Policy Significance Statement
Conventional decision-making methods fall short in recommending validated and evidence-based policies during outlier events. During such events, Artificial Intelligence methods provide a viable alternative for better decision and policy making at medical institutions. We present three methods that provide data-driven guidance during healthcare crises; the methods aim at helping hospital managers and public health officials in preparing their budgets, identifying optimized economic plans for distribution of health relevant items (such as vaccines and masks), and overall resource allocation.
1. Introduction and Motivation
Healthcare resources consist of materials, personnel, facilities, and anything else that can be used for providing a healthcare service to patients at hospitals. Studies in healthcare economics have suffered from lack of relevance to practical realities on the ground. Potential deployments of such economic reevaluations have proven that the reality is different from theory (Niven, Reference Niven2002). In the wake of a global pandemic, policy making has been scrutinized and public trust in opinion-based policy making has been diminished. It is more important than ever to begin integrating Artificial Intelligence (AI) systems into the policy evaluation and advocacy process. Evidence-based approaches facilitate policy making in a manner that is more relative to the scientific method, beginning with initial hypotheses and evaluating them based on experimentation. Accordingly, in this study, we compare: (a) conventional (unnormalized, no learning, no optimization), with (b) AI-based (normalized, unsupervised learning, with optimization) methods, and derive recommendations to hospital managers accordingly. Healthcare management and executive teams often face the challenge of resources’ management but end up compromising decisions to avoid issues with public health standards/laws and other safety compliances. That strategy is however destined to fail due to the many hidden consequences; for example, a survey identified that most finance directors in Scottish hospitals were unaware of the cost of back injuries among nurses (McGuire et al., Reference McGuire, Silbersweig and Wright1995). The vast majority of hospitals run their budgets and economics based on previous experiences and heuristic intuitions of management. Moreover, the Economic Rate of Return is very difficult to quantify in the context of health, given the multidimensional aspects that feed into the budget. For instance, patient satisfaction, readmission rates, hospital costs and budgets, and many other factors are important to consider during the measurement of the efficiency and quality of service.
Moreover, decision-making’s difficulty exacerbates during outlier events that directly affect healthcare services. Multiple factors influencing healthcare resource allocation in such scenarios arise from economic, geographic, or demographic dynamics. Each outlier event enforces a wide variety of factors which makes its analysis for healthcare policy extremely complicated. In order to extricate the process from such difficulties, in this paper, we present implementations of AI methods, such as Reinforcement Learning (RL), and Genetic Algorithms (GAs) to evaluate different policy scenarios and point to the best possible outcome. By using the mentioned methods, we incorporate the varying factors of healthcare services along with the Traveling Salesman Problem (TSP), during conventional times as well as during outlier events to find an optimized equilibrium of resources’ allocation and sharing paths. We argue that AI methods produce improved results over existing traditional methods. The research hypotheses that our study aims to evaluate are twofold: (a) AI-based methods can provide superior results (fitness value) when compared to conventional nonoptimized methods for resources allocation; (b) as hospitals are grappling with datasets that they collect, real-world data (collected from veteran affairs and other sources presented in Section 3.1) can be used to test the presented methods and develop an interactive dashboard that presents the outcomes for decision support at a hospital.
This paper is structured as follows: the next section reviews existing resource allocation and sharing methods, as well as similar AI examples, Section 3 introduces the three methods, Section 4 confers the results and other discussions, and lastly, Section 5 presents the conclusions.
2. Background and Related Work
2.1. Resource allocation in healthcare
Pandemics (such as Covid-19) and other mass casualty events place enormous demands on public health and health systems (Christian et al., Reference Christian, Sprung and King2014; Bell et al., Reference Bell, Abir and and Choi2018). They can cause a shortage of hospital beds and medical equipment. They are also likely to affect the availability of the medical workforce, since doctors and nurses likely become ill or quarantined. Such demands create the need to ration the allocation of scarce resources. If preparedness is inadequate, the foundation of all disaster response efforts can crumble with subsequent adverse patient outcomes (Sandrock, Reference Sandrock2014).
In disasters, while some in “hot spots” have found their resources rapidly depleted, others have found themselves managing largely empty intensive care units (ICUs) waiting for an inevitable surge of critically ill patients. A coordinated nationwide response is needed for an effective and responsive strategy as the outlier event moves across the country. However, other than in the case of organ transplants, governments at all levels have unfortunately had little experience in engaging stakeholders in priority setting, rationing lifesaving resources, and implementing policies (Ramachandran et al., Reference Ramachandran, Swamy, Kaul and Agrawal2020). Since the New York attacks of 2001, the hurricane season of 2005, and the Ebola and Covid-19 outbreaks, significant resources have been used in the United States to improve the processes behind care provided in a disaster or pandemic (Romney et al., Reference Romney, Fox and and Carlson2020). However, even the well-designed allocation guidelines that have been proposed so far by various professional societies present challenging problems in real-time decision-making and implementation. To help clinicians navigate these challenges, institutions have employed triage officers, physicians in roles outside direct patient care, or committees of experienced physicians and ethicists, to assist in applying guidelines and making timely decisions at the bedside (Emanuel et al., Reference Emanuel, Persad and and Upshur2020). A lottery system has also been proposed to better promote equity and social justice by removing the likelihood of people being given preferential treatment on the basis of social or economic advantages (Silva, Reference Silva2020). According to the National Institutes of Health (NIH), healthcare disparity can be recognized by analyzing the relationship between healthcare utilization and household assets; outcomes of resource allocation can cause different levels of disparities (Gómez et al., Reference Gómez, Jungmann and Lima2018).
One of the commonplace methods for resource allocation originated from the United Kingdom and is currently implemented in multiple health systems of Low- and Middle-Income Countries such as South Africa (Love-Koh et al., Reference Love-Koh, Griffin and and Kataika2020). A regional health-funding formula is created by either taxation or insurance values; this method promotes both vertical and horizontal equities: for instance, regions with the same health needs are provided with similar resources (horizontal), while regions with different health needs are provided with dissimilar resources (vertical).
Another method of resource allocation is through the use of Health Benefits Packages (HBPs). This method is most beneficial in resource-constrained settings because it offers an alternative to traditional formulas when defining area-level allocations (Schreyögg et al., Reference Schreyögg, Stargardt, Velasco-Garrido and Busse2005). HBPs are funded based on the expected costs of providing services and the expected target patient population.
