Introduction
Major depressive disorder (MDD) is a highly prevalent and debilitating mental disorder, which is expected to be a leading contributor to global disease burden by 2030 [Reference Le, Wong, Lu, Vasudeva, Gill and Badulescu1–Reference Maj, Stein, Parker, Zimmerman, Fava and De Hert3]. Findings from extant literature indicate MDD is associated with reduced quality of life and increased economic burden [Reference Cui, Li, Wang, Wu, Liu and Yu4–Reference Thaipisuttikul, Ittasakul, Waleeprakhon, Wisajun and Jullagate6].
While monoaminergic antidepressants (e.g., selective serotonin reuptake inhibitors [SSRIs] and serotonin-norepinephrine reuptake inhibitors) are first-line pharmacotherapies for persons with MDD, a substantial proportion of patients fail to achieve a clinically meaningful response [Reference Le, Wong, Lu, Vasudeva, Gill and Badulescu1, Reference Saragoussi, Touya, Haro, Jönsson, Knapp and Botrel7, Reference Singh, Hazari, Mittal, Yadav, Kumar and Mishra8]. Persons with MDD and an inadequate response to two or more antidepressants are classified as having treatment-resistant depression (TRD) [Reference McIntyre, Alsuwaidan, Baune, Berk, Demyttenaere and Goldberg9].
Ketamine and esketamine have demonstrated rapid, robust and sustained antidepressant effects in TRD [Reference Calder, Kwan, Teopiz, Wong, Rosenblat and Mansur10–Reference Quintero, Bustos, Lechtig-Wassermann, Beltran and Zarate12]. Ketamine and esketamine’s antidepressant mechanism of action is posited to include N-methyl-D-aspartate (NMDA) receptor antagonism on GABAergic interneurons, resulting in glutamatergic disinhibition and increased AMPA receptor expression and activity [Reference Aleksandrova and Phillips13–Reference McIntyre and Jain16].
However, findings from extant literature suggest that NMDA receptor antagonism may not fully account for ketamine and esketamine’s antidepressant effects [Reference Antos, Żukow, Bursztynowicz and Jakubów17–Reference Zanos and Gould19]. Emerging evidence suggests potential involvement of the opioidergic system [Reference Adzic, Lukic, Mitic, Glavonic, Dragicevic and Ivkovic20, Reference Grasso, Tennyson, Airan and Di Ianni21]. Specifically at subanesthetic doses, ketamine and esketamine nonselectively interact with mu-opioid (MORs), delta-opioid (DORs), and kappa-opioid (KORs) receptors, suggesting that their antidepressant effects may involve opioid signaling pathways [Reference Niesters, Martini and Dahan22, Reference Williams, Heifets, Blasey, Sudheimer, Pannu and Pankow23]. Furthermore, preliminary evidence suggests that the pathophysiology of depression may involve dysregulation of endogenous opioid signaling (e.g., reduced endogenous MOR availability and KOR hyperactivation) [Reference Jawad, Alnefeesi, Lui, Ceban, Chen-Li and Teopiz24–Reference Nummenmaa, Karjalainen, Isojärvi, Kantonen, Tuisku and Kaasinen26].
Herein, the present systematic review aims to comprehensively and critically evaluate available literature on whether opioid signaling may contribute to ketamine- and/or esketamine-associated clinical symptom outcomes. This area of research holds critical implications for elucidating the mechanisms underlying ketamine and/or esketamine’s antidepressant efficacy, as well as informing safety guidelines for potential abuse liability.
Methods
Search strategy
This review followed the 2020 Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [Reference Page, Moher, Bossuyt, Boutron, Hoffmann and Mulrow27]. The systematic search was conducted by retrieving articles from databases, including PubMed/MEDLINE, Cochrane Library, Embase, AMED, PsycINFO and Joanna Briggs Institute (JBI) EBP databases. The search string employed for each database included terms relevant to ketamine and esketamine, depression, opioid systems/mechanisms, and human and animal studies (Supplementary Table S1). The search occurred from inception to September 27, 2025. In addition, no search filters were applied to retrieve all potentially relevant studies.
Study selection and eligibility criteria
Covidence was used to screen studies [28]. Two independent reviewers (A.L. and H.X.) conducted title and abstract screening for relevant studies, followed by full-text screening. Only articles deemed relevant according to the eligibility criteria (Table 1) were included for data extraction and analysis. Any conflicts were resolved between the two independent reviewers. While Covidence removed duplicate studies, a few additional duplicates were manually removed.
