The debate over the dietary requirement for vitamin D continues, despite the Institute of Medicine's re-evaluation of dietary requirements, released in 2011(Reference Aloia1), with continuing concern that recommendations fall short of actual vitamin D requirements(Reference Heaney and Holick2, Reference Holick, Binkley and Bischoff-Ferrari3). Uncertainty and lack of consensus over biomarkers of sufficient and insufficient vitamin D status, and growing evidence for the role of vitamin D in diseases beyond Ca and bone health, further emphasise the importance of continued research on vitamin D nutrition. For individuals with low serum 25-hydroxyvitamin D (25(OH)D), and certain at-risk populations, such as the elderly or those with dark skin or little sunlight exposure, supplements may be advised(4). Concerns over the risks of hypervitaminosis D also continue, and the Institute of Medicine report concluded that serum concentrations of 25(OH)D >125 nmol/l come with an increased risk of negative effects(Reference Aloia1, 4, Reference Ross, Manson and Abrams5). One potential side effect of excess vitamin D intake is the increased risk of hypercalciuria, but despite this fact, only a few studies have examined 24 h Ca excretion.
One of the limitations of strategies to improve nutrient status through the use of vitamin supplements is poor compliance with daily supplementation(Reference Smith, Gardner and Locke6). For this reason, large doses taken less frequently may offer advantages to some people. To assess the efficacy and safety of such an approach, we evaluated the effects of vitamin D supplementation in daily and weekly doses, and a high weekly dose followed by monthly doses, on circulating 25(OH)D and vitamin D metabolites and on Ca and related indices in healthy adults. We studied vitamin D3 supplementation given the general consensus that this is more effective(Reference Armas, Hollis and Heaney7–Reference Tripkovic, Lambert and Hart9), despite conflicting literature(Reference Holick, Biancuzzo and Chen10). Although only vitamin D2 is available by prescription, the Internet has opened the door to a wide availability of many doses and forms. We did achieve the aim of evaluating the efficacy and safety of daily, weekly and weekly/monthly doses of vitamin D3 in a healthy adult population, and we describe their effectiveness at maintaining serum 25(OH)D status and discuss their likelihood of increasing urinary Ca excretion and producing vitamin D toxicity.
Subjects and methods
The present study was conducted at the Johnson Space Center in Houston, Texas, from June 2008 to September 2009. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Johnson Space Center Committee for the Protection of Human Subjects. Participants were recruited from local advertising at Johnson Space Center. Written informed consent was obtained from all subjects.
After passing a general physical examination, subjects were screened for serum 25(OH)D; blood urea N; serum creatinine, Mg, P and Ca; and 24 h urinary Ca excretion. Subjects who had urinary Ca >7·5 mmol/d or a BMI >30 kg/m2, who took >40 μg/d (>400 IU/d) of supplemental vitamin D or who smoked were excluded from participation. After screening, forty-eight subjects (n 31 men and n 17 women) met the eligibility criteria and were randomly assigned to one of four dosage groups: placebo, or vitamin D3 as 50 μg/d (2000 IU/d), 250 μg (10 000 IU) once per week or 1250 μg (50 000 IU) once per week for 4 weeks and then once per month for 2 months for a total of 3 months. For reporting purposes in the tables, this group will be referred to as the group receiving 1250 μg/month. The placebo group was randomised to placebo daily, once per week and once per week for 4 weeks and then once per month for 2 months (n 4 per group), to match the vitamin D supplement groups to make sure subjects could not know their treatment group simply by knowing their dosing regimen. Subjects with an initial serum 25(OH)D concentration >85 nmol/l were assigned to the placebo group (n 5). Given that these five subjects were not randomly assigned in the present study, this is a study design limitation.
Vitamin D3 capsules in the amounts of 50, 250, 1250 μg or the placebo (identical in appearance and excipient to the vitamin D capsules) were from Tishcon. The analysis of vitamin D content of the supplements by Covance showed the actual contents of 70·1 (sd 2·0), 330·5 (sd 8·7) and 1544 (sd 30) μg for the 50, 250 and 1250 μg supplements, respectively. The 50 and 250 μg vitamin D and placebo capsules were packaged in blister-sealed pouches to enable compliance tracking. The 1250 μg capsules were taken under supervision.