In the United Kingdom, the Resource Allocation Working Party (RAWP) manages the process (Smith, Reference Smith2008). Historically, resources were allocated based on precedent, which created geographical bias toward areas such as London and South East England (something we address in Method #3). This produced an imbalance in the healthcare system in the United Kingdom. Recently, however, health resources are disaggregated into a small number of disease categories that are based on the World Health Organization (WHO)’s international classification of disease. This approach allowed for the notion of “weighted capitation” to surface.
RAWP utilized a predefined set of variables as follows (Gillie et al., Reference Gillie, Slater and Biswas1988): first, per capita need is calculated by disaggregating the population by age and gender. Corresponding healthcare utilization of each demographic group is approximated by using the national average hospital bed utilization (per capita). RAWP broke down healthcare into a smaller number of broad categories of conditions and their index of approximate need of care. That is determined through the application of standardized mortality ratios and the population of geographical areas. The formula that was generated from this process relies on five variables: illness ratios, mortality ratios, proportions of economically active yet unemployed citizens, proportions of pensionable-age and “living-alone” citizens, and the proportion of dependents per household.
In the United States, the challenge of healthcare expenditures has been a major part of policy debates. For example, nearly half of Americans have at least one chronic condition. Direct medical costs for chronic conditions are >$750 billion annually (Batarseh et al., Reference Batarseh, Ghassib, Chong and Su2020). Out of the four possible healthcare models for a country (The Beveridge, Bismarck, Out-of-Pocket, and National Insurance), a model such as the Affordable Care Act (ACA—i.e., Obamacare) provides guarantees for the pursuit of preventive healthcare, but no pointers to healthcare access, resource allocation, or other geographical-relevant issues such as medical deserts in the United States. Additionally, in recent years, the ACA has been deployed with high costs, the program has low adoption rates, and it still suffers from partial public’s rejection.
Based on our extensive search for a “gold standard” in medical resources sharing, no standard was found that is agreed upon in the research community or in practice. Generally, every hospital system has their own network and resources, especially in the case of private institutions. In the case of public institutions, such as veteran affairs hospitals presented in our study, resources sharing is based on policies by the government, and is mostly an opinion/expertise-based process that is maneuvered based on the context. Even in AI research, no existing methods are found that are dedicated to healthcare resources management. A key objective of quantitative healthcare economic analyses is to uncover relationships—for example, number of beds, patients, and staff—for use in making predictions or forecasts of future outcomes. However, current systems that generate forecasts for decision-making tend to use ad hoc, expert-driven, personal partisanship, linear, factor, and nonlinear models. Multiple factors influencing healthcare economics in outlier scenarios arise from nutritional, geographic, or demographic variables and each policy comes with a wide variety of such factors. Work presented in this paper aims to facilitate that and provide an intelligible alternative.
2.2. Review of existing AI deployments
As it is established, measuring the health of economy is a long-lasting science; however, only recently, the economy of health is a gaining traction and is a rising research area. Successful national models in healthcare have varied between public and private. Previous studies (Friedman and Oren, Reference Friedman and Oren1995) have shown that policy making for resource allocation is complex and requires thorough data analysis. AI, namely, RL and GA can provide improved suggestions over existing traditional methods. During major economic shifts, AI can capture hidden trends and provide real-time recommendations that conventional heuristic-based approaches may fail to arrive at. However, the application of AI in healthcare economics is still at its inception; in Table 1 we review “similar” methods and illustrate a chronological review (1982–2020) of RL methods applied in comparable decision-making scenarios.
Table 1. Reinforcement Learning (RL) for decision-making
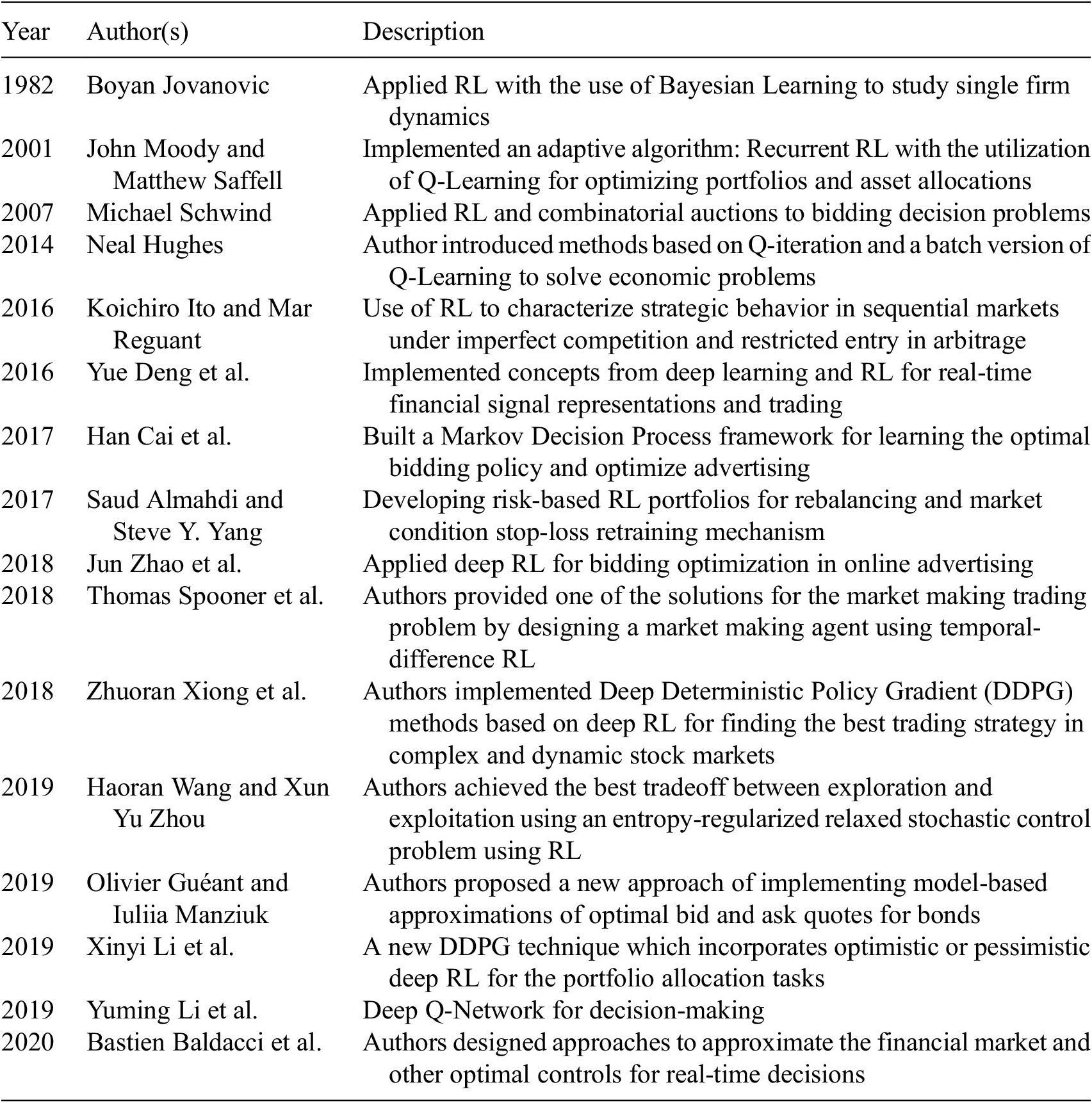
As shown in Table 1, different researchers have tried applying RL methods to assisting with decision-making, but to the best of our knowledge, none have provided AI-based methods for policies and decisions for resource allocation during outlier events. In this project, we present AI-based methods for that goal.
