaced by grasslands, and the riparian trees increased, suggesting relatively humid conditions and damp soils at MAR-1.
Interestingly, pollen evidence from higher latitudes of the south Balkans indicates tree-population crashes during MIS 12a (∼442–424 ka), suggesting particularly cold and dry conditions. Especially, pollen data from Tenaghi Philippon document a total collapse of tree taxa (AP = 0% min.) in MIS 12a, the only one recorded at Tenaghi Philippon during the past 1.3 Ma (e.g., Tzedakis et al., Reference Tzedakis, Hooghiemstra and Pälike2006; Koutsodendris et al., Reference Koutsodendris, Dakos, Fletcher, Knipping, Kotthoff, Milner and Müller2023). Likewise, Koutsodendris et al. (Reference Koutsodendris, Kousis, Peyron, Wagner and Pross2019) report no forest expansion events (i.e., lowest mean AP abundances = ∼9% and min. 1.6%) in the catchment area of Lake Ohrid during MIS 12a, as well as the lowest temperature estimates for the entire MIS 12 (i.e., 0.2°C mean annual and 13.9°C mean warmest temperatures). These distinct differences in vegetation responses during MIS 12a, even within the south part of the Balkan Peninsula between its higher (i.e., Lake Ohrid and Tenaghi Philippon) and lower latitudes (i.e., the Megalopolis Basin; AP = ∼20% mean, 5% min.; mesophilous 15.4% mean in-between ∼438–424 ka), reflect regional-scale patterns concerning tree-population expansions and contractions. In addition, the mean summer temperatures at Megalopolis, as inferred by ostracods (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021), were higher by 1.1°C compared with those reported for Lake Ohrid. These lines of evidence suggest that climate conditions at the southernmost point of the Balkans were less severe during MIS 12a, thus allowing mesophilous and thermophilous trees to persist in Megalopolis; however, new climate-proxy records from MAR-1 and the Megalopolis Basin in general are necessary to draw robust conclusions about the climate, and particularly, the precipitation regime, as well as the responses of the Megalopolis ecosystem to climate oscillations during MIS 12a.
Despite prevailing climatic conditions that influenced the vegetation development at Megalopolis, local environmental parameters also controlled the wetland ecosystem at MAR-1 during MIS 12a. Specifically, the mosaic of habitats and mainly riparian trees (from ∼433 ka onwards; Fig. 3) was developed during a period marked by low precipitation (Fig. 8). A plausible scenario could be that the presence of a small spring/stream (surface water) close to MAR-1 during MIS 12a (Bludau et al., Reference Blackwell, Sakhrani, Singh, Gopalkrishna, Tourloukis, Panagopoulou and Karkanas2021) fed or recharged the groundwater table through seasonal seepage from its channel, thereby regulating periodically wet conditions in MAR-1 during the period of hominin occupation at the site. Furthermore, in the case of wetland taxa, their constant persistence could have also been facilitated by increasing oxygenation (Mn/Fe) and mesotrophic conditions (low algae content) in the water column (Figs. 4 and 6), given that a clear water environment with ample underwater light is an essential precondition for their growth. Such conditions are confirmed by the enhanced presence of diatoms and gastropods and the rich diversity of ostracods in the corresponding deposits (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021) in MIS 12a.
Towards the MIS 12/11 transition (termination V), the increasing forest cover and the establishment of alluvial woodlands (riparian trees; Fig. 3) at ∼424 ka point to a shift towards temperate conditions and sufficient edaphic humidity in the riparian zone. Peak values of Salix at ∼422.5 ka are synchronous with the rise of mesophilous trees and increased precipitation (Rb/Sr ratios; Figs. 4 and 5), also reflecting adequate moisture availability at Megalopolis. A similar pattern of Salix pollen has been recorded at Lake Ohrid between ∼427 and 424 ka (Kousis et al., Reference Kousis, Koutsodendris, Peyron, Leicher, Francke, Wagner, Giaccio, Knipping and Pross2018). According to those authors, this evidence signals the onset of the early interglacial forest phase that is characterised by increasingly warmer (wetter) climate and the presence of nutrient-poor soils. Considering that Salix is a pioneer tree with a competitive advantage for growth on poor soils, its dominance in MARPZ8 might be related to the onset of the MIS 11 interglacial period and increasing temperatures in the Megalopolis Basin.