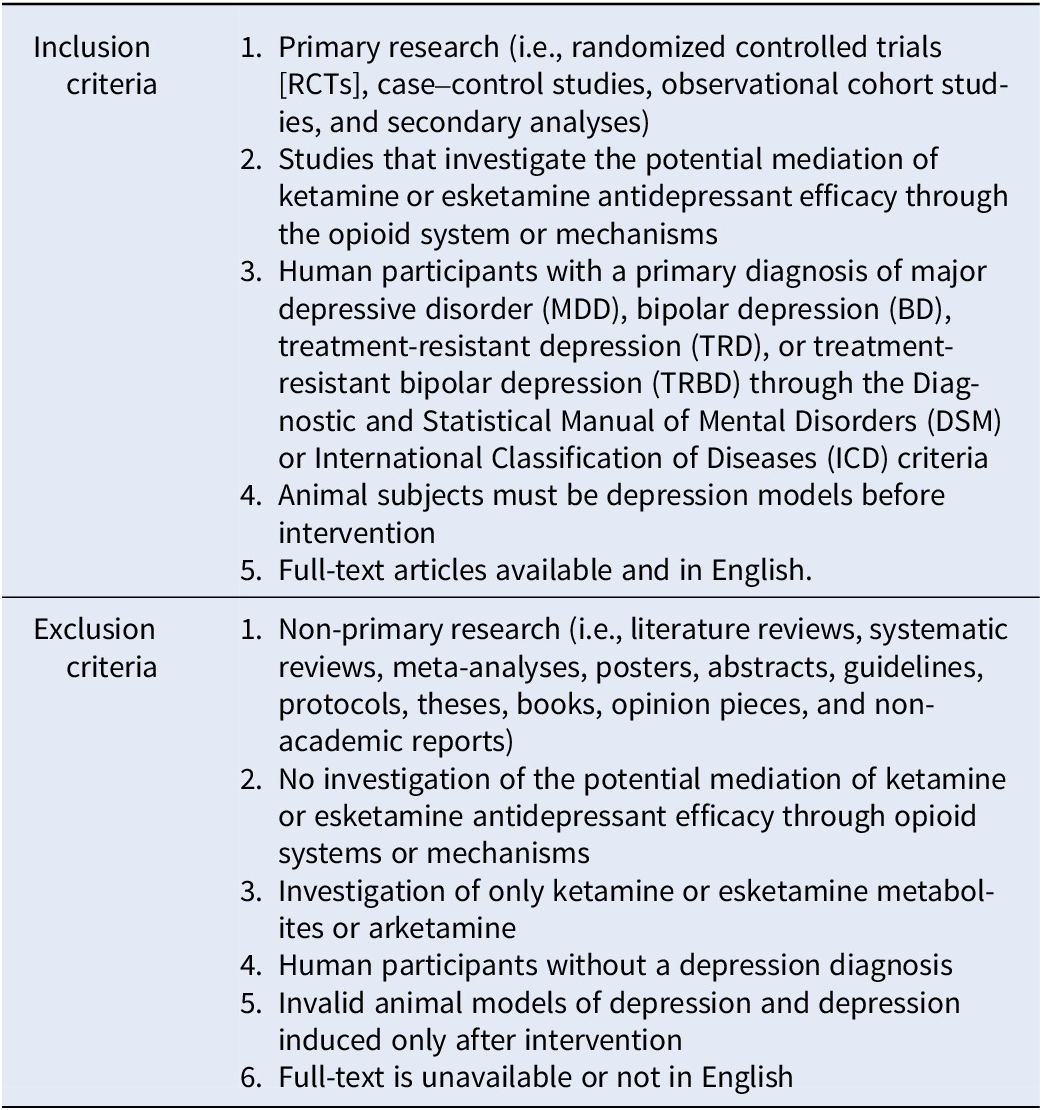
Table 1. Eligibility criteria

Studies were eligible for inclusion if they reported primary research examining the potential role of opioid systems or mechanisms in mediating the antidepressant efficacy of ketamine or esketamine. Human participants must have been diagnosed with a depressive disorder (i.e., MDD, bipolar depression [BD], TRD, or treatment-resistant BD). For animal studies, a validated depression model must have been employed before the administration of ketamine or esketamine.
Data extraction
Data extraction was conducted by two independent reviewers (A.L. and H.X.). Extracted study characteristics for clinical/human studies were established a priori and included: (1) the first author and year of publication, (2) study design, (3) primary diagnosis, (4) total sample size (included in relevant analyses), (5) control, (6) dosage with sample size, (7) dosing frequency, (8) measure(s) (for depression), and (9) antidepressant efficacy outcomes (Table 2). Similar characteristics were extracted for preclinical/animal studies; the type of model, behavioral outcomes, and brain slice findings were extracted (Table 3).
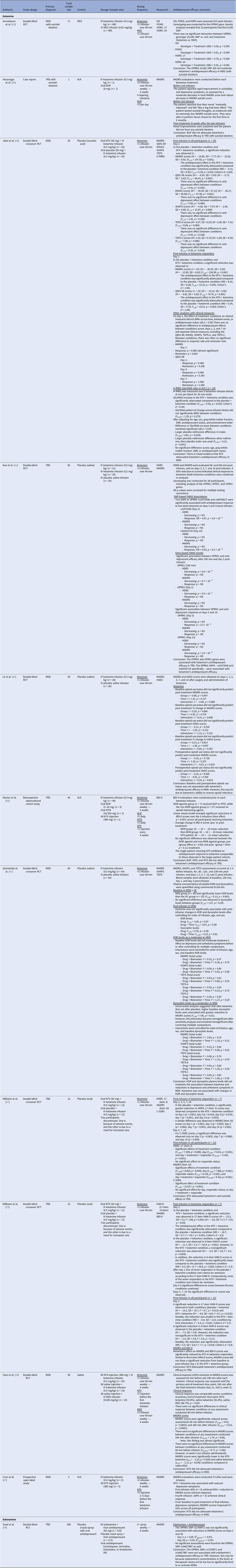
Table 2. Characteristics of clinical studies investigating the potential mediating effect of the opioid system on antidepressant efficacy of ketamine or esketamine in persons with depressive disorders (n = 12)

Abbreviations: ACC, anterior cingulate cortex; BDI-II, Beck Depression Inventory-Second Edition; BUP, buprenorphine; C-SSRS, Columbia Suicide Severity Rating Scale; ELISA, enzyme-linked immunosorbent assay; Glx, combined measure of glutamate and glutamine; GWAS, genome-wide association studies; HADS, Hospital Anxiety and Depression Scale; HAM-D, Hamilton Depression Rating Scale; HC, healthy controls; HDRS, Hamilton Depression Rating Scale; H-fMRS, proton functional magnetic resonance spectroscopy; IM, intramuscularly; IN, intranasal; IV, intravenous; KOR, kappa opioid receptor; M, mean; M3VAS, Maudsley 3-item Visual Analogue Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; MD, mean difference; MDD, major depressive disorder; MDZ, midazolam; MOR, mu opioid receptor; MTD, methadone; NR, not reported; NS, not significant; NTX, naltrexone; OR, odds ratio; POMS, Profile of Mood States; QIDS-SR, Quick Inventory of Depressive Symptomatology Self-Report; RCT, randomized controlled trial; SD, standard deviation; SHAPS, Snaith–Hamilton Pleasure Scale; SNP, single nucleotide polymorphism; SNR, signal-to-noise ratio; SSI, Beck Scale for Suicidal Ideation; TEPS, Temporal Experience of Pleasure Scale; TEPS-A, TEPS anticipatory subscale; TEPS-C, TEPS consummatory subscale; tNAA, N-acetylaspartate + N-acetylaspartylglutamate; TRD, treatment resistant depression.