On study day 1, the subjects arrived after fasting for 8 h. Venous blood was drawn and then subjects were given their first placebo or vitamin D supplement. The subjects were then instructed to collect all urine voided for the next 24 h into the containers provided and to return the samples, packed in a bag with ice packs provided, on the following day. Collection of fasting blood and a 24 h urine sample (starting immediately after a morning vitamin D dose) was repeated after 30, 60 and 90 d of supplementation. Additional weekly blood and 24 h urine samples were collected from some subjects during the first 21 d of the study, but results were not used in the statistical analyses.
Subjects were trained to complete a 7 d food questionnaire focusing on food sources of vitamin D, modified from a previously described questionnaire(Reference Smith, Gardner and Locke6, Reference Zwart, Mehta and Ploutz-Snyder11), and they completed this questionnaire for 6 d before and 24 h after each blood collection.
Exposure to UV radiation was measured using polysulfone film dosimeters as described previously(Reference Hall, Kimlin and Aronov12). Subjects were asked to wear the dosimeters (approximate size 5 × 5 cm) on their shoulder over their clothes for two 24 h periods, the first preceding the first day of sample collection and the second preceding the 2-month blood draw.
After collection, blood was allowed to clot and was centrifuged, and serum was aliquoted into cryotubes. Serum Ca, blood urea N, serum creatinine and the liver enzymes aspartate transaminase and alanine transaminase were measured within 4 d of blood collection. Serum for other analyses was frozen at − 70°C and batch-analysed at the end of the study.
Biochemical analytes were measured as described previously(Reference Smith, Gardner and Locke6, Reference Zwart, Mehta and Ploutz-Snyder11). Our laboratory participated in blinded proficiency testing by the College of American Pathologists for serum Ca, as well as the vitamin D external quality assurance scheme that monitors and reports the accuracy of 25(OH)D and 1,25-dihydroxyvitamin D measurements. Our laboratory also participated in the Accuracy Based Vitamin D Survey by the College of American Pathologists. Liver enzymes, serum creatinine and blood urea N were analysed in a College of American Pathologists-accredited clinical laboratory at the Johnson Space Center.
Levels of 24,25-dihydroxyvitamin D (24,25(OH)2D) were measured as described previously(Reference Ding, Schoenmakers and Jones13) using Waters MassLynx 4.1 (Waters Corp.), with quantification performed with Waters QuanLynx. For HPLC separation, a solvent gradient of 2 mm-ammonium formate with 0·1 % formic acid in water (A) and 0·1 % formic acid in methanol (B) at 0·3 ml/min was used, beginning with 95 % A/5 % B for 2 min, then 40 % A/60 % B for 2·1 min, then 12 % A/88 % B for 5 min, and then 2 % A/98 % B for 3 min. Multiple reaction monitoring consisted of a transition of 574·3 to 298·0 for 24,25(OH)2D with a collision energy at 18 V and a cone voltage at 25 V. Ar was used as a collision gas.
Statistical analyses
Biochemical markers of vitamin D, Ca and bone metabolism were assessed using a two-way repeated-measures ANOVA followed by a post hoc Bonferroni t test to determine differences from baseline when there was a main effect (time) or a significant interaction term. The α-level for statistical significance was set at P= 0·05. Multiple linear regression was used to determine the influence of vitamin D intake and UV light exposure on serum 25(OH)D concentration. A two-way repeated-measures ANOVA followed by a Bonferroni t test were used to determine the effects of weekly 1250 μg supplementation and BMI during the first 30 d of the study. A relative-risk test was performed to compare the relative risk of hypercalciuria in the group taking the 1250 μg dose and the placebo group once supplementation had started. For parathyroid hormone, two data points were lower than the lower limit of detection. For statistical analyses (and mean and standard deviation calculations), we used the lower limit of detection as the value for those data points.
All data reported are means and standard deviations, and all statistical analyses were completed with SigmaPlot 12.0 (Systat Inc.), unless noted otherwise.