Climate-proxy records from the broader area of the south Balkans evidence a similar climate regime to Megalopolis during the MIS 12/11 transition. Specifically, in Lake Ohrid (Fig. 1), this transition is marked by an abrupt increase in the mean annual precipitation (600 mm) and temperature (7°C) (Kousis et al., Reference Kousis, Koutsodendris, Peyron, Leicher, Francke, Wagner, Giaccio, Knipping and Pross2018). Likewise, the Tenaghi Philippon X-ray fluorescence and biomarker records indicate increasing precipitation from 425.9 ka onwards (Koutsodendris et al., Reference Koutsodendris, Dakos, Fletcher, Knipping, Kotthoff, Milner and Müller2023) and a sharp increase in the mean annual air temperatures (from 8°C to 16.5°C) from 431 ka (Ardenghi et al., Reference Ardenghi, Mulch, Koutsodendris, Pross, Kahmen and Niedermeyer2019).
The charcoal data suggest increased local fire occurrences in MIS 12a compared with the earlier periods. Local fires in MAR-1 were likely favoured by sufficient accumulations of local vegetation biomass for burning in the catchment area, as inferred by high abundances of both upland and wetland vegetation (Fig. 7) and wood-decay fungi; high Gelasinospora values also confirm local fire occurrence (Fig. 7). This notion is further strengthened by palaeobotanical data from MAR-1 that identify large wood charcoals from willows and alders (riparian), oaks, elms, and reeds (Field et al., Reference Field, Ntinou, Tsartsidou, van Berge Henegouwen, Risberg, Tourloukis, Thompson, Karkanas, Panagopoulou and Harvati2018), attesting to local biomass burning. Moreover, between ∼431 and 428 ka—the period including the archaeological layers—alkaline conditions (increasing Mg/Ti ratios; Fig. 4) documented in MAR-1’s sediments might be (partly) associated with local fires, given that fire alters soil chemistry through ash towards increasing alkalinity (see Shumilovskikh et al., Reference Shumilovskikh, O’Keefe, Marret, Marret, O’Keefe, Osterloff, Pound and Shumilovskikh2021). Local milder microclimate (temperatures) would have favoured fires during MIS 12a, and the precipitation regime might have also accounted for local fire events at MAR-1 towards the MIS 12/11 transition; mudflows identified in the corresponding sediments (UB3) are attributed to storm events (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021), which in turn could have triggered lightning strikes (the most common cause of fire; Pausas and Keeley, Reference Pausas and Keeley2009), promoting ignition. If so, the fuel would not have been saturated enough to prevent wildfires (Sadori and Giardini, Reference Sadori and Giardini2007).