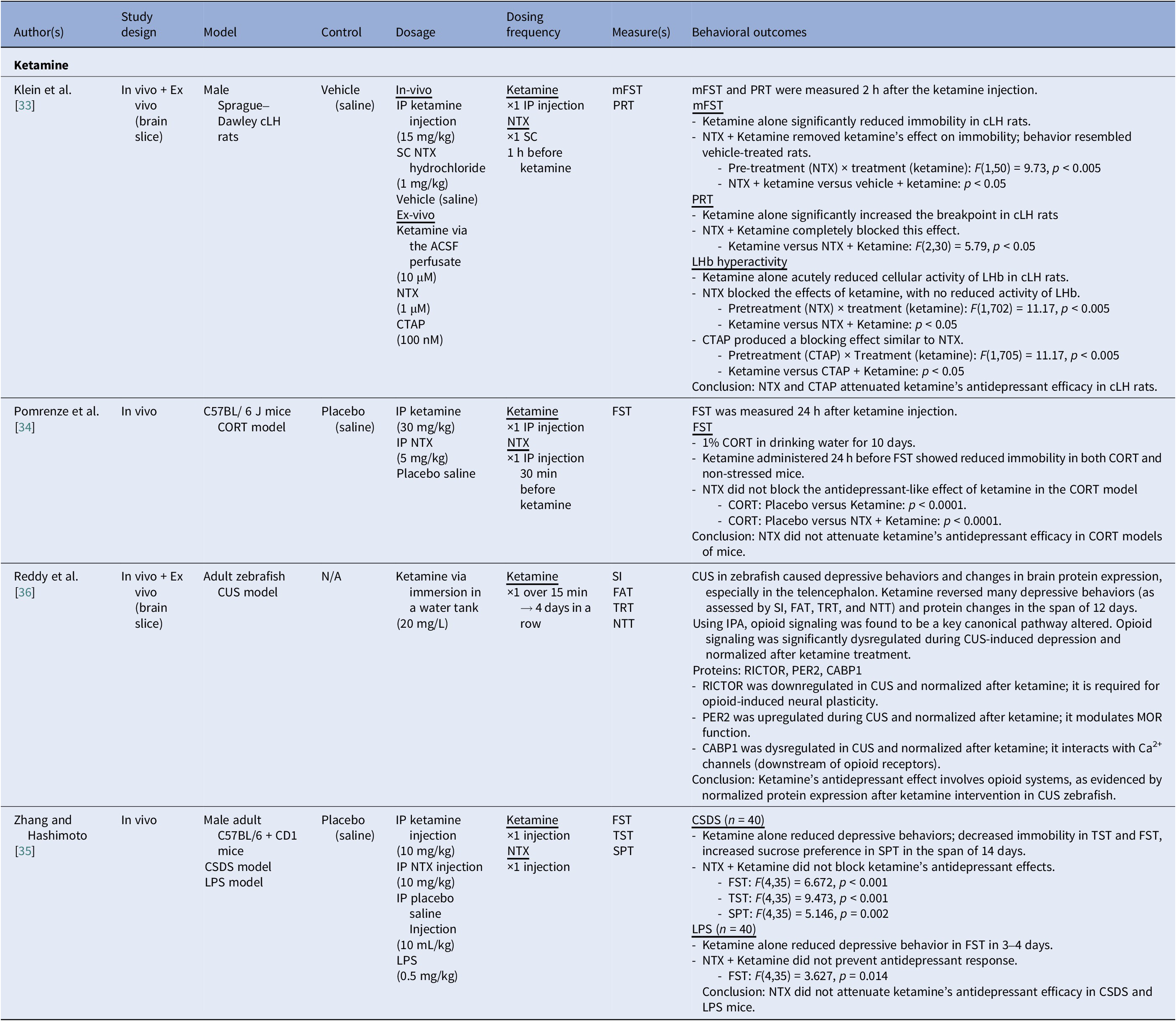
Table 3. Characteristics of preclinical studies investigating the potential mediating effect of the opioid system on behavioral outcomes of ketamine in depressed animal models (n = 4)

Abbreviations: ACSF, artificial cerebrospinal fluid; cLH, congenital learned helplessness; CORT, corticosterone; CSDS, chronic social defeat stress; CTAP, D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2; CUS, chronic unpredictable stress; FAT, feed approach test; FST, forced swim test; IP, intraperitoneal; IPA, Ingenuity Pathway Analysis; LHb, lateral habenula; LPS, lipopolysaccharide-induced inflammation; mFST, modified forced swim test; NTT, novel tank test; NTX, naltrexone; PRT, progressive ratio task; SI, social interaction test; SPT, sucrose preference test; SC, subcutaneous; TRT, threat response test; TST, tail suspension test; WT, wild type.