Results
No significant group differences were found in age, BMI, body weight, dietary vitamin D intake or UV exposure (Tables 1 and 2). Compliance, as determined by pill counts and subject questioning, was >96 % for all groups. The 1250 μg supplements and respective placebos were taken under supervision.
Table 1 Subject demographic data before and throughout the supplementation study (Mean values and standard deviations)

* The 1250 μg/month group took one 1250 μg pill each week for the first 30 d and then one pill per month for the next 2 months. There were no significant changes in body weight over time, nor were there baseline differences between the groups for age or BMI.
Table 2 Daily UV light exposure and dietary vitamin D intake for 6 d before and 1 d after blood draws (Mean values and standard deviations, n 12 per group)

MED, minimal erythemal dose; NA, not available.
* This intake did not include the study supplements. A two-way repeated-measures ANOVA was performed on vitamin D intake data that did not include the study supplements, and there were no differences between the groups or over time.
† The 1250 μg/month group took one 1250 μg pill each week for the first 30 d and then one pill per month for the next 2 months.
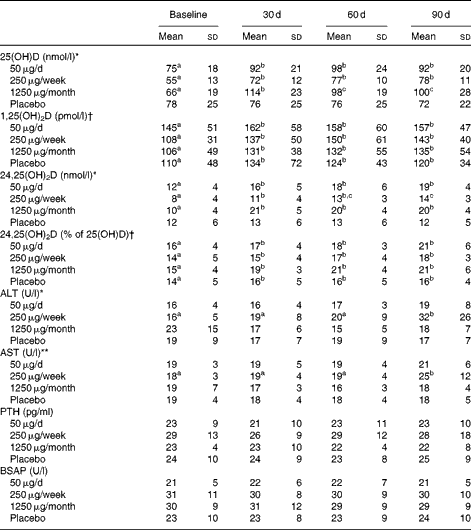
Serum 25(OH)D (Table 3) increased significantly over time (P< 0·001) and was significantly higher after 30 d than at baseline in all the three groups taking vitamin D, but not in the placebo group. Serum 25(OH)D was best predicted from dietary vitamin D intake assessed at baseline and 60 d (P< 0·001); UV exposure in the 24 h before blood collection had no influence on 25(OH)D (data not shown).
Table 3 Serum vitamin D status and serum concentrations of liver enzymes, parathyroid hormone and bone-specific alkaline phosphatase for subjects assigned to the different doses of vitamin D or placebo for 3 months‡ (Mean values and standard deviations)

25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; ALT, alanine transaminase; AST, aspartate transaminase; PTH, parathyroid hormone; BSAP, bone-specific alkaline phosphatase.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05; post hoc Bonferroni t test).
There was a significant group × time interaction effect: * P< 0·001, ** P< 0·05.
† There was a significant time effect (P< 0·01).
‡ Statistical analyses were performed on the monthly data (baseline and 30, 60 and 90 d time points, n 12). The 7, 14 and 21 d data are included in the table, but were not analysed statistically because of the smaller sample sizes (n 8/9 for the 50 μg group, n 5/6 for the 250 μg group, n 12 for the 1250 μg group and n 9 for the placebo group for the weekly time points). The 1250 μg/month group took one 1250 μg pill each week for the first 30 d and then one pill monthly for the next 2 months.
Serum 24,25(OH)2D (Table 3) was higher at 30, 60 and 90 d of supplementation than at baseline in all of the groups taking vitamin D; at 90 d, it was significantly higher in the groups taking 50 or 1250 μg vitamin D than in those taking 250 μg vitamin D. As a percentage of 25(OH)D, 24,25(OH)2D was significantly greater at 30, 60 and 90 d of the study than at baseline (P< 0·01).
Vitamin D supplementation had no effect on serum parathyroid hormone (Table 3). However, as expected, serum parathyroid hormone was inversely correlated with 25(OH)D (r − 0·22, P< 0·001) and 24,25(OH)2D (r − 0·24, P< 0·001).