The co-occurrence of micro- and mesoscopic particles points to regional wildfires; however, at least a portion of them could be a product of secondary fragmentation of larger particles, because the area was subjected to a minor postdepositional reworking process identified at the base of UB4 (Giusti et al., Reference Giusti, Tourloukis, Konidaris, Thompson, Karkanas, Panagopoulou and Harvati2018). Nonetheless, ascertaining microcharcoals’ origin in archaeological deposits is difficult, as natural and human-induced distortions should be considered (Lebreton et al., Reference Lebreton, Bertini, Ermolli, Stirparo, Orain, Vivarelli, Combourieu-Nebout, Peretto and Arzarello2019). According to these latter authors, in addition to potential taphonomic processes, increased charcoal fragmentation by hominin and animal trampling should be considered before, during, and after the main phase of occupation. In MAR-1, CFS suggest herbivore presence from MIS 12c, which peaks in MIS 12a (see Herbivore presence and environmental interactions at Marathousa 1). Thus, we assess, particularly in-between ∼431–428 ka (MARPZ7/archaeological horizon), that micro- and mesoscopic particles mirror regional and, to some extent, local origin of fires, even though the available data do not allow for determining the proportion. Moreover, significant microcharcoal input is anticipated in case of fire use by hominins (Lebreton et al., Reference Lebreton, Bertini, Ermolli, Stirparo, Orain, Vivarelli, Combourieu-Nebout, Peretto and Arzarello2019). Given the limitations of the pollen-slide method, it is difficult to evaluate the use of fire by hominins in MAR-1. So far, bones, artefacts, and macroscopic charcoal recovered from the site have not provided evidence of anthropogenic burning. Nevertheless, the potential role of hominins (or not) on the local fire regime in the Megalopolis Basin can also be evaluated by the nature of fire events, frequency, and intensity using our new sediment charcoal record (charcoal particles > 200 µm) from the MAR-1 sequence, to be assessed in our future work.
Within the early part of MIS 11, the increasing forest cover in the Megalopolis catchment points to a climatic amelioration with a shift towards warmer but still dry conditions, as deduced by the development of Mediterranean elements and the rise of pioneer shrubs (Fig. 5). The concurrent high concentrations of algal blooms (i.e., high productivity in the water column; Fig. 6) attest to higher temperatures in the Megalopolis catchment, similarly to the MIS 13a palynological evidence. In addition, at the lowlands of the basin in MAR-1, a continuous drying of the habitat is evidenced by shifts in the composition of wetland vegetation and increased accumulations of fungal indicators of dry environment (Figs. 3 and 6). Moisture deficit at Megalopolis is further indicated by elemental data (low Rb/Sr ratios; Fig. 4) from the corresponding sediments, which suggest low precipitation during MIS 11c.
Further evidence of increasingly warm and dry conditions during MIS 11c is provided from the broader region. Pollen data from Lake Kopais (southeast Greece; Fig. 1) show the expansion of Mediterranean woodlands (mainly comprised of Olea and Q. ilex), suggesting particularly warm and dry conditions at that time (Okuda et al., Reference Okuda, Yasuda and Setoguchi2001). In contrast, the Lake Ohrid (Kousis et al., Reference Kousis, Koutsodendris, Peyron, Leicher, Francke, Wagner, Giaccio, Knipping and Pross2018) and Tenaghi Philippon (Koutsodendris et al., Reference Koutsodendris, Dakos, Fletcher, Knipping, Kotthoff, Milner and Müller2023) pollen records show warm but wet conditions at that time. This evidence implies a N-to-S precipitation gradient in the south Balkans during MIS 11c.
Charcoal data also corroborate such climate conditions. Specifically, local fire occurrence, as deduced by the macrocharcoal peak at ∼421 ka, was likely favoured by the prevailing warm and dry climate. In addition to biomass availability, aridity is another necessary ecological parameter for natural fires, as dry conditions convert potential fuels to available ones (Pausas and Keeley, Reference Pausas and Keeley2009). The presence of Neurospora (Fig. 7) attests to local fire occurrence at that time, considering that it grows a few minutes after being subjected to moist heat (van Geel and Aptroot, Reference van Geel and Aptroot2006). At ∼416 ka, the last documented fire phase suggests an extralocal to regional fire signal, given the lack of macrocharcoals in MARPZ9.
However, some micro- and mesoscopic charcoals might reflect local sources, considering that microscopic particles can be produced by burning herbs and shrubs (Scott, Reference Scott, Cerda and Robichaud2009). Indeed, from ∼420 ka onwards, the transition from a marsh to peatland (characterized by high proportions of ferns and reeds) could have yielded high amounts of microcharcoals at MAR-1; fires occur naturally in peatlands (Link et al., Reference Link, Mclaughlin, Stewart, Strahm, Varner, Word and Wurster2023). Thus, adequate vegetation biomass at MAR-1, in addition to favourable climate conditions for fires, corroborates the hypothesis for local sources at ∼416 ka. However, due to limitations of the pollen-slide method, taxonomic identification of charcoal particles was not feasible, and any linkage to pollen taxa or (and) vegetation types that were burnt should be considered with caution.
