Introduction
Cloeosiphon Grube, Reference Grube1868 is one of the three genera belonging to Aspidosiphonidae de Quatrefages, Reference de Quatrefages1866. The genus has a complex taxonomic history that began when Diesing (Reference Diesing1851) proposed Loxosiphon to include a single sipunculan species, Loxosiphon elegans (formerly Sternapis elegans Chamisso and Eysenhardt, Reference Chamisso and Eysenhardt1821). Later, de Quatrefages (Reference de Quatrefages1866) described from Île de France (=Mauritius) an additional species within the genus, Loxosiphon aspergillum. He explained that in both species, the introvert was inserted laterally into the anal shield. Subsequently, Grube (Reference Grube1868) suggested reassigning L. aspergillum to a new genus, Cloeosiphon. This decision was based on his observation that, while L. elegans exhibited a lateral insertion of the introvert relative to the anal shield, L. aspergillum had a central insertion into the anal shield, contrary to the earlier interpretation by de Quatrefages (Reference de Quatrefages1866). Overlooking Grube’s proposal, Sluiter (Reference Sluiter1883) assigned L. aspergillum to a new genus, Echinosiphon, based on the presence of ‘calcareous papillae’, a character absent in all other sipunculan species. However, 3 years later, he acknowledged that this genus name was a synonym of Cloeosiphon (Sluiter Reference Sluiter1886).
When de Quatrefages (Reference de Quatrefages1866) described L. aspergillum, he described a specimen measuring 6 cm in length with an anal shield characterized by large, elongate spines, which he observed after dissolving the calcareous material with nitric acid; however, he did not dissect the specimen. In contrast, Selenka (Reference Selenka1883) provided a more detailed account of the external morphology and the hooks of Cloeosiphon aspergillum. Furthermore, Selenka & Bülow in Selenka (Reference Selenka1883) examined specimens collected from various localities and proposed a new species from the Philippines, Cloeosiphon mollis, distinguishing it from C. aspergillum by arguing that the hooks in C. mollis were smaller than those in C. aspergillum. Over subsequent years, three additional species were described within Cloeosiphon: Cloeosiphon javanicum Sluiter, Reference Sluiter1886 (from Java), Cloeosiphon japonicum Ikeda, 1904 (from Ryukyu and Amami-Ōshima Islands, Japan), and Cloeosiphon carolinum Ikeda, 1924 (from Palau).
Sluiter (Reference Sluiter1902) synonymized his newly described species C. javanicum with C. aspergillum, while Augener (Reference Augener1903) proposed that C. javanicum and C. mollis were varieties of C. aspergillum. Later, Fischer (Reference Fischer1922) listed C. javanicum, C. japonicum and C. mollis as synonyms, and finally, Satô (Reference Satô1935) synonymized C. carolinum with C. aspergillum. The morphological differences among these species are further discussed below in the Remarks section.
In their monograph, Stephen and Edmonds (Reference Stephen and Edmonds1972) made two significant points. First, they clarified that the correct specific epithet should be C. aspergillus, not C. aspergillum, because the genus name is masculine. Second, they agreed with the synonymization of C. mollis, C. javanicum, C. japonicum, and C. carolinum with C. aspergillus after examining several specimens from Australia. They concluded that C. aspergillus is ‘a well-known species of coral reefs in the Indo-Pacific region’.
Saiz-Salinas (Reference Saiz-Salinas1984) designated a lectotype and paralectotype for C. aspergillus, providing detailed illustrations of its internal and external anatomy, as well as an isolated hook. Later, Cutler (Reference Cutler1994) compiled all available data on this species, reaffirming the earlier conclusion of Stephen and Edmonds (Reference Stephen and Edmonds1972). He agreed that Cloeosiphon is a monotypic genus, characterized by a pineapple-shaped anal shield, associated with coral reefs, and distributed throughout the Indo-Pacific region. Currently, this genus is recognized as a monotypic within sipunculans and has been consistently placed in the family Aspidosiphonidae, from the earliest phylogenetic analyses (Cutler and Gibbs Reference Cutler and Gibbs1985; Gibbs and Cutler Reference Gibbs and Cutler1987) to more recent studies (Kawauchi et al Reference Kawauchi, Sharma and Giribet2012; Lemer et al Reference Lemer, Kawauchi, Andrade, González, Boyle and Giribet2015).