Quality assessments
Using the National Institutes of Health tools, independent reviewers (A.L. and H.X.) appraised the included clinical/human studies. In particular, this review utilized the Quality Assessment of Controlled Intervention Studies, the Quality Assessment of Observational Cohort and Cross-Sectional Studies, and the Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group [Reference Ma, Wang, Yang, Huang, Weng and Zeng29, 30]. In addition, the JBI Critical Appraisal Tool for Case Reports was used [Reference Ma, Wang, Yang, Huang, Weng and Zeng29, 31]. For preclinical/animal studies, the SYRCLE’s Risk of Bias tool was utilized [Reference Hooijmans, Rovers, de Vries, Leenaars, Ritskes-Hoitinga and Langendam32]. Any disagreements in assessments were resolved through discussion.
Results
Search results
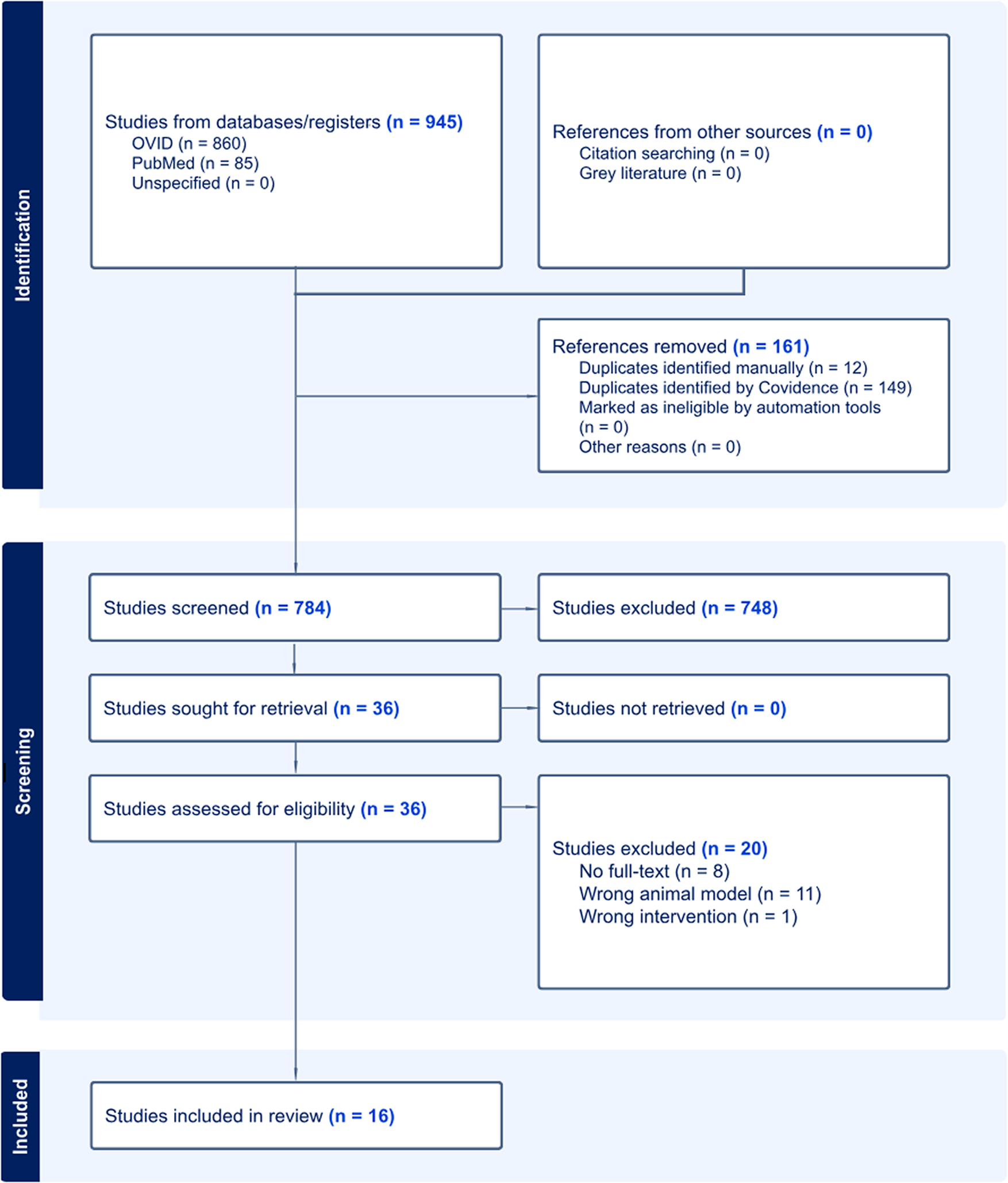
The search strategy yielded 784 studies after removing duplicates (n = 161). Using the eligibility criteria for screening (Table 1), 36 studies remained after title and abstract screening, and 16 studies were included in this review after full-text screening (n = 4 preclinical studies; n = 12 clinical studies). Reasons for excluding studies in full-text screening included the wrong animal model (n = 11), no full-text (n = 8), and the wrong intervention (n = 1). Other details can be found in the PRISMA flow diagram (Figure 1).

Figure 1. PRISMA flow diagram of literature search.
Source: Page MJ, et al. BMJ 2021;372:n71. doi: 10.1136/bmj.n71 [28].