3. Methods
The results of data mining endeavors are majorly driven by data quality. Throughout these deployments, serious show-stopper problems are usually unresolved, such as data collection ambiguities, data imbalance, hidden biases in data, the lack of domain information, and data incompleteness. In a traditional data science lifecycle, outliers, bias, variance, boosting, over and under sampling, and data wrangling are measures that can be tuned to mitigate output quality issues and misinformation. In our work, we performed data collection from multiple sources to mitigate issues relevant to one dataset. Additionally, normalization, descriptive analytics, hyperparameter selection, and other quality-control methods are implemented.
3.1. Data collection
Real-world data of this study are collected from Centers for Medicare and Medicaid Services (CMS) including data on patient complications and deaths, hospital general information, cost, and value of care for each hospital (Centers for Medicare and Medicaid Services, 2020). Additionally, data for hospital beds are collected from Open Data DC—the Office of the Chief Technology (Open Data DC, 2020). Covid-19 patients’ data are collected from Centers for Disease Control and Prevention (CDC) (National Center for Health Statistics, 2020). CDC data are important in this context because hospitals in the United States follow CDC regulations when it comes to reporting healthcare-related practices. U.S. Veterans Affairs (VA) medical centers information is collected as well from the U.S. Department of Homeland Security (2020); the VA system constitutes the main real-world example of this paper—due to federal regulations, patients’ data, and hospital network information are scarce and difficult to acquire. Nonetheless, VA hospital performance star rating is also gathered (U.S. Department of Veterans Affairs, 2018) to be used as part of the input for training the models. Data from the U.S. Census Bureau provided the files for geo-visualization, and other studies (Voinsky et al., Reference Voinsky, Baristaite and Gurwitz2020) have provided the estimated recovery rates for Covid-19 patients based on gender and age group (U.S. Census Bureau, 2019).
For all data sources, normalization is performed to standardize and smooth the statistical distributions of different datasets. The values are scaled to match a range. For instance, data are normalized by performing simple linear scaling from the original scale to a 0–1 scale, or a 0–100 scale. LinearScaling (an R package) is used for normalizing the range of values of a continuous variable using a linear scaling within the range of the variable, using:
LinearScaling(x, mx = max(x, na.rm = T), mn = min(x, na.rm = T)),
where x is a vector with numeric values, mx is the maximum value of the continuous variable being normalized (defaults to the maximum of the values in x), and mn is the minimum value of the continuous variable being normalized.
All datasets, code, and scripts used for the experiment are available on a GitHub public page: https://github.com/ferasbatarseh/EconHealthAI
3.2. Method #1: Resource management using RL
RL is a subfield of AI; it involves the process of learning of an agent by using a trial and error approach that maximizes rewards by interacting with dynamic environments (Kaelbling et al., Reference Kaelbling, Littman and Moore1996; Szepesvári, Reference Szepesvári2010). RL is different from supervised and unsupervised learning. In supervised learning, a dataset is available with predefined labels that represent knowledge; while in unsupervised learning, the labels are not initially provided, and the outputs are not predetermined. In contrast, RL is goal-oriented, it processes learning from the interactions as compared to other approaches of AI (Sutton and Barto, Reference Sutton and Barto2018). Chades et al. (Reference Chades, Chapron and Cros2017) noted the application of RL to decision-making such as in the forest management problem; which inspired part of the work presented here (Method #1). We use a form of RL referred to as Markov Decision Process (MDP), which is suitable for time series data and does not require a simulation environment like Q-Learning, (another form of RL). Forest management consists of two contrasting objectives: maintaining an old forest for wildlife preservation, and/or making money by selling the forests’ wood (Cros et al., Reference Cros, Peyrard and Sabbadin2016). This tradeoff is often the case with decision-making. The concept can be applied to healthcare resource allocation as follows: keep the resources (i.e., conserve wildlife) versus share resources with other hospitals in your region (i.e., cut the trees). Such decision questions exacerbate during externalities—such as outlier events—that directly affect healthcare services and other hospital processes. In economics, an equilibrium of economic outcomes is usually modeled to study tradeoffs and support policy and decision-making.
In optimization problems, algorithms are performed in an iterative fashion until the best result is found. This concept works well with RL (Li and Malik, Reference Li and Malik2016). In RL, based on the policy chosen, the agent will take an action, the action will change the environment, and the outcome is then evaluated. As the environment changes, the policy will be updated based on a reward or a punishment due to the policy change, and then the model will make a transition into a “better” new state (Mousavi and Li, Reference Mousavi and Li2020).
In the RL model developed for resource allocation and sharing, multiple stages are deployed. At the beginning of each stage, an action is selected and evaluated by the algorithms (good decisions are rewarded, and “bad” decisions are punished). For instance, in the forest example:
-
• If the action is “cut”—which equates to the “share” option in our study, the loop goes back to stage 1; otherwise it moves to the next stage.
-
• If a wildfire occurs, the loop goes back to stage 1—requires a different decision process.
-
• The algorithm rewards the decision when the forest trees are old, and presents a cut or wait (which equates to “idle” in Section 4—the results of our study) suggestion, which in turn, each has a different reward.
-
• The goal is to maximize the reward by choosing the appropriate action at each stage.