The main objective of this study is to provide a detailed redescription of Cloeosiphon aspergillus based on type material and additional specimens to document variations in hook morphology along the ringed zone. Furthermore, we describe a new species of Cloeosiphon from the southern Mexican Pacific.
Material and methods
Specimens from the following collections were reviewed: Museum of Comparative Zoology (MCZ), Harvard University, Massachusetts, USA; National Museum of the Natural History (USNM), Smithsonian Institution, Washington, USA; and Muséum National d’Histoire Naturelle (MNHN), Paris, France.
The redescription was primarily based on type material, with additional comments on other specimens to assess potential intraspecific variations. Standardized descriptions included both external and internal anatomy, following the terminology proposed by Cutler and Cutler (1989), Cutler (Reference Cutler1994), and Silva-Morales and Carrera-Parra (Reference Silva-Morales and Carrera-Parra2025).
Introvert hooks and trunk papillae were extracted with fine forceps and examined under a compound light microscope. Hooks were sampled from three different regions of the introvert (anterior, median, and posterior), while papillae were described from three different regions of the trunk (anterior, median, and posterior).
Digital photographs of taxonomically important internal and external features were obtained using a Canon X6 digital camera mounted on a dissecting stereomicroscope. To enhance depth of field, images were processed by combining a series of optical focal planes with HeliconFocus v6.7.1.
Results
Systematics
Order Sipuncula Sedgwick, Reference Sedgwick1898
Family Aspidosiphonidae de Quatrefages, Reference de Quatrefages1866
Genus Cloeosiphon Grube, Reference Grube1868
Loxosiphon (de Quatrefages, Reference de Quatrefages1866 [partim]
Type species. Loxosiphon aspergillum de Quatrefages, Reference de Quatrefages1866, by monotypy.
Diagnosis. Introvert inserted centrally in the anal shield. All hooks Type A, compressed, bidentate, arranged in rings with a complex clear streak. Anal shield pineapple form in lateral view. Conical units in all Zone A. Longitudinal muscle continuous.
Cloeosiphon aspergillus (de Quatrefages, Reference de Quatrefages1866)
Loxosiphon aspergillum de Quatrefages, Reference de Quatrefages1866: 605, pl. 20, fig. 20.
Cloeosiphon aspergillum (Grube Reference Grube1868: 47–49).
Cloeosiphon mollis Selenka & Bülow in Selenka, Reference Selenka1883: 128, figs. 217–218.
Echinosiphon aspergillum Sluiter, Reference Sluiter1883: 26–38, pl. 1, figs. 1–15.
Cloeosiphon javanicum Sluiter, Reference Sluiter1886: 473.
Cloeosiphon aspergillus (Stephen and Edmonds Reference Stephen and Edmonds1972: 268, figs. 32A–D).
Diagnosis
Introvert inserted centrally in the anal shield. All hooks with conspicuous secondary tooth. Basal margin of the anal shield with a row of conspicuous spherical units.
Material examined
Lectotype. MNHN 423a, V24, Île de France (=Mauritius), Mr. Desjardins. A’ (R)- 1868. Paralectotype. MNHN 423b, V24, same data as holotype.