Methodological quality assessment
Preclinical/animal studies were evaluated as a moderate risk of bias [Reference Klein, Chandra, Sheriff and Malinow33–Reference Reddy, Babu, Das, Bhattacharya, Murthy and Kumar36]. These evaluations were largely due to the lack of reporting of several study design details. Among clinical studies, controlled intervention studies had a low risk of bias [Reference Williams, Heifets, Blasey, Sudheimer, Pannu and Pankow23, Reference Williams, Heifets, Bentzley, Blasey, Sudheimer and Hawkins37–Reference Saad, Hibar, Fedgchin, Popova, Furey and Singh44]. Similarly, Marton et al. [Reference Marton, Barnes, Wallace and Woolley45] was an observational cohort study, and Hosanagar et al. [Reference Hosanagar, Schmale and LeBlanc46] was a case report; they were deemed moderate and low risk of bias, respectively. In contrast, Yoon et al. [Reference Yoon, Petrakis and Krystal47] was a pre-post study with no control group, and the only study assessed as high in risk of bias. All assessments can be found in the Supplementary Materials (Supplementary Tables S2–S6).
Overview of preclinical and clinical study characteristics
There were four preclinical studies that examined ketamine with animal models of depression; none of the studies examined esketamine. A subanesthetic dose of ketamine was administered either through intraperitoneal injection (i.e., 10–30 mg/kg) [Reference Klein, Chandra, Sheriff and Malinow33–Reference Zhang and Hashimoto35], artificial cerebrospinal fluid perfusate (i.e., 10 μM) [Reference Klein, Chandra, Sheriff and Malinow33], or immersion in a water tank (i.e., 20 mg/L) [Reference Reddy, Babu, Das, Bhattacharya, Murthy and Kumar36].
Across all 12 clinical studies (n = 9 controlled intervention, n = 1 observational cohort, n = 1 pre-post with no control group, and n = 1 case report), a total of 706 participants (n = 264 MDD and n = 526 TRD) were included. Eleven studies investigated a subanesthetic dose of intravenous (IV) ketamine (i.e., 0.2 or 0.5 mg/kg) [Reference Williams, Heifets, Blasey, Sudheimer, Pannu and Pankow23, Reference Williams, Heifets, Bentzley, Blasey, Sudheimer and Hawkins37–Reference Grunebaum, Galfalvy, Liu, Huang, Marcott and Burke43, Reference Marton, Barnes, Wallace and Woolley45, Reference Hosanagar, Schmale and LeBlanc46, Reference Yoon, Petrakis and Krystal47]. Intranasal esketamine (i.e., 84 mg) was investigated in only one study [Reference Saad, Hibar, Fedgchin, Popova, Furey and Singh44].
Preclinical/animal studies
Effect of opioid antagonists on ketamine
Naltrexone (i.e., NTX; a nonselective opioid antagonist) attenuated ketamine’s effects in congenitally learned helplessness rats (i.e., rat model of depression) [Reference Klein, Chandra, Sheriff and Malinow33]. Significantly greater immobility time in the forced swimming test (FST) was observed in depressed rats receiving NTX combined with ketamine compared to ketamine-placebo (p < 0.05) [Reference Klein, Chandra, Sheriff and Malinow33]. In line with the foregoing trend, ketamine significantly increased effort exerted to obtain rewards in the progressive ratio task, indexing improvements in anhedonia symptom severity; however, this effect was not observed in the NTX-ketamine group (F = 5.79, p < 0.05) [Reference Klein, Chandra, Sheriff and Malinow33]. Furthermore, NTX also inhibited ketamine-induced reductions in lateral habenula (LHb) activity (i.e., LHb hyperactivity is associated with depression) (p < 0.05) [Reference Klein, Chandra, Sheriff and Malinow33].
Separately, NTX did not attenuate ketamine’s antidepressant effects in stress-induced mice, wherein a nonsignificant between-group difference in immobility time was observed [Reference Pomrenze, Vaillancourt, Llorach, Rijsketic, Casey and Gregory34]. Comparably, in other stress-induced models, ketamine produced antidepressant-like effects across the FST (F = 6.672, p < 0.001; F = 3.627, p = 0.014), tail suspension test (TST) (F = 9.473, p < 0.001), and sucrose preference test (F = 5.146, p = 0.002), with no observed attenuation by NTX coadministration [Reference Zhang and Hashimoto35].
Beyond NTX, D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (opioid antagonist) also inhibited ketamine-induced reductions in LHb activity (p < 0.05) [Reference Klein, Chandra, Sheriff and Malinow33].
Regulation of opioid signaling by ketamine
In a chronic unpredictable stress model of zebrafish, ketamine elicited improved performance in the social interaction test, feed approach test, novel tank test and TST [Reference Reddy, Babu, Das, Bhattacharya, Murthy and Kumar36]. These behavioral effects were accompanied by normalization of dysregulated opioid signaling. Ketamine restored the expression or function of opioid-related proteins, including RICTOR, PER2 and CABP1, to baseline levels [Reference Reddy, Babu, Das, Bhattacharya, Murthy and Kumar36].
Clinical/human studies
Effect of NTX on ketamine
In a crossover randomized controlled trial (RCT), NTX significantly attenuated both the overall antidepressant and anti-suicidal effects of ketamine in patients with TRD (n = 12) [Reference Williams, Heifets, Blasey, Sudheimer, Pannu and Pankow23, Reference Williams, Heifets, Bentzley, Blasey, Sudheimer and Hawkins37]. Among ketamine responders (n = 7), reductions in Hamilton Depression Rating Scale (HDRS) total scores were significantly greater when administered placebo-ketamine compared to NTX-ketamine (HDRS 17-item: F = 43.7, p < 0.001; HDRS 6-item: F = 29.8, p = 0.002) [Reference Williams, Heifets, Blasey, Sudheimer, Pannu and Pankow23]. No significant difference was observed on days 5, 7 and 14 post-treatment [Reference Williams, Heifets, Blasey, Sudheimer, Pannu and Pankow23]. In addition, five out of seven responders achieved remission (a score of ≤7 on the HDRS) when administered placebo-ketamine, whereas none met the criteria when administered NTX-ketamine [Reference Williams, Heifets, Blasey, Sudheimer, Pannu and Pankow23]. The attenuation effect of NTX was consistent in the Montgomery-Åsberg Depression Rating Scale (MADRS), Beck Depression Inventory Second Edition (BDI-II), and Columbia Suicide Severity Rating Scale (C-SSRS) scores, and in all participants [Reference Williams, Heifets, Blasey, Sudheimer, Pannu and Pankow23, Reference Williams, Heifets, Bentzley, Blasey, Sudheimer and Hawkins37]. Notably, among responders, reductions differed significantly between groups for HDRS suicidal ideation (SI) score (all p ≤ 0.02), MADRS SI score (all p ≤ 0.002), and C-SSRS (all p ≤ 0.006) [Reference Williams, Heifets, Bentzley, Blasey, Sudheimer and Hawkins37].