Vitamin D supplementation had no effect on serum Ca, vitamin D-binding protein, N-telopeptide, Mg, high-sensitivity C-reactive protein, urea, P or creatinine (data not shown). A significant group effect occurred for bone-specific alkaline phosphatase, but the post hoc Bonferroni t test yielded no significant differences between the groups (Table 3). The liver enzymes alanine transaminase and aspartate transaminase were elevated in the 250 μg/week group after 90 d of supplementation (P< 0·001 and P< 0·05, respectively; Table 3). Of the twelve subjects, four who took 250 μg vitamin D had an alanine transaminase concentration>0·83 μkat (50 U)/l (normal range 0·10–0·67 μkat (6–40 U/l), and three of these four subjects also had elevated serum aspartate transaminase.
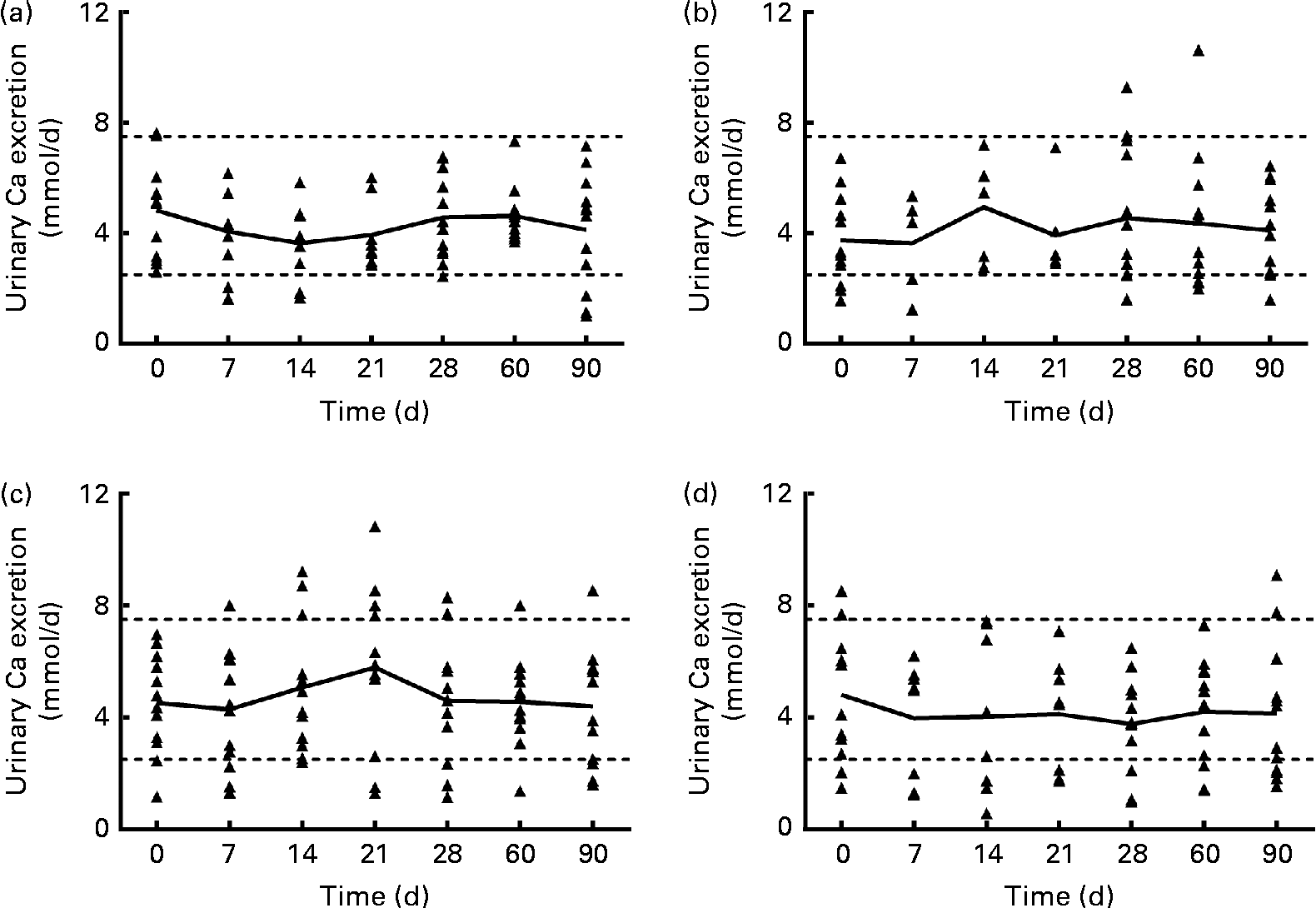
Urinary Ca excretion (Fig. 1) was above the upper limit of normal for twelve samples from subjects in the 1250 μg vitamin D group when expressed as nmol/d (but not when normalised to creatinine), three samples from the 250 μg group, two samples from the 50 μg group and four samples from the placebo group. However, no significant effect of group was found for mean urinary Ca, creatinine, N-telopeptide, P or Mg excretion at any time (data not shown). When the urinary Ca data (nmol/d) from the 1250 μg/month group were analysed by BMI subgroup over the first 30 d of supplementation (the time when the supplementation was weekly in this group), mean urinary Ca increased significantly only in subjects with a higher BMI (26–30 kg/m2, n 6), not in subjects with a lower BMI (20–25 kg/m2, n 6). When the urinary Ca data normalised to creatinine were analysed, a similar trend was found, but the interaction was not significant (P= 0·052). The data were analysed using a two-way repeated-measures ANOVA (P< 0·05), and a post hoc Bonferroni t test revealed that after 3 weeks of supplementation, urinary Ca excretion was significantly greater in the group with a higher BMI. The relative risk of urinary Ca excretion being >7·5 mmol/d was compared between the 1250 μg and placebo groups, and the likelihood that urinary Ca exceeded 7·5 mmol/d was higher in the 1250 μg group (P= 0·01).