Other material examined
USNM 33052, 2 specimens, Nosy Be, Lokobe Point, Madagascar, 13°20′S, 48°15′E, coll. E. Cutler, 13 July 1964, 2.6–8.6 m depth, Sta. EC 14. MCZ 130163, 1 specimen, St. Lucia Co., Perrier’s Rock, KwaZulu-Natal, South Africa, coll. R. Biseswar, 18 October 2002, under oyster shells (Saccostrea cuccullata), intertidal. MCZ 130164, 1 specimen, Recif du Prony, New Caledonia, 6 m depth, coral rubble, 22°15′60″S, 166°19′37″E, 15 November 2007, coll. G.Y. Kawauchi, C. Tiago.
Redescription
Lectotype MNHN 423a.
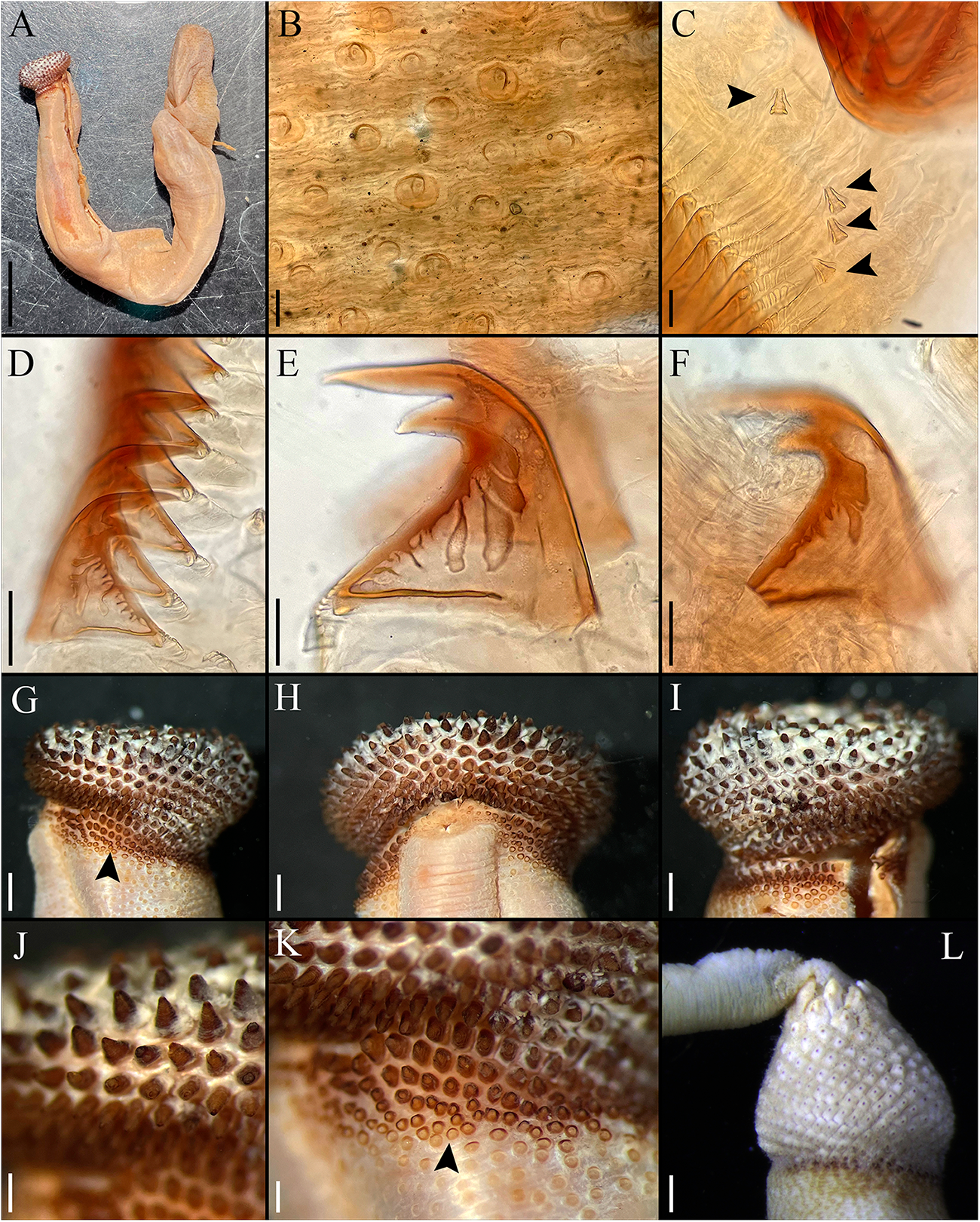
External morphology. Trunk 50 mm in length (Figure 1A); smooth, light brown with opaque body wall; trunk with semicircular papillae of different sizes, non-conglomerate (50–100 µm length), without platelets, arranged scattered throughout the trunk (Figure 1B).