Herbivore presence and environmental interactions at Marathousa 1
The first herbivore presence in the CFS record is documented between ∼473 and 464.5 ka within a steppe landscape where shallow, mesotrophic aquatic environments were present in the lowlands. Such a local setting possibly favoured the fauna, providing food sources and potable water under particularly dry conditions during MIS 12c.
Interestingly, the influx rates of Sporormiella-type peak at ∼437.5 ka. However, this peak is associated with a flood event that caused the hiatus in MAR-1’s sequence in-between ∼464.5–438 ka (see Vegetation and fire dynamics in response to palaeoenvironment and climate changes) rather than with a strong herbivore signal. Given that CFS can be transported from a non-local area during flooding (Lee et al., Reference Lee, van Geel and Gosling2022), this explains the surge of Sporormiella-type at that time.
Herbivore presence appears enhanced between ∼432 and 423 ka (high CFS influx; Fig. 8)—the period encompassing the archaeological horizon—suggesting that herbivore populations were concentrated near the MAR-1 wetland. This is corroborated by the rich faunal assemblage containing remains from micro- and macro-fauna (fishes, amphibians, birds, reptiles, mammals) and at least two individuals of the extinct straight-tusked elephant Palaeoloxodon antiquus (Doukas et al., Reference Doukas, van Kolfschoten, Papayianni, Panagopoulou and Harvati2018; Michailidis et al., Reference Michailidis, Konidaris, Athanassiou, Panagopoulou and Harvati2018; Konidaris et al., Reference Konidaris, Athanassiou, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018, Reference Konidaris, Athanassiou, Panagopoulou and Harvati2022) excavated at the archaeological horizon.
Within this interval, the mosaic of habitats developed in the Megalopolis’s catchment (as mentioned above) possibly played a critical role in the survival of terrestrial and semi-aquatic fauna during MIS 12a. Wetland vegetation creates habitats that promote faunal diversity, providing shelter, nesting locations, and food sources; for instance, the floating-leaved blades of N. alba and Nuphar lutea and several parts of wetland sedges and grasses (e.g., rhizomes, nutlets, seeds, tubers, leaves, and culms) serve as animal food (Mishra et al., Reference Mishra, Tripathi, Tripathi, Chauhan, Azooz and Ahmad2016; Klok and van der Velde, Reference Klok and van der Velde2017). Moreover, the two short-lived forest expansion events—having ∼1 ka duration each—point to temporarily denser vegetation cover that likely attracted herbivores to the site during MIS 12a. A plausible scenario could be that the higher availability of food sources favoured browsing or grazing activities by herbivores at MAR-1, thereby reflecting their preference for areas with higher vegetal biomass during glacial periods. The persistence of Mediterranean taxa corroborates the refugial character of MAR-1 in this interval.
Concerning water availability, shoreline reconstructions at MAR-1 indicate that the area was a floodplain during the deposition of MARPZ6 (∼436.5–432 ka), where seasonal (MARPZ7) to permanent (MARPZ8) ponds developed between ∼432 and 421.5 ka (Table 1). These aquatic environments that persisted for ∼15 ka in the region and provided habitat to several aquatic organisms (Bludau et al., Reference Bludau, Papadopoulou, Iliopoulos, Weiss, Schnabel, Thompson and Tourloukis2021) would also have furnished food sources and access to potable water for the terrestrial fauna. The rich marshy vegetation around the pond and the enhanced upland vegetation would have protected the waterbody by eliminating sediments and other pollutants from water runoff. This notion is confirmed by the corresponding NPP assemblages, which mirror a shallow freshwater environment at MAR-1.