The initial values for rewards and the probability of wildfire are used through the MDPtoolbox package in R (Chades et al., Reference Chades, Chapron and Cros2017). Additionally, a real-time web application is developed to change these values in real-time and to understand how actions and rewards changes for each state based on the “wildfire” probability. Execution data are generated using the mdp_example_forest() function from within MDPtoolbox. The tool, results, and other outcomes are presented in the results section.
3.3. Method #2: Resource management using GA
GA is a subfield of evolutionary algorithms; they are inspired by the process of biological natural selection. By using bioinspired methods such as crossover, mutation, and selection, GAs have been found to be one of the relatively effective algorithms for optimization problems. In this study, we deploy GAs independently, as well as in coordination with algorithms such as TSP (Kim et al., Reference Kim, Lee and Kim2013)—an R package, GA is used. There are three types of GAs: binary, real-valued, and permutation (Scrucca, Reference Scrucca2013, Reference Scrucca2017). Binary GAs are used to solve binary representation of decisions such as whether the hospital should share resources or not, while permutation involves reordering a list of objects, which is suitable with TSP for choosing/sorting which hospitals are best to share with due to distance and other external factors—we experiment with both methods (permutation and binary).
In GA, the input data constitutes the initial random population, or as it is called, the first generation. The fitness of the generation will be measured; “parents” will be randomly selected based on a fitness function. During the reproduction state, by crossing over of “genes,” two “children” will be produced, and mutations occur with a given rate. If a mutation occurs, one gene will be randomly changed, which then leads to a new generation. The process continues until the termination condition is met and the population is more “fit” (Woodward and Kelleher, Reference Woodward and Kelleher2016).
GAs are controlled by multiple hyperparameters; we set the following values (deemed as defaults) throughout: Probability of mutation: 50%; Population size: 1,000; Maximum iterations: 250 (without improvement); Default iterations: 1,000.
Healthcare variables that are inputted to the GAs as determinants of generational updates are: hospital performance, death rates, and number of beds. Additionally, two types of fitness functions are used for comparison: a fitness function with all the mentioned variables + hospital ratings (FF1), and fitness function without hospital rating (FF2). Based on Lewis et al. (Reference Lewis, Pham, Batarseh, Batarseh and Yang2020), hospital ratings, reputation and ranking has a major effect on the quality of service. (Batarseh and Yang, Reference Batarseh and Yang2020) Therefore, we aimed at exploring that aspect.
In both fitness functions: the following constants are deployed: α, β, and γ. The constants aim to weight each input variable differently (if needed according to policy). Data used in this analysis have been normalized. Fitness functions 1 (FF1) and 2 (FF2) are presented below:
 $$ \mathrm{FF}1\hskip1.5pt =\hskip1.5pt \mathrm{Hospital}\ \mathrm{rating}\times \left(\frac{\;\alpha \times \mathrm{number}\ \mathrm{of}\ \mathrm{beds}}{\beta \times \mathrm{death}\ \mathrm{rate}}+\frac{1}{\gamma \times \mathrm{cost}}\right), $$
$$ \mathrm{FF}1\hskip1.5pt =\hskip1.5pt \mathrm{Hospital}\ \mathrm{rating}\times \left(\frac{\;\alpha \times \mathrm{number}\ \mathrm{of}\ \mathrm{beds}}{\beta \times \mathrm{death}\ \mathrm{rate}}+\frac{1}{\gamma \times \mathrm{cost}}\right), $$
The quality of service is an important indicator of whether the hospital is ready for outlier events and whether it is well equipped. However, if Methods #1 and #2 can aid with presenting a sharing recommendation, how can a hospital manager find the best/nearest/readiest hospital to share with or share from? Method #3 addresses that question.
3.4. Method #3: Resource management using TSP
TSP is an important combinatorial optimization problem in the field of theoretical computer science and operations research (Lu et al., Reference Lu, Fang, Shao and Liu2007). The purpose statement of TSP is simple: “to find the best route to visit each location once and return to the original position”, TSP belongs to a family of nondeterministic polynomial-time problems (NP) (Calude, Reference Calude, Calude and Queen2013; Hillar and Lim, Reference Hillar and Lim2013).
By using the concepts of TSP, different factors have been implanted into the traditional algorithms and used by a fitness function through GA. The goal is to optimize the best route for resources delivery during outlier events. That is done while not merely considering distances, but also other factors, such as availability of resources. Due to the lack of patients’ locations at the city and county levels, the algorithm is not based on specific addresses, rather, on hospital geographical locations and state centers (by longitude and latitude). We began by using the state center. The variables were used to create an index to maximize “good” sharing of resources and minimize “bad” resources’ allocation. TSP is coded into two fitness function of the GA algorithm (to allow the GA to consider distance and traveling optimizations as it is parsing and learning from the data), one with healthcare variables (FF3), and other with geographical distance (FF4). Both formulas are shown below:
FF3 variables have data collected from Covid-19 patients and scenarios to illustrate an outlier situation. Cost in the fitness functions is based on the following formula:
In the experiment, only contiguous U.S. states (excluding Alaska and Hawaii) and the District of Columbia have been included. For FF4, due to the lack of patients’ data in Minnesota and Wyoming, these two states are excluded as well. GAs are controlled by multiple hyperparameters; we set the following values throughout, by trial and error, and they provided the best statistical accuracy:
-
1. Probability of mutation: 20%
-
2. Population size: 100
-
3. Maximum number of iterations: 5,000
-
4. Default iterations: 1,000
In addition to the GA package, two more R packages are used for mapping: rgdal is used to collect data from the Geospatial Data Abstraction Library (GDAL), and tidygeocoder was used for mapping the results (Bivand et al., Reference Bivand, Keitt and Rowlingson2020; Cambon, Reference Cambon2020).
Results for FF3 and FF4 as well as all three methods (#1, #2, and #3) are presented in the next section.
4. Models’ Results, Assurance, and Discussions
This section presents the results of the three methods (RL, GA, and TSP) and discusses the outcomes in light of deployability and policy.
4.1. RL results
Method #1 is deployed through a real-time R-Shiny web application (shown in Figure 1). But how does this apply to outlier events? As of September 7, 2020, there have been 6,280,400 patients suffering from Covid-19 in the United States (Johns Hopkins University & Medicine, 2020). To suitably manage the resources for patients, it can be essential to understand the pandemic’s hot zones and severities. For this purpose, we used the Pandemic Severity Assessment Framework (PSAF) proposed by the CDC in 2014 (Centers for Disease Control and Prevention, 2016). PSAF is driven by two factors—clinical severity and transmissibility—which determine the overall impact of a pandemic (Reed et al., Reference Reed, Biggerstaff and Finelli2013). The presented web application is to be used at a hospital for real-time decision-making support. The RL algorithm is connected to the application, and the model could be retrained in real time when new data are available. A hospital manager can use the “hospitalization ratio,” a parameter in clinical severity (aggregate of patients’ situation) for determining the amount of resources needed. The mentioned RL stages are represented as scales based on transmissibility and clinical severity. The outcomes (i.e., actions) are assigned as either “Idle” or “Share”—which represents the recommendation that the RL algorithm generates.