Figure 1. Cloeosiphon aspergillus (de Quatrefages Reference de Quatrefages1866). Lectotype MNHN 423a (A, G–K), Paralectotype 423b (B–F), non-type USNM 33052 (L). (A) Adult complete specimen, lateral view; (B) Papillae of the trunk, median region; (C) Conical papillae, median rings zone, arrows point papillae; (D) Hooks, median ring; (E) Hook, median ring; (F) Hook, last ring; (G, I) Anal shield, lateral view, arrow point spherical units zone; (H) Anal shield, dorsal view; (J) Anal shield, zoom; (K) Basal margin shield/trunk, arrow point spherical units zone; (L) Anal shield covered with calcareous material, lateral view. Scale bars: A, 5 mm; B, 100 µm; C, 20 µm; D, 50 µm, E–F, 25 µm; G–I, 1 mm; J–L, 0.5 mm.
Introvert entirely retracted, 33% trunk length, protrudes from centre of shield. Introvert papillae conical (Figure 1C), smaller anteriorly, taller posteriorly (20–25 µm tall), arranged in rings. Tentacles not observed.
Introvert with all hooks (95–100 µm tall, n = 100 hooks). Type A (compressed, bidentate) arranged in 23 rings (Figure 1D–E). Ringed area covering 10% of introvert length. All hooks with the angle between line X and Y of 90° or less, main and secondary teeth sharp and main tooth exceeds the base length of the hook in most cases. Clear streak complex, but the most posterior hooks with less complexity (Figure 1F).
Anal shield dark brown without grooves. Conical units covering all shield (Figure 1G–I), tip of conical units oriented upwards (Figure 1J). Spherical units on the basal margin (Figure 1K). Shield with pineapple-like form in lateral, ventral, and dorsal view. Conical units covered by calcareous material produced by the specimen (Figure 1L). Caudal shield absent.
Internal morphology. A pair of nephridia opening posterior to anus level, attached in all their length, occupying 90% trunk length. Longitudinal muscle layer continuous. A pair of retractor muscles attached to the body wall in 92% of the trunk length. Wing muscle, fixing muscle, caecum, and eyespots not observed. Spindle muscle attached posteriorly.
Habitat
Mainly on coralline rock; under oyster shells, 2.6–8.6 m depth.
Distribution
Western and Central Indo-Pacific Ocean.
Variations
Trunk length (17–50 mm, n = 6); Hook rings (15–23, n = 3); Nephridia length (75–90% trunk length, n = 6) Retractor muscle attachment (75–92% trunk length, n = 6).
The lectotype has a few tears that, at first glance, resemble longitudinal muscle bundles (LMB). However, these are not true LMB, as they result from fractures in the body wall.
The shape of the anal shields may vary depending on the degree of contraction of the animal; therefore, these differences should not be considered diagnostic characters.
Taxonomic remarks
In the jar V24 of the MNHN, there are two small vials containing specimens of L. aspergillum, currently recognized as Cloeosiphon aspergillus. The first vial bears the label ‘Lectotype, studied by Saiz-Salinas, 1868-423a’. Although the original label is broken, its content remains legible. The specimen in this vial had already been dissected. The second vial is labelled ‘Paralectotype, studied by Saiz-Salinas. M. Desjardins, Île de France’. This specimen is in poor condition, it is broken into two parts, with the retractor muscle loose inside the vial, although the anal shield remains intact. There is no doubt that both specimens were examined by de Quatrefages (Reference de Quatrefages1866).