In contrast to MIS 12a, our data suggest herbivore biomass decline at MAR-1 during the beginning of the MIS 11 interglacial period. The postulated faunal decline (MARPZ9) is contemporaneous with climate and local hydrologic shifts. Climate and hydrologic regimes can affect the composition and abundance of fungal flora and might prevent the preservation of Sporormiella fungus on the site (Davis and Shafer, Reference Davis and Shafer2006; Wood and Wilmshurst, Reference Wood and Wilmshurst2013). Humidity is the most crucial factor in the growth of coprophilous fungi (Lee et al., Reference Lee, van Geel and Gosling2022). In MAR-1, local dry conditions are evidenced by the decline of riparian trees and wetland taxa and the rise of fungi, indicators of dry environments (Figs. 4 and 6) from ∼422 ka onwards. It could be that the local moisture deficit (i.e., soil moisture) affected the growth and preservation of CFS in MAR-1’s sediments during that time. If so, the CFS decline does not reflect a reduced faunal presence in the greater area of the basin but particular taphonomic conditions at MAR-1.
Alternatively, the dry area might have been no longer attractive to the fauna, which thus visited MAR-1 less often. From the ecological perspective, the degraded aquatic environment characterised by stagnant, anoxic waters and eutrophic conditions by ∼422 ka (Fig. 6) rendered MAR-1’s habitat inhospitable to certain taxa, supporting this interpretation. Furthermore, charcoal data seem to support the scenarios for local environmental stressors. Specifically, the CFS decline at ∼422 ka coincides with the termination of the most intense fire phase documented in the MAR-1 sequence (see Vegetation and fire dynamics in response to palaeoenvironment and climate changes). The more extended burning in the vicinity of the site—starting at ∼432 ka (MARPZ7) and culminating within MARPZ8—in conjunction with the overall climate and environmental oscillations, probably negatively affected the ecosystem, thereby forcing faunal populations to abandon the site.
On the other hand, the precipitation regime points to overall arid conditions in the Megalopolis catchment during MIS 11c. Geochemical proxies from the corresponding sediments have shown contrasting patterns in precipitation (fluctuating Rb/Sr ratios; Fig. 4) since ∼432 ka (MARPZ7). Particularly, moderate Rb/Sr ratios indicate a relatively stable state of moisture availability at the site between ∼432 and ∼425 ka when CFS present their maximal influx values. On the contrary, an abrupt increase in precipitation at ∼423 ka (high Rb/Sr ratios, upper MARPZ8) was followed by a sharp decrease from ∼422 ka onwards (low Rb/Sr ratios, MARPZ9), the time when CFS influx decreases. These rapid shifts in precipitation regime during MIS 11c—which diverged from the previous relatively steady state during MIS 12a—likely promoted changes in Megalopolis’s terrestrial and aquatic ecosystem, leading to a drastic herbivore population decrease in the region. Cooper et al. (Reference Cooper, Turney, Hughen, Brook, McDonald and Bradshaw2015) examined megafauna species along with major megafaunal transitional events (widely distributed geographically across Eurasia and North America) during the past 56,000 yr against the Greenland ice core record. Those authors suggested that the regional turnover of megafauna species was promoted during the onset of interstadial periods when the most rapid climate shifts in the late Pleistocene occurred. Our hypothesis for herbivore decline associated with abrupt shifts in the Megalopolis climate regime appears to agree with the proposed hypothesis of these authors; however, we underline that our hypothesis needs further analysis to draw a solid conclusion.