Conversely, in an observational cohort study of TRD patients, a patient (n = 1) received NTX-ketamine and reported BDI-II scores comparable to patients (n = 34) receiving ketamine alongside buprenorphine (i.e., partial MOR agonist), methadone (i.e., full MOR agonist), or no MOR agent [Reference Marton, Barnes, Wallace and Woolley45]. Consistent with the foregoing findings, an open-label study of patients with MDD and alcohol use disorder (AUD) (n = 5) received NTX-ketamine and all achieved clinical response by the fourth infusion (50% reduction in MADRS scores), demonstrating MADRS reductions ranging from 57 to 92% after 4 weeks of treatment [Reference Yoon, Petrakis and Krystal47]. Similarly, in an RCT study, patients with MDD and AUD (n = 58) received NTX-ketamine, saline-ketamine or midazolam-ketamine and showed no significant differences in clinical response (p = 0.63) [Reference Yoon, Pittman, Ralevski, Petrakis and Krystal38]. MADRS scores were similar across conditions 60 min before (F 10,214 = 0.77, p = 0.66) and 240 min after infusion (F 10,213 = 1.79, p = 0.06) [Reference Yoon, Pittman, Ralevski, Petrakis and Krystal38].
In contrast, a crossover RCT of patients with MDD (n = 26) compared NTX-ketamine with placebo-ketamine and reported mixed evidence for opioid-mediated antidepressant effects [Reference Jelen, Lythgoe, Stone, Young and Mehta39]. In all participants, reductions in MADRS scores significantly differed between conditions on day 1 of post-infusion (F 1,74 = 5.39, p = 0.023) [Reference Jelen, Lythgoe, Stone, Young and Mehta39]. However, the foregoing finding was not replicated on days 1, 3 and 7 in self-report measures, including the Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR), Maudsley 3-item Visual Analogue Scale, Snaith Hamilton Pleasure Scale (SHAPS), and Temporal Experience of Pleasure Scale anticipatory anhedonia subscale (TEPS-A) and consummatory anhedonia subscale (TEPS-C) (all p > 0.05) [Reference Jelen, Lythgoe, Stone, Young and Mehta39]. Among ketamine responders, the attenuation effect of NTX was more robust (MADRS: F 1,38 = 15.33, p < 0.001; QIDS-SR: F 1,38 = 10.51, p = 0.003) [Reference Jelen, Lythgoe, Stone, Young and Mehta39]. Response and remission rates were generally comparable between conditions, except for a lower MADRS remission in the NTX-ketamine condition on day 1 of post-infusion (p = 0.034) [Reference Jelen, Lythgoe, Stone, Young and Mehta39]. In a neuroimaging subsample (n = 24) using proton functional magnetic resonance spectroscopy, Glx/tNAA (glutamate + glutamine/N-acetylaspartate + N-acetylaspartylglutamate) ratios in the anterior cingulate cortex (ACC) increased significantly during ketamine infusion, but this increase was attenuated in the NTX-ketamine condition compared with placebo-ketamine (F 1,253 = 4.83, p = 0.029) [Reference Jelen, Lythgoe, Stone, Young and Mehta39].
Effect of opioid use on ketamine
In a case report of a patient with TRD and active SI, Hosanagar et al. [Reference Hosanagar, Schmale and LeBlanc46] reported a robust decrease in MADRS total and SI score after the first and second infusion of ketamine-buprenorphine. Mood improvements were sustained, with no report of SI 4 weeks post-treatment [Reference Hosanagar, Schmale and LeBlanc46]. Similarly, Marton et al. [Reference Marton, Barnes, Wallace and Woolley45] did not observe a significant difference in reductions in BDI-II scores between TRD patients administered ketamine with oral buprenorphine or methadone (n = 7) compared to ketamine only (n = 27) (p = 0.11).
In a separate double-blind RCT study of patients with MDD (n = 40), baseline opioid use status did not significantly predict post-treatment MADRS scores (t = −0.40, p = 0.686), % change in MADRS scores (t = 0.25, p = 0.806), post-treatment Hospital Anxiety and Depression Scale (HADS) scores (t = 1.15, p = 0.251), and % change in HADS scores (t = 0.85, p = 0.397) [Reference Lii, Flohr, Okada, Cianfichi, Hack and Schatzberg40]. In addition, postoperative opioid use status did not significantly predict post-treatment MADRS (t = −0.23, p = 0.819) and HADS scores (t = 0.24, p = 0.814) [Reference Lii, Flohr, Okada, Cianfichi, Hack and Schatzberg40].