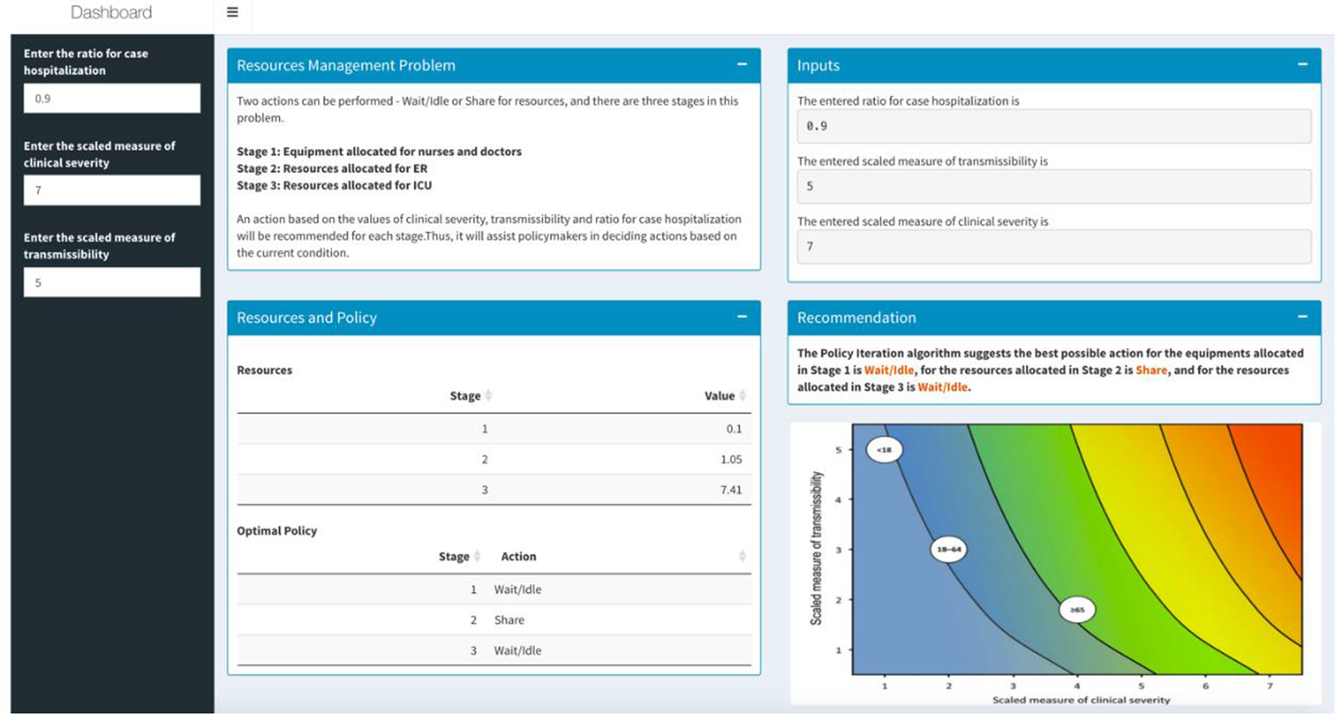
Figure 1. Resources management dashboard using Reinforcement Learning.
The MDP RL model intakes all parameters (such as budget and staff availability) and provides a table indicating a value matrix, where every value is associated with a scale. A negative value indicates an “Ask” action for more resources, while a higher positive value indicates a surplus in resources (Table 2). RL is a dynamic classification approach, and it is deemed as one of the most successful for real-time, in real environments, and for binary outcomes—we apply that for hospital resources allocation, a notion that has not been deployed prior. Our prototype can be used as an early failure detection system that can guide hospital policy makers to make resource sharing decisions in a proactive manner.
Table 2. Resources management decisions using Reinforcement Learning (RL) (based on the Pandemic Severity Assessment Framework scale)

4.2. GA results
The algorithm in Method #2 provides policy makers suggestions of whether the hospital should request additional resources based on the current conditions of the hospital (Table 3 shows the top 10 hospitals in terms of fitness). As it is noted in Batarseh and Yang (Reference Batarseh and Yang2020), hospital rankings are often not specific to certain goals such as ranking in readiness for resource allocation—which is part of the overall readiness for an outlier event. Using GAs (two different fitness functions) and based on the variables discussed, in Table 3, we present the readiness ranking for hospitals. If such results are embedded into a data dashboard for hospital management, managers can see which hospitals are best candidates for resources sharing during the time of need. The number of beds is generated as a decimal by the algorithm for accuracy, but could be rounded up in practice.