Despite the abovementioned scenarios, one can argue that the low temporal resolution of MARPZ9 is possibly masking the CFS signal in the record or, alternatively, that the otherwise increased influx of semi-CFS (Cercophora-type and Coniochaeta-type), along with the presence of few CFS (e.g., Sordaria-type) that grow primarily on dung, suggests persistence of faunal populations in the site. While such a hypothesis cannot be rejected, it is worth mentioning that this interval corresponds to the deposition of LIIIa (UB1) (Karkanas et al., Reference Karkanas, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018) and the peatland development at MAR-1. Consequently, given that the former semi-CFS primarily grow on peat deposits, the synchronous presence of the latter is associated with the prevailing vegetation rather than with herbivore presence (see Supplementary Table 2 for preferential substrates of CFS).
Notwithstanding the proposed hypotheses for the CFS decline at the onset of MIS 11, the interpretation is still equivocal; the putative faunal decline might have been specified locally to MAR-1 or might reflect absence at the broader catchment of Megalopolis or even could mirror taphonomic bias in the signal of the CFS record. Therefore, only by applying a multi-core approach (Lee et al., 2022), that is, studying material from different coring sites within the basin covering the same interval, we can have better insights into whether the CFS data show the very local or regional signal of herbivore presence/absence in the Megalopolis Basin..
Could the Megalopolis Basin have served as a “safe haven” for the biota during the MIS 12 glaciation?
The pollen analysis of the MAR-1 sequence shows the local presence of tree populations in the Megalopolis Basin during MIS 12c and MIS 12a. More specifically, the persistence of mesophilous (and thermophilous in MIS 12a) trees, even in low abundances, indicates the persistence of moist environmental conditions in the lower altitudes of the catchment, reflecting the refugium character of MAR-1 and the Megalopolis Basin in general. The tree populations were possibly restricted to areas with well-drained soils near the riparian zone, which served as a protected microhabitat (small refugium) where the local microclimate and topography provided constant moisture availability, mediating their persistence. In addition, the continuous presence of aquatic vegetation and wet meadow herbs at the site, even during periods of a very shallow water environment, witnesses the capability of MAR-1 to retain water and support life within or in the nearby damp soils. Médail and Diadema (Reference Médail and Diadema2009) stressed the need to also consider herbaceous species when examining the potential of a site as a refugium, because their local survival and distribution patterns might vary from those of tree species, as phylogeographic studies have shown (e.g., Abbott and Comes, Reference Abbott and Comes2003; Cozzolino et al., Reference Cozzolino, Cafasso, Pelegrino, Musacchio and Widmer2003). Wetlands have also served as refugia for boreal herbs, such as marshes in northern Italy, southern France, and northeastern Algeria (see Médail and Diadema, Reference Médail and Diadema2009). Therefore, the MAR-1 wetland habitat, which was located near the palaeolake that existed in the Megalopolis Basin during the Pleistocene, allowed a diverse flora to survive during the glaciation of MIS 12 and to expand after its termination, as deduced by the tree-pollen abundance documented in MARPZ9 during MIS 11c (Fig. 5).
In addition to this long-term vegetation development during MIS 12, two distinct periods of changes in the vegetation composition can be identified in pollen data based on fluctuations in the arboreal pollen/ non-arboreal pollen (AP/NAP) ratios. In particular, the first period, spanning almost the entire MIS 12c, indicates drastic tree-population reductions, reflecting particularly dry conditions at that time. In contrast, during the second period (∼438–424 ka; MARPZ6–MARPZ8), which also includes the archaeological horizon, two short-term enhancements of tree populations—in addition to the increased average AP values—imply sufficient edaphic humidity and milder conditions at MAR-1 during the otherwise particularly cold and dry MIS 12a. Moreover, changes in floristic composition are also observed between these two periods ( see archaeological horizon in the Vegetation and fire dynamics in response to palaeoenvironment and climate changes subsection ). This is in agreement with the sedimentological evidence (Karkanas et al., Reference Karkanas, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018) that indicates completely different depositional environments between the upper (UB5–UB1) and the lower part (UB10–UB6) of the sequence.