Molecular biomarkers: KOR and dynorphin plasma levels
In a double-blind crossover RCT of MDD patients (n = 64), no significant drug × time × plasma levels of soluble KORs interaction was found for MADRS (F = 0.36, p = 0.70), SHAPS (F = 0.02, p = 0.98), TEPS total score (F = 0.60, p = 0.55), and TEPS-A (F = 0.23, p = 0.79) or TEPS-C (F = 1.27, p = 0.29) [Reference Quintanilla, Medeiros, Greenstein, Yuan, Johnston and Park41]. In line with the foregoing trend, a nonsignificant interaction was observed for plasma levels of dynorphins in MADRS (F = 1.19, p = 0.31), SHAPS (F = 0.48, p = 0.62), TEPS total score (F = 1.10, p = 0.34), and TEPS-A (F = 1.28, p = 0.29) or TEPS-C (F = 1.08, p = 0.35) [Reference Quintanilla, Medeiros, Greenstein, Yuan, Johnston and Park41].
Ketamine and opioid gene variants
In a double-blind RCT of TRD patients (n = 65), Kao et al. [Reference Kao, Tsai, Su, Li, Lin and Hong42] observed a significant association between the OPRD1 gene and reductions in HDRS (p ≤ 0.049) and MADRS scores (p = 0.047) post-treatment of ketamine. In addition, the OPRM1 gene was associated with a clinical response (50% reduction) in HDRS (p = 0.033) and MADRS scores (p = 0.026) [Reference Kao, Tsai, Su, Li, Lin and Hong42]. Notably, the OPRM1 variant rs2473546 was associated with clinical response in HDRS scores (odds ratio [OR] = 4.07, p = 0.0069); the rs9479827 variant was also associated with clinical response in MADRS scores (OR = 6.04, p = 0.0083) [Reference Kao, Tsai, Su, Li, Lin and Hong42].
However, in a double-blind RCT of MDD patients with active SI (n = 71), Grunebaum et al. [Reference Grunebaum, Galfalvy, Liu, Huang, Marcott and Burke43] observed no significant interaction between the OPRM1 A118G polymorphism and ketamine response, particularly in HDRS-17 (t = 0.78, p = 0.439), HDRS-24 (t = 0.55, p = 0.582), Beck Scale for Suicidal Ideation (t = 0.59, p = 0.554), and Profile of Mood States scores (t = 0.61, p = 0.544).
Esketamine and opioid gene variants
In a double-blind RCT of TRD patients (n = 406), Saad et al. [Reference Saad, Hibar, Fedgchin, Popova, Furey and Singh44] observed that the OPRM1 variant rs1799971 was not significantly associated with reductions in MADRS scores on days 2 and 28 post-treatment of esketamine combined with an antidepressant (p = 0.69, R2 partial = < 0.5%; p = 0.34, R2 partial = < 0.5%). In accordance, no significant association with esketamine response was observed for the OPRM1 variant rs34427887 [Reference Saad, Hibar, Fedgchin, Popova, Furey and Singh44].
Discussion
To our knowledge, this is the first systematic review to comprehensively evaluate the role of opioid signaling in the mechanisms of action of ketamine’s and esketamine’s antidepressant efficacy. Studies reported that opioid antagonists (e.g., NTX) may attenuate ketamine’s antidepressant efficacy, particularly in small, randomized crossover trials. In contrast, larger randomized trials generally found no consistent moderation by opioid-related gene expression (i.e., OPRM1 and OPRD1) or plasma biomarkers (KOR and dynorphin). Separately, opioid use (e.g., buprenorphine and methadone) did not significantly alter response to ketamine. Although definitive conclusions cannot be drawn, trends across preclinical and clinical evidence support a partial mediating effect of the opioid system on ketamine’s and esketamine’s antidepressant effects in persons with TRD.
Separately, all but one study involving persons with MDD did not observe any support for a mediation effect. The foregoing distinction may be attributed to neuropathophysiological differences between TRD and MDD populations, potentially accounting for differential treatment response [Reference McIntyre, Alsuwaidan, Baune, Berk, Demyttenaere and Goldberg9, Reference Gutierrez, Swainson, Ravindran, Lam, Giacobbe and Karthikeyan48–Reference Zolghadriha, Anjomshoaa, Jamshidi and Taherkhani54]. In particular, TRD is associated with lower ACC GABA levels and smaller hippocampal volumes than MDD, indicating greater GABAergic dysfunction [Reference Abdallah, Jackowski, Sato, Mao, Kang and Cheema55]. More pronounced GABAergic deficits in TRD may render ketamine’s antidepressant effects increasingly dependent on opioid-mediated mechanisms, thereby causing NTX antagonism to be consistently disruptive. In contrast, in MDD, relatively preserved GABAergic function may subserve partial antidepressant efficacy, notwithstanding opioid receptor antagonism.
Differences in the pharmacological profiles of ketamine and esketamine, as well as the hypothesized distinct roles of MOR, DOR and KOR receptors in depression pathophysiology and antidepressant response, may also contribute to the observed variability in our findings (Supplementary Table S7) [Reference Niesters, Martini and Dahan22, Reference Williams, Heifets, Blasey, Sudheimer, Pannu and Pankow23, Reference Jelen, Stone, Young and Mehta25]. In addition, extant literature notes that subanesthetic ketamine and esketamine doses exhibit low binding affinity for opioid receptors, which may limit receptor engagement and the detection of opioid-mediated effects [Reference Bonaventura, Lam, Carlton, Boehm, Gomez and Solís56–Reference Fricker, Osman, Gupta, Gomes and Devi60]. In particular, recent mechanistic studies suggest that ketamine and its metabolites act as positive allosteric modulators of MORs at standard antidepressant doses, enhancing opioid signaling rather than directly activating the receptor [Reference Fricker, Osman, Gupta, Gomes and Devi60]. The foregoing framework may reconcile our mixed clinical findings by positing that the antidepressant effects depend on individual differences in receptor availability or opioid system responsiveness, rather than direct MOR activation [Reference Fricker, Osman, Gupta, Gomes and Devi60].