Table 3. Top 10 hospitals in terms of resource sharing readiness using Genetic Algorithms
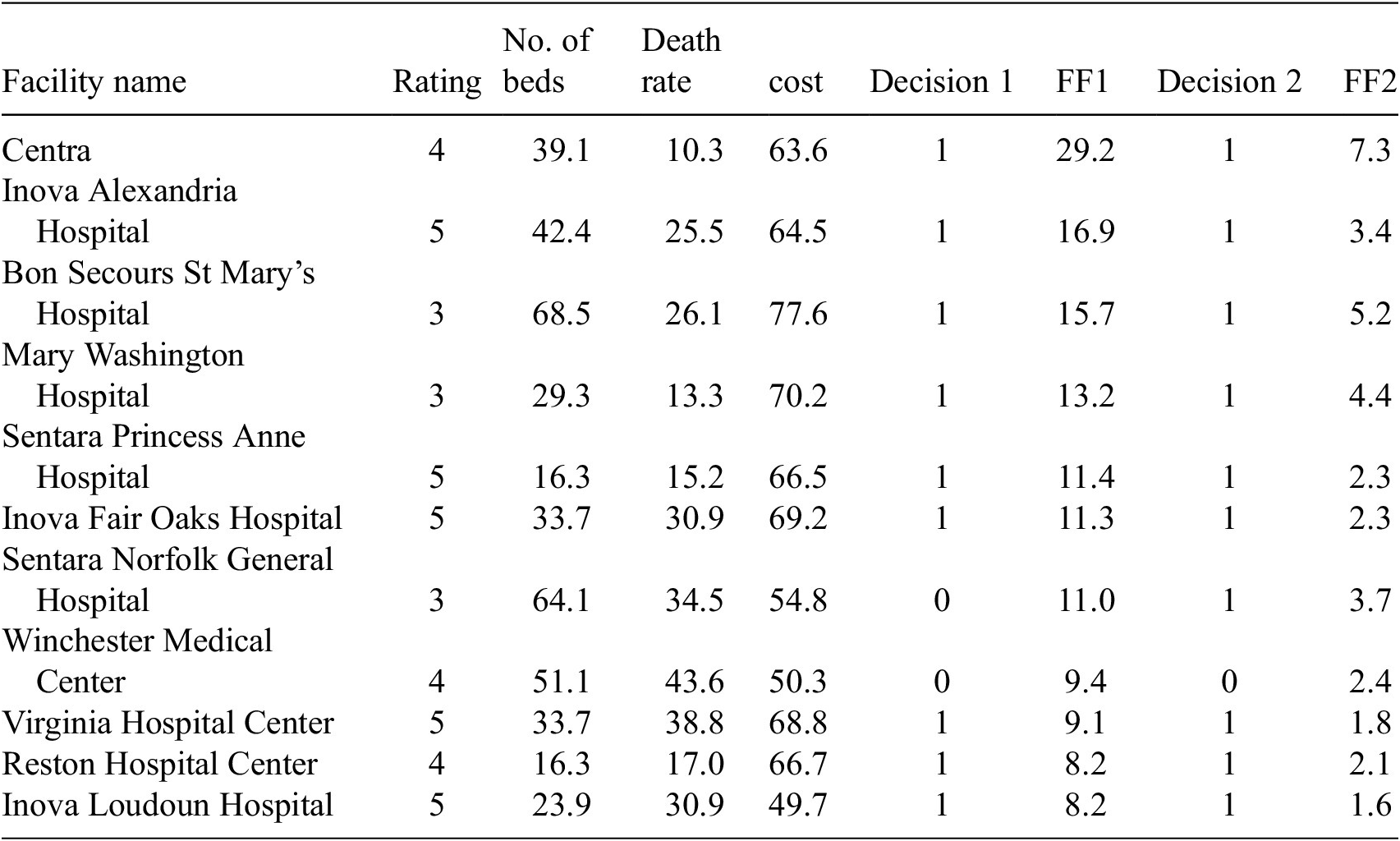
FF1 and FF2 use the number of beds, death rate, cost, and hospital ratings as inputs. No correlation between the input variables is observed. Table 3 presents results for Method #2. Decision 1 is based on FF1, the fitness values are shown in the FF1 column, and decision 2 is based on FF2, the values are shown in the FF2 column (both columns are highlighted). We aim to analyze the outcomes to specify which independent variables had the most effect on the outcomes, for instance, methods such as Gain, DeepLift, and Attributions can be applied to provide such insights.
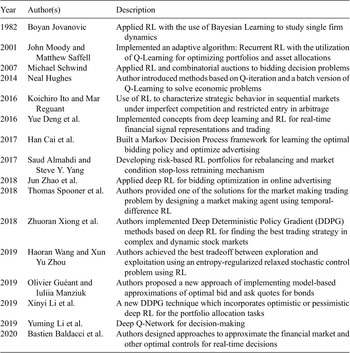
In Figure 2, green bars indicate which hospitals should receive additional beds in the current context of healthcare variables (exacerbated by an outlier event), while the red bars represent the hospitals that should not receive additional beds. The height of the bars represents the fitness value of the hospital. Figure 2a shows the results of FF1, and Figure 2b shows the results after using FF2. In both figures, the blue, orange, gray, and purple marks and line represent number of beds, death rate, cost, and hospital rating for each hospital, respectively. Figure 2a,b indicates how different measures can produce different rankings, which is commonly a major reason to considering multiple fitness functions and data management approaches as presented. The results show that by using FF1, 25% (32% for FF2) of those with high fitness values are suggested to not receive additional beds, and 8% (12% for FF2) of hospitals with low fitness values show as they should receive additional beds. FF1 performed better overall, which aligns well with studies such as in Batarseh and Yang (Reference Batarseh and Yang2020) and elects the function as the most accurate resource allocation that are on a national scale—other functions are expected to perform better in more limited geographies (such as state level), a question that we aim to answer in our future work.