Regarding the names synonymized with C. aspergillus, when Selenka & Bülow in Selenka (Reference Selenka1883) proposed C. mollis, they based it on two specimens measuring 13–14 mm from the Philippines. They argued that the hooks were smaller (40 µm tall) compared to those of C. aspergillus (90–100 µm tall) and that the shape of the anal shield units in their new species was irregular, in contrast to those of C. aspergillus. Unfortunately, the holotype cannot be re-examined to confirm this because it is lost. Additionally, regarding the morphology of the anal shield, we have observed that a single specimen can have both perfectly rhomboidal and irregular shaped units. Furthermore, Stephen and Edmonds (Reference Stephen and Edmonds1972) examined 25 specimens from the Great Barrier Reef in Australia and, although they did not provide data on trunk or hook size, they stated that the variation observed in the hooks of C. mollis falls within the range of C. aspergillus. In conclusion, we agree that C. mollis is a synonym of C. aspergillus.
When Sluiter (Reference Sluiter1886) described C. javanicum, he argued that his new species exhibited muscle bands and that its hooks had a different patterns and shapes compared to C. aspergillus. However, he did not provide specific details about these differences. The type material of C. javanicum is apparently lost, but several years after, after collecting numerous specimens during the Siboga Expedition (Sluiter Reference Sluiter1902), he synonymized his own species upon realizing that the morphological variation within the population fell within the range of variation observed in C. aspergillus. Herein, we have described that C. aspergillus may exhibit divisions in the body wall, but these should not be mistaken for muscle bands. In conclusion, we agree that C. javanicum is a synonym of C. aspergillus.
Augener (Reference Augener1903) examined material from Ambon Island, he identified two specimens, one as C. aspergillum var. mollis and the other as C. aspergillum var. javanicum. In his discussion, he clearly stated that the internal anatomy of C. aspergillum, C. aspergillum var. javanicum, and C. aspergillum var. mollis was identical and that the differences in the structure of the shield units and hook size were so minimal that they both species should be considered varieties of C. aspergillum. Augener (Reference Augener1903) described C. aspergillum var. javanicum as having a dark yellow to greyish or even transparent coloration, while C. aspergillum var. mollis was more yellowish. However, he did not provide details on the shape of the hooks or the size of the specimens he examined.
Ikeda (Reference Ikeda1904) proposed C. japonicum, stating that, in terms of internal anatomy, it was identical to C. aspergillum but differed in having irregular shaped units in the anal shield and papillae of the introvert. We have not been able to access the type material housed in Japan, but based on Ikeda’s detailed illustrations, we consider that C. japonicum may indeed represent a different species, which could be reinstated after a revision of the type material or topotypic specimens. Ikeda´s illustrations depict cylindrical introvert papillae, which were not observed in the type material of C. aspergillus or in any specimens we examined. Additionally, the internal structure of the hooks in C. japonicum appears to have a complex clear streak, but only in the anterior region of the hook, not across the entire internal area as seen in C. aspergillus.
Fischer (Reference Fischer1922) listed C. mollis, C. javanicum, and C. japonicum as synonyms of C. aspergillum after examining material from the Java Sea, Fiji, and Sumatra, as well as topotype material of C. aspergillus from Mauritius. He conducted a thorough analysis of morphological characters and found that all three species fell within the range of variation of C. aspergillus. However, as we noted above, C. japonicum differs in the clear streak pattern of its hooks.
Ikeda (1924) proposed another new species from Palau, C. carolinum, but Satô (Reference Satô1935) later synonymized it with C. aspergillum, as the primary diagnostic characteristic Ikeda used to differentiate C. carolinum was the presence of calcareous material. Satô examined several specimens from Palau and confirmed that C. carolinum was indistinguishable from C. aspergillus. In conclusion, C. carolinum should also be considered a synonym of C. aspergillus, as its differentiation was primarily based on the presence of calcareous material in the anal shield, an aspect that has already been discussed both here and in previous studies.