A first concise comparison of the MAR-1 record with other pollen archives from the south Balkans (see Vegetation and fire dynamics in response to palaeoenvironment and climate changes subsection)) suggests, particularly for the MIS 12a, that the Megalopolis ecosystem responded differently to prevailing climate compared with Tenaghi Philippon and Lake Ohrid (Fig. 1), reflecting less severe dry conditions at the southernmost part of the Balkans. This evidence appears to confirm our assumption about the role of Megalopolis as a glacial refugium during MIS 12, with an emphasis on MIS 12a, where all the archaeological evidence is found, representing a refugium within a refugium at that time. Nevertheless, in the presence of a significant lithological hiatus in the MAR-1 sequence spanning the entire middle part of MIS 12 (i.e., MIS 12b), we underline the fact that further research in the site examining high-resolution and continuous pollen records is necessary to explore in detail the vegetation dynamics of the Megalopolis ecosystem throughout MIS 12, and comparisons with other climate-proxy records are necessary to comprehend how climate influenced the ecosystem dynamics in the basin.
Concerning fauna, the fact that MAR-1’s wetland ecosystem attracted and sustained herbivores—even in low abundances—during MIS 12c, the period when Megalopolis was marked by the most intense forest contractions and a particularly dry climate regime, further supports the refugial character of the site. During the hominin occupation, the herbivore populations became abundant under wetter (milder) conditions and increased vegetation cover in the basin during MIS 12a. Considering the pollen evidence for severe tree-population contractions (and particularly cold conditions) at the higher latitudes of the south Balkans during MIS 12a, our CFS data, in addition to pollen data, also confirm the refugium hypothesis for the Megalopolis Basin at that time.
Consequently, the natural features of the site, such as ecosystem diversity (aquatic, semi-aquatic, and terrestrial), would also have provided essential food resources to hominins, such as plants and animals, as well as water and shelter from cold and arid conditions in the interval between ∼436.5 and 428 ka. Notably, the wide variety of plants (MARPZ6–8) might have created a potential for a broad diet. Phytolith analysis from the Theopetra Cave (central Greece) suggests that sedge rhizomes were consumed by MP hominins (Tsartsidou et al., Reference Tsartsidou, Karkanas, Marshall and Kyparissi-Apostolika2015). Despite the lack of evidence of plant exploitation either in MAR-1’s pollen record or in palaeobotanical remains retrieved from the corresponding sediments (Field et al., Reference Field, Ntinou, Tsartsidou, van Berge Henegouwen, Risberg, Tourloukis, Thompson, Karkanas, Panagopoulou and Harvati2018), this middle Pleistocene wetland was rich in plant resources, including edible taxa for hominins like seeds and rhizomes of Nymphaea and Nuphar, S. lacustris rhizomes, or Bolboschoenus (synonym = Scirpus) tubers (see Hillman et al., Reference Hillman, Madeyska, Hather, Wendorf, Schild and Close1989; Wollstonecroft et al., Reference Wollstonecroft, Hroudová, Hillman and Fuller2011). It could have offered the potential to hominins to utilise the vegetation directly, for example, by consuming fruits, seeds, and tubers/rhizomes, or indirectly, using the hard or fibrous parts of plants (e.g., reed stems) to produce tools for various purposes (see also Guibert-Cardin et al. [Reference Guibert-Cardin, Tourloukis, Thompson, Panagopoulou, Harvati, Nicoud and Beyries2022] for the use of MAR-1 artefacts on both animal and plant materials). Furthermore, hominins would have been attracted by the herbivore presence on the site. Indeed, abundant herbivore populations congregated in MAR-1 would have provided hominins a wide range of subsistence opportunities. Cut-marked elephant and other mammal bones from MAR-1 were found in association with lithic remains documenting human exploitation through butchering activity at the site (Konidaris et al., Reference Konidaris, Athanassiou, Tourloukis, Thompson, Giusti, Panagopoulou and Harvati2018; Guibert-Cardin et al., Reference Guibert-Cardin, Tourloukis, Thompson, Panagopoulou, Harvati, Nicoud and Beyries2022). In addition to the ecosystem diversity, the Megalopolis Basin had the potential to serve as a glacial refugium for the survival of all the biotic components of MAR-1’s ecosystem (i.e., to sustain wetland habitats and furnish potable water for faunal and hominin populations) due to its capability to retain water at its lowlands (see also Cuthbert et al. [Reference Cuthbert, Gleeson, Reynolds, Bennett, Newton, McCormack and Ashley2017] for the groundwater hydro-refugia) under the otherwise cold and dry conditions during MIS 12. We therefore hypothesise that the Megalopolis Basin could have acted as a long-term refugium offering survival over the course of the Pleistocene. However, further investigation from other sites with different temporal scales within the basin (see Konidaris et al., Reference Konidaris, Athanassiou, Panagopoulou and Harvati2022) is necessary to test this hypothesis.