Clinical implications
Our inconclusive findings warrant further investigation, with a specific focus in clarifying ketamine’s and esketamine’s antidepressant mechanisms and informing the development of novel therapeutics. However, our findings highlight several considerations. The lack of attenuation by opioid receptor antagonists suggests that receptor activation may not be essential for antidepressant response. Notwithstanding, the observed consistent attenuation in other studies involving TRD and the aforementioned factors that may underlie variability in our results, more likely suggest that the opioid system may function as a context-dependent modulator rather than a uniform mediator. The foregoing distinction underscores the need for stratified research designs that differentiate between MDD and TRD and account for individual variability in opioid receptor availability and expression, prior opioid exposure, and genetic polymorphisms.
In addition, at subanesthetic doses of IV ketamine, there is a concern for potential abuse liability [Reference Andrade61–Reference Trujillo and Iñiguez64]. A scoping review by Le et al. [Reference Andrade61] included four clinical studies and reported that patients with TRD showed no compelling evidence of dependence or diversion after a single or repeated ketamine administration in a professionally controlled setting. Similarly, results from pharmacovigilance and other inquiries have not provided evidence of drug abuse, misuse, diversion or gateway activity with ketamine or esketamine when administered under clinical supervision [Reference Kwan, Lakhani, Rosenblat, Mansur, Rhee and Teopiz65–Reference Kwan, Rosenblat, Mansur, Teopiz and McIntyre67]. The Food and Drug Administration (FDA)―mandated Risk Evaluation and Mitigation Strategy for esketamine has also likely contributed to the reduced risk profile observed [Reference Roncero, Merizalde-Torres, Szerman, Torrens, Vega and Andres-Olivera68]. However, clinical evidence remains limited, warranting further investigation [Reference Levinstein, Carlton, Di Ianni, Ventriglia, Rizzo and Gomez59, Reference Le, Cordero, Jawad, Swainson, Di Vincenzo and Jaberi62].
Limitations and future directions
Our review was limited to studies that investigated ketamine and esketamine in persons with depressive disorders and animal models of depression. Thus, we excluded studies on arketamine or ketamine metabolites. We also excluded preclinical studies during full-text review that used nondepressed animal models, most of which reported opioid-mediated mechanisms or irrelevant outcomes [Reference Bonaventura, Lam, Carlton, Boehm, Gomez and Solís56, Reference Levinstein, Carlton, Di Ianni, Ventriglia, Rizzo and Gomez59, Reference Langreck, Chen, Luna, Nelson, Turi and Hill69–Reference Di Ianni, Ewbank, Levinstein, Azadian, Budinich and Michaelides77]. Therefore, our results cannot be generalized to other human populations, animal models or glutamatergic modulators. Studies varied considerably, such as in study designs, sample sizes, routes of administration, dosage, dosing schedule, and outcome measures, limiting direct comparisons (Supplementary Tables S6–S10). Although our review included a comparable number of MDD and TRD studies, the sample was skewed toward TRD due to one large esketamine trial. However, findings remained inconclusive as it was the only esketamine study. The inclusion of only one esketamine study also limited opportunities for direct comparison with ketamine.
Further research should adopt rigorously controlled, stratified and dose-escalation RCT designs using opioid receptor-selective probes to more precisely delineate the role of the opioid system. It is also recommended that further mechanistic research investigate esketamine’s opioid interactions because of its FDA approval for depression and more widespread clinical use compared to ketamine [Reference Aguilar, Beauregard, Conroy, Khatiwoda, Horsford and Nichols78, Reference Vekhova, Namiot, Jonsson and Schiöth79].
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1192/j.eurpsy.2026.10157.
Data availability statement
This systematic review did not generate any new data. All extracted and analyzed data are provided in the Supplementary Materials and presented within the tables and figures of this article. Data from the original studies can be accessed through their respective publications or repositories.
Author contributions
Conceptualization: AL and RSM. Data curation: AL. Investigation: AL and HX. Methodology: AL. Project administration: AL. Resources: AL. Supervision: GHL and RSM. Validation: AL and HX. Visualization: AL. Writing – original draft preparation: AL. Writing – review and editing: All authors. All authors agree to be accountable for all aspects of the work. All authors have read and agreed to the submitted version of the manuscript.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
Dr. Roger S. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Neurawell, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, Bristol Myers Squibb (BMS) Teva, Adhere Tech, GH Research, Autobahn Theapeutics and Atai Life Sciences. Dr. Taeho Greg Rhee was supported in part by the National Institute on Aging (#R21AG070666, R21AG078972, and R01AG088647), National Institute of Mental Health (#R01MH131528), National Institute on Drug Abuse (#R21DA057540), and Health Resources and Services Administration (#R42MC53154–01-00). Dr. Rhee serves as a review committee member for the National Institutes of Health (NIH), Patient-Centered Outcomes Research Institute (PCORI), and Substance Abuse and Mental Health Services Administration (SAMHSA), and has received honoraria payments from NIH, PCORI, and SAMHSA. Dr. Rhee has also served as a stakeholder/consultant for PCORI and received consulting fees from PCORI. Dr. Rhee serves as an advisory committee member for the International Alliance of Mental Health Research Funders (IAMHRF). Dr. Guillen-Burgos has received research grant support from the Ministry of Science, Technology, and Innovation (Minciencias) in Colombia, UKRI in the United Kingdom; and speaker fees from Abbott, GSK, Roche, Pfizer, and Synergy R&D. All other authors declare no competing interests.


Comments
No Comments have been published for this article.