Cloeosiphon mexicanus sp. nov
Material examined
Holotype. USNM 1199527, 1 specimen, Sta. 2564/648, Acapulco, near Barra de Terocha, Guerrero, Mexico, 21 April 1964, coll. A. Guerra.
Etymology
The name of the species refers to the country Mexico where the specimen was collected.
Diagnosis
Introvert inserted centrally in the anal shield. Hooks from the last ring with inconspicuous secondary tooth. Basal margin of the anal shield lacks any type of units.
Description
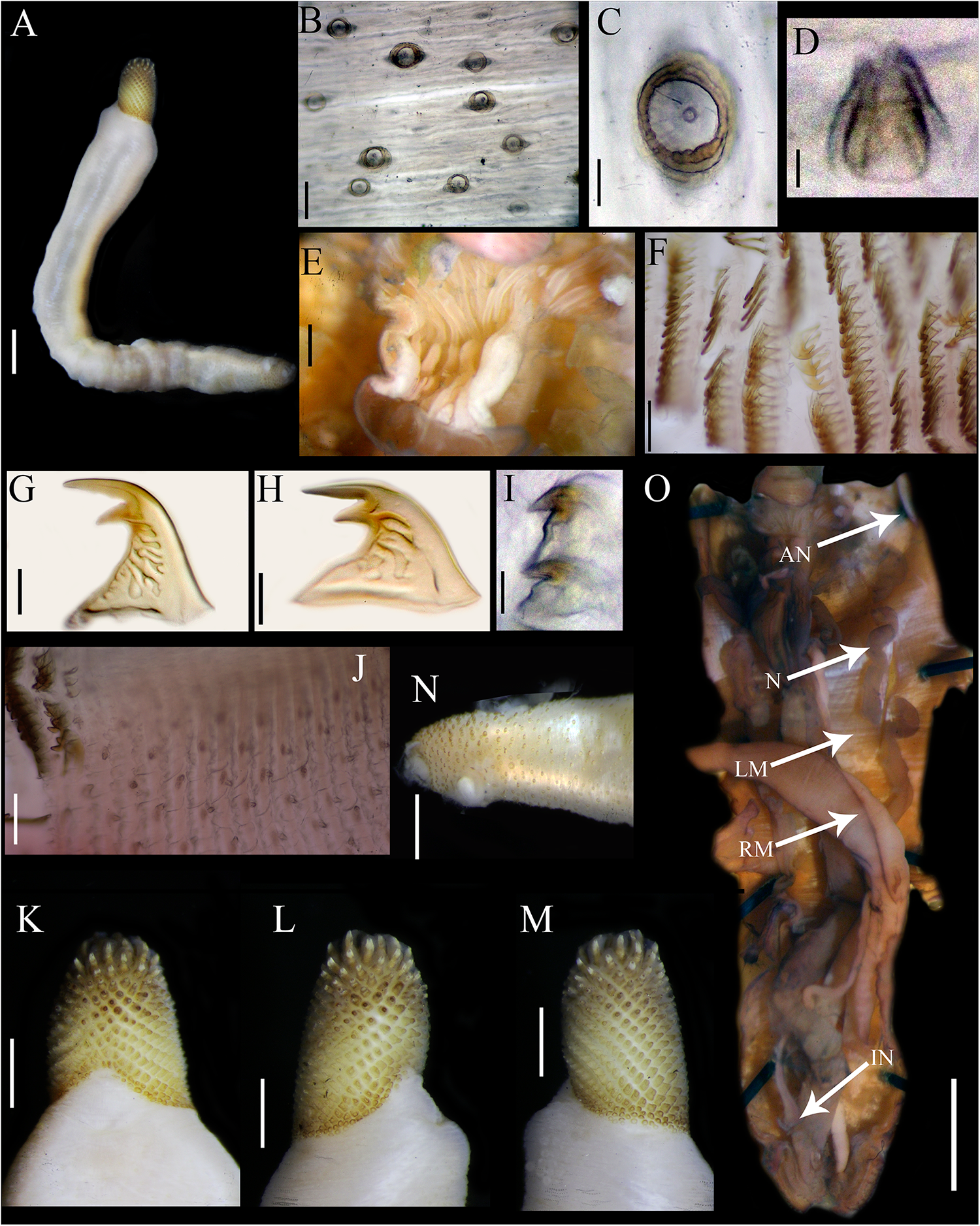
External morphology. Trunk 20 mm in length (Figure 2A); smooth, light brown with opaque body wall; trunk with semicircular papillae of different sizes, non-conglomerate (30–75 µm length), without platelets, arranged scattered throughout the trunk (Figure 2B–C).