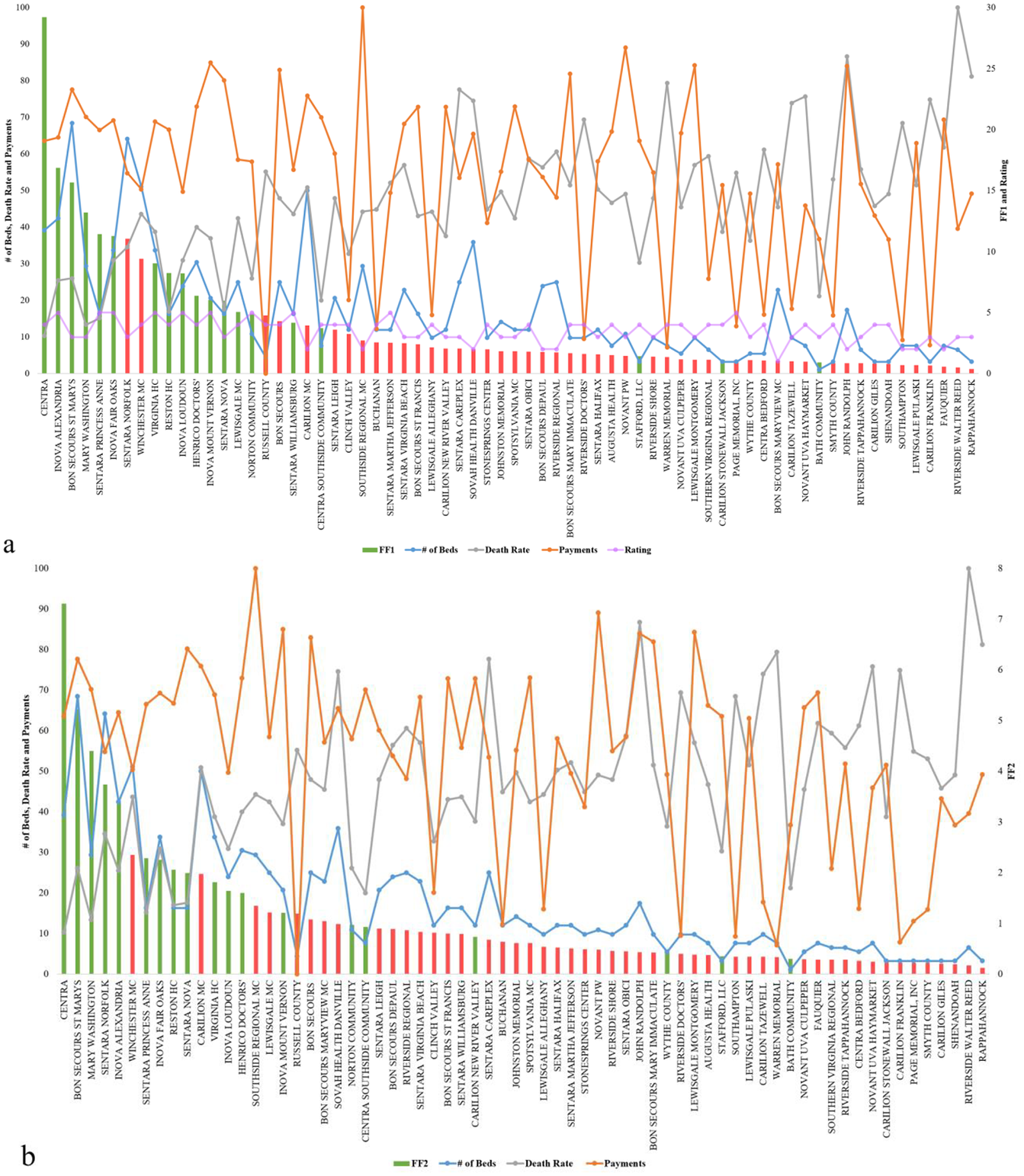
Figure 2. Resource allocation inputs and outputs for Genetic Algorithms using (a) FF1 and (b) FF2.
In Method #2, based on FF1 and FF2, the algorithms provide two different suggestions (Figure 3). Even though overall the results are highly consistent between the two fitness functions, ~9% of the hospitals have opposite suggestions on whether the hospitals should request additional beds or not during an outlier event. The bars represent the fitness values; to the left is FF1 and to the right is FF2. Green bars indicate a recommendation to requesting beds, while red recommends stopping and not requesting beds.

Figure 3. Beds allocation for Veterans Affairs hospitals based on Method #2.
In every cycle of the GA algorithm, getting new beds naturally increases the value of the input variable: number of beds, which then also increases the cost value. That leads—in the next iteration (i.e., generation) of the algorithm—to assigning some of the high-value hospitals a lower fitness value, or a slight increase to their fitness that is not representative in that context. Adjusting the number of generations set in the algorithms for instance would yield slight changes to that premise.
4.3. TSP results and Validation
Method #3 is especially relevant in the case of outlier events. For instance, during the current Covid-19 crises, hospitals are facing shortages on medical resources and medical professionals. A tradeoff in decisions is presented as follows: delivering the necessary limited resources to hospitals in the shortest amount of time, saving as many lives as possible, and doing so at a relatively low cost.
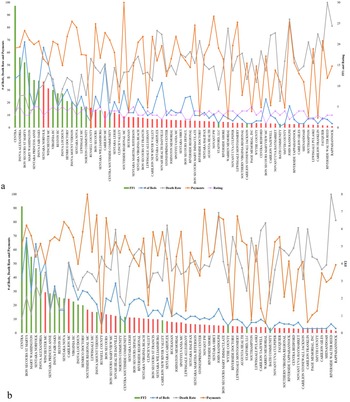
The fitness value of the result can vary based on different factors, such as the total number of locations, whether the data are normalized or not, and different fitness functions (presented in Table 4). It is clear that using results with state centers (using geographical longitudes and latitudes) is more straightforward and simpler; versus when using locations of hospitals (Figure 4a,b). However, using hospitals’ locations is obviously more realistic and useful. The fitness value different is 68% higher when comparing routes between state centers (48 mainland states), or with VA hospitals (141 locations). FF3 seems to provide suggestion with lower fitness value compared to using FF4 (Table 4). However, when comparing FF3 using normalized data with using unnormalized data, the fitness value improves drastically.
Table 4. Fitness values with different fitness functions

Figure 4. Resource allocation routes using Traveling Salesman Problem with Genetic Algorithm. (a) Veterans Affairs medical centers using unnormalized data with FF3; (b) state centers using unnormalized with FF3; (c) state centers using unnormalized data with FF4; (d) state centers using normalized data with FF4; and (e) state centers using K-means with FF4 with normalized data.
To improve the results further, we deployed clustering of the states by using K-means clustering (the value of k is selected using the elbow method, with the least sum of squared error). In reality, VA facilities are clustered into groups based on geographical locations, such as the Veterans Integrated Service Networks system used in VA medical facilities that categorizes the United States into 10 geographical areas. However, by deploying K-means clustering, the number of locations/region has been changed based on statistical measures and the size of k, which lead to a higher average fitness value of the algorithm. Figure 4a provides route suggestion between VA medical centers using FF4 (all maps’ starting point is in Pennsylvania); each point represents a VA medical center, sized by hospital rating. Figure 4b illustrates route suggestions between state centers using FF4; each point represents a state center. Both 4a and b use data that are not normalized; because distance is the only variable considered in FF4. In Figure 4c–e, state centers have been used (didnot provide results that are transferable to hospitals given the sparsity of outcomes); the size of the points indicate the sum of the hospital performance rating in the state. Figure 4c presents the route suggestion using unnormalized data using FF3. Figure 4d presents the routes using normalized data with the same fitness function (FF3), and Figure 4e presents the function with K-means clustering of the states into four regions (presented by four different colors).
In the resources’ allocation routes experiment (Method #3), Alaska, Hawaii, and other U.S. territories have been removed from the discussion due to their geographical nature. Since the distances between those locations to the contiguous United States are significant, the output will be greatly influenced and so we elected to remove them to avoid disturbance in results. In Figure 4, some routes show the shortest distance between two locations (a straight line). That leads to routes that go through Canada, the Great Lakes, and the Gulf of Mexico (which is troublesome). It is important to note that performing similar analysis on the state level or for within a hospital network will yield more related outcomes to hospitals, albeit a national recommendation model as the one presented in Figure 4 is beneficial for systems like the U.S. VA that are national in nature. Based on the result, using FF3 with normalized data yielded the best outcomes, and is recommended for resources’ allocation routes suggestions.