Conclusions
This is the first study to examine a palynological record encompassing pollen, NPPs, and charcoal to infer the potential of a middle Pleistocene archaeological study in Greece to have served as a glacial refugium. The present record from MAR-1, Megalopolis Basin (southern Greece), provides new insights into the local environment during the early and late part of the glacial stage of MIS 12. We find that open vegetation predominated the catchment of the basin, where mainly mesophilous trees and aquatic vegetation persisted in the low-lying areas of the basin during MIS 12c and MIS 12a. Two short-term tree-population expansions documented within the archaeological horizon reflect local wetter and milder conditions between ∼436.5 and 428 ka. Various NPPs and hydrophilous taxa indicate fluctuating water levels throughout the studied interval, suggesting that the site was periodically inundated by groundwater supply or enhanced precipitation. Sufficient moisture was likely the most crucial parameter determining the survival and certain diversity of the biota in MAR-1 during MIS 12c and MIS 12a.
Herbivore presence is documented from ∼473 ka in MAR-1 and become intensified between ∼435 and 423 ka, within the archaeological horizon, as evidenced by our CFS record. The mosaic of plant habitats within the basin would have provided shelter and food to both fauna and hominins at that period. CFS decline is recorded from ∼423 ka onwards, under prevailing warm and dry conditions in MIS 11c. Nonetheless, the direct cause of this observation and its implications for the megafaunal populations is unclear.
The present pollen-slide charcoal analysis is our first attempt to detect the major trends of the Megalopolis fire regime and its relation to climate and the local ecosystem during MIS 12. The charcoal data reveal a linkage between climate, available vegetation biomass, and fires. Local fire occurrences were mainly documented towards the transitions of MIS 13/12 and MIS 12/11, when climate conditions and plant biomass availability could have favoured natural fires in the catchment. Despite the limitations of the pollen-slide technique for reconstructing palaeofires, it allowed the investigation of fires in response to significant vegetation and climate changes.
In conclusion, our results support the role of the Megalopolis Basin as a glacial refugium within a refugium that enabled the survival of flora, fauna, and hominins, most likely due to its capability to retain water during the dry and cold conditions of MIS 12c and MIS 12a, and possibly also during the other glacial intervals of the Pleistocene.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2024.59.
Acknowledgments
Excavation at Marathousa 1 was conducted under a permit granted to the Euphoria of Paleoanthropology Speleology, Greek Ministry of Culture. Support was provided by the European Research Council ERC Consolidator Grant ERC-CoG-724703 (“CROSSROADS”) and the ERC Starting Grant ERC-StG-283503 (“PaGE”), both awarded to KH. KH and VT are also supported by the Deutsche Forschungsgemeinschaft (DFG FOR 2237; DFG HA 5258 “Early Human Adaptations in Megalopolis”) and the (ERC AdG 101019659 “FIRSTSTEPS”). A. Koutsodendris and one anonymous reviewer are gratefully acknowledged for their constructive comments that improved this article.
Competing Interests
The authors declare that they have no conflicts of interest.