Figure 2. Cloeosiphon mexicanus sp. nov. Holotype USNM 1199527 from southern Mexican Pacific: (A) Adult complete specimen, lateral view; (B) Papillae of the trunk, median region; (C) Papillae, Zoom; (D) Conical papillae, median ring zone; (E) Tentacles; (F) Hooks, median rings; (G) Hook, median ring; (H) Hook, posterior ring; (I) Hooks, last posterior ring; (J) Papillae after rings of hooks; (K) Anal shield, dorsal view; (L–M) Anal shield, lateral view; (N) Caudal region of the trunk, lateral view; (O) Internal anatomy. Abbreviations: AN, anus; IN, intestine; LM, longitudinal muscle layer; N, nephridia; RM, retractor muscles. Scale bars: A, O, 3 mm; B, F, J, 100 µm; C, I, 15 µm; D 5 µm; E, 0.025 mm; G, H, 25 µm; I, 15 µm; K–N, 1 mm.
Introvert entirely retracted, 25% trunk length, protrudes from centre of the shield. Introvert papillae conical (Figure 2D), smaller anteriorly, longer posteriorly (15–20 µm tall), arranged in rings. Tentacles digitiform (Figure 2E).
Introvert with most anterior hooks (75–100 µm tall, n = 100 hooks) Type A (compressed, bidentate) arranged in 20 rings (Figure 2F–H). Ringed area covering 20% introvert length. All bidentate hooks with the angle between line X and Y of 90° or less, main and secondary teeth sharp and main tooth does not exceed the base length of the hook in most cases. Clear streak complex. Last ring with small hooks (24–25 µm tall) and with secondary tooth inconspicuous (Figure 2I). Rings of hooks following by smaller conical papillae (10–15 µm tall), arranged in rings (Figure 2J).
Anal shield brown without grooves. Conical units covering all shield (Figure 2K–M), tip of conical units oriented upwards. Without units on the basal margin. Shield with pineapple-like form in lateral and dorsal view. Caudal shield absent (Figure 2N).
Internal morphology (Figure 2O). A pair of nephridia opening posterior to anus level, attached in all their length, occupying 75% trunk length. Longitudinal muscle layer continuous. A pair of retractor muscles attached to the body wall in 80% of the trunk length. Wing muscle, fixing muscle, caecum and eyespots not observed. Spindle muscle attached posteriorly.
Type locality
Acapulco, Guerrero, southern Mexican Pacific.
Distribution
Only known from the type locality.
Taxonomic remarks
Cloeosiphon mexicanus sp. nov. differs from C. aspergillus by having an inconspicuous secondary tooth on the hooks of the last ring (Figure 2I), and its basal margin of the anal shield lacks any type of units (Figure K–M). In contrast, C. aspergillus has bidentate hooks with a conspicuous secondary tooth, including on the last ring (Figure 1E–F), and the basal margin of its anal shield features a row of conspicuous spherical units (Figure 1G, K). Regarding hook morphology, no evidence of fractures was observed, indicating that the inconspicuous secondary tooth result from wear or friction. Compared with C. japonicum, which possesses a complex clear streak restricted to the anterior region of the hook and cylindrical papillae in the posterior region of the introvert, C. mexicanus sp. nov. exhibits a complex clear streak extending over more than half of the hook and conical papillae in the posterior region of the introvert.