5. Conclusions and Discussions
The resource management R dashboard using RL can help hospital administrations know whether they have additional resources that they may be able to share with others in the near future. The binary GA model provides administrators unbiased information to suggest whether the hospital should receive additional resources or not. Finally, the TSP with GA can provide unbiased route suggestion based on cost, emergency, distance, and other factors. These models can be used together but may also be used independently during outlier events. Instead of using merely historical data to provide suggestions for future events, these models collect current data and provide solutions based on the current context.
Due to the Covid-19 pandemic, telehealth service has increased dramatically (more algorithmic and real-time systems became more relevant). The algorithm for resources’ allocation routes can be applied to homecare providers and others such as prescription drug delivery service. Besides distance, some patients might have more emergence conditions compared to others; therefore, using the system can provide the best route suggestion while balancing all factors.
Resource allocation strategies (even using AI) strive to maximize, treat people equally, promote and reward instrumental value, and give priority to the worst off (Emanuel et al., Reference Emanuel, Persad and and Upshur2020). The need to balance all these values for various interventions and in different circumstances is likely to lead to differing judgments about how much weight to give each value in particular cases (i.e., where hospital managers can then use the constants presented in our methods). The choice to set limits on access to resources is a necessary response to the overwhelming effects of mass casualty events but the question is how to do so in an ethical and systematic way, rather than basing decisions on individual institutions’ approaches or a clinician’s intuition in the heat of the moment (Emanuel et al., Reference Emanuel, Persad and and Upshur2020).
Pandemics have shaken healthcare systems around the globe, turning attention and resources away from patients who need other types of care. Providers defer elective and preventive visits. Patients decline emergency room (ER) visits, hospitalizations, and elective procedures. Children go unvaccinated; blood pressure is left uncontrolled; cancer survivors miss their checkups. A decline of 42% was reported by the CDC in ER visits because of the Covid-19 pandemic (Hartnett et al., Reference Hartnett, Kite-Powell and and DeVies2020), a decline of 42% in VA hospitals admissions (Baum and Schwartz, Reference Baum and Schwartz2020) and 33.7% in hospital admissions for eight other acute care hospitals (Oseran et al., Reference Oseran, Nash and Kim2020), a decline of 38–40% in myocardial infarctions (Garcia et al., Reference Garcia, Albaghdadi and and Meraj2020), and a decline in the use of stroke imaging by 39% (Kansagra et al., Reference Kansagra, Goyal, Hamilton and Albers2020). Sicker admissions of non-Covid-19 patients to the ICU are noted (Fadel et al., Reference Fadel, Al-Jaghbeer and and Kumar2020), confirming a trend where patients most often reached sicker health status, seeking care later and avoiding hospitals due to fear of the Covid-19 infection. To mitigate the risks for decompensation, morbidity, and loss to follow-up that could result from delayed care for patients, providers are now converting, when possible, in-person visits to telemedicine visits (Gadzinski et al., Reference Gadzinski, Gore and and Ellimoottil2020; Mann et al., Reference Mann, Chen and and Chunara2020). Our models could be merged with some telehealth services and provide a more comprehensive view to hospital management. Telehealth and other digital services come along with additional benefits for both the patient and the provider including potential faster care, cost savings, time savings, increased access to healthcare for rural areas, limiting the spread of Covid-19, and help preserve the limited supply of Personal Protective Equipment (Kichloo et al., Reference Kichloo, Albosta and and Dettloff2020). Patient satisfaction for telemedicine care has been consistently high (Kruse et al., Reference Kruse, Krowski and Rodriguez2017). A recent report, evaluating 400 patients scheduled for office consultation, showed that about 95% of the patients were at increased risk for a severe outcome of Covid-19 and that 85% of them would have favored telemedical consultation during the pandemic (Novara et al., Reference Novara, Checcucci and and Crestani2020). However, there are also some potential negatives about telemedicine services, including less access for those without basic technology or computer skills, the potential of paying out-of-pocket, interstate licensure challenges and other regulatory issues that may vary by state, the need to address sensitive topics, especially if there is patient discomfort or concern for privacy, and that not all conditions are appropriate for online treatment (Hjelm, Reference Hjelm2005; Ruiz Morilla et al., Reference Ruiz Morilla, Sans, Casasa and Giménez2017). Properly trained, a learning algorithm can augment physician judgment about when to offer or subtract lifesaving care and how to allocate scarce resources in challenging mass critical illness (Alimadadi et al., Reference Alimadadi, Aryal and Manandhar2020; Allam and Jones, Reference Allam and Jones2020). We anticipate that our methods will help in these scenarios; however, the following limitations are also areas where we wish to improve on: (a) testing the system with data from other health institutions and (b) trying different AI models to evaluate if they overperform RL and GA. (c) Although healthcare data are scarce and difficult to gather, we aim to inject more health variables into our algorithms to improve their accuracy and usability. Additionally, as part of future work, (d) we will test the mentioned methods presented in real-world scenarios, at hospitals such as Massachusetts General Hospital, the U.S. VA network, and other potential locations. Besides testing feasibility, deploying the system at a hospital will help evaluate its sanity and confirm its accuracy and relevance. It is evident that human judgment will remain important and ultimate (Garratt and Schneider, Reference Garratt and Schneider2019; Shah et al., Reference Shah, Kendall and and Khozin2019), but it can be supported with the dispassionate independent score-keeping capabilities of AI. This will be a logical extension of the risk-assessment tools used today in medicine.
Funding Statement
No funding was provided for this study.
Competing Interests
The authors declare no competing interests exist.
Author Contributions
C.-H.H. led the project’s experiments, wrote the manuscript, and developed the maps. F.A.B. designed the research plan, developed the idea and its implementation, and validated and evaluated the experimental work. A.B. developed the medical analysis and helped with writing, as well as provided medical and healthcare insights to the paper. A.K. and P.-H.S. collected data, developed the AI models (RL and GA), developed the dashboard, and designed the figures. J.A. wrote several sections of the paper and developed R code for the AI models.
Data Availability Statement
The data that support the findings of this study, code, and the data dashboard are openly available in a GitHub public repository, https://github.com/ferasbatarseh/EconHealthAI.








Comments
No Comments have been published for this article.