Regarding the distribution, C. mexicanus is only known from its type locality in the southern Mexican Pacific. In contrast, C. aspergillus is primarily distributed in the Western and Central Indian Ocean, while C. japonicum occurs in southern Japan, specifically around the Ryukyu and Amami-Ōshima Islands.
Discussion
The results presented in this study provide a comprehensive review of the taxonomic status of Cloeosiphon aspergillus and the description of a new species, C. mexicanus sp. nov., thereby contributing to a better understanding of morphological diversity within the genus Cloeosiphon. The detailed redescription of the type material and additional specimens of C. aspergillus allowed the identification of notable intraspecific variation in characters such as trunk length, number of hook rings, and proportions of internal structures like nephridia and retractor muscles. These variations were interpreted as part of the species’ normal morphological range, supporting its recognition as a valid taxon with a broad Western and Central Indo-Pacific distribution.
Furthermore, the critical review of historically proposed synonyms of C. aspergillus confirms its monospecific status, with the exception of C. japonicum, which exhibits unique morphological features that may justify its reinstatement as a valid species. These include the distinct morphology of the introvert papillae and the pattern of the clear streak in the hooks. This highlights the need for future taxonomic revisions using type or topotypic material, ideally complemented by molecular data.
The discovery of C. mexicanus sp. nov. is a significant addition to the known Sipuncula fauna of the Mexican Pacific. Its distinction from C. aspergillus is based on consistent diagnostic features, such as the absence of spherical units along the basal margin of the anal shield and the reduced secondary tooth in the posterior hooks, which cannot be attributed to wear or breakage. Silva-Morales and Carrera-Parra (Reference Silva-Morales and Carrera-Parra2025), following a comprehensive revision of diagnostic characters, highlighted the hooks and anal shield as key features for distinguishing species within Aspidosiphonidae. Molecular analyses in Sipuncula have consistently shown that even subtle morphological differences reflect genetically distinct lineages (Kawauchi and Giribet Reference Kawauchi and Giribet2010, Reference Kawauchi and Giribet2014; Silva-Morales Reference Silva-Morales2020; Silva-Morales and Carrera-Parra Reference Silva-Morales and Carrera-Parra2025; Silva-Morales et al Reference Silva-Morales, López-Aquino, Islas-Villanueva, Bastida-Zavala and Ruiz-Escobar2019). The restricted occurrence of this new species in the southern Mexican Pacific suggests a pattern of regional endemism, extending the known geographical range of the genus Cloeosiphon and emphasizing the importance of detailed taxonomic studies in underexplored regions.
Therefore, in addition to the redescription of C. aspergillus based on both type and non-type material, we consider the description of the new Cloeosiphon species a significant contribution toward documenting sipunculans diversity, which remains underestimated.
Acknowledgements
We thank Adam Baldinger (MCZ), Tarik Meziane (MNHN), Karen Reed and Karen Osborn (USNM) for kindly lending and managing the specimens examined. The careful review by Rolando Bastida-Zavala and an anonymous referee resulted in a significant improvement in this final version.
Author contributions
Both authors formulated the research question(s), designed the study, carried out the study, analysed the data, interpreted the findings and wrote the article.
Funding
During this research, ISM was supported by a scholarship from CONAHCYT (951069). Additionally, the following scholarships were awarded for museum research stays: Ernest Mayr Grant, Museum of Comparative Zoology, Harvard University; Kenneth Jay Boss Fellowship, National Museum of Natural History, Smithsonian Institution.
Competing interests
The authors declare none.
Ethical standards
Not applicable.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.