Fig. 1 Urinary calcium excretion (24 h) of each subject, for each vitamin D dose group: (a) 50 μg/d (2000 IU/d), (b) 250 μg/week (10 000 IU/week), (c) 1250 μg/week (50 000 IU/week) for 4 weeks and then 1250 μg/month for 2 months and (d) placebo. — Connects the mean for each time point; Δ represents individual subject data; - - - represents the normal range for urinary calcium (2·5–7·5 nmol/d(Reference Wu23)). n 8/9 for the 50 μg/d group, n 5/6 for the 250 μg/week group, n 12 for the 1250 μg/month group and n 9 for the placebo group for the 7, 14 and 21 d time points. n 12 for all groups at baseline and at 28, 60 and 90 d time points.
Discussion
The dosing regimens of 50 μg/d, 250 μg/week and 1250 μg/month for 4 weeks and then 1250 μg/month for 2 months were all similarly effective in raising circulating 25(OH) vitamin D concentrations. Of the subjects, one-third taking the weekly rather than the daily dose of vitamin D ended the 90 d study with high levels of alanine transaminase, but this effect was not noted in the subjects taking the monthly dose. One pathway by which the body protects itself against vitamin D toxicity is the conversion of 25(OH)D to 24,25(OH)2D. Vitamin D supplementation did increase serum 24,25(OH)2D concentrations in all of the supplemented groups. The mean 24 h urinary Ca excretion was not significantly different after 30, 60 or 90 d of taking vitamin D or placebo, but the urinary Ca excretion of all subjects was positively correlated with their serum 25(OH)D concentration (P <0·001). Further, more subjects in the 1250 μg vitamin D group than in the other groups had 24 h urinary Ca excretion above the normal range at some time during the study, with 40 % (n 5/12) of the subjects in this group showing 24 h urinary Ca excretion typically defined as hypercalciuric (i.e. >300 mg/d) by study day 30. Subjects in the placebo group who had elevated urinary Ca (Fig. 1) at baseline had serum 25(OH)D concentrations of 91 and 78 nmol/l. When we examined the group receiving the 1250 μg/month dose in the first 30 d of the study, we noticed that some subjects’ urinary Ca increased steadily over the first 3 weeks of supplementation, and others’ urinary Ca did not. When the subjects in that dose group were divided into categories of higher or lower BMI (greater or less than 26 kg/m2, which was the median BMI), only the subjects with a higher BMI had an elevation in urinary Ca after the third week of 1250 μg vitamin D3 supplementation. This interesting result will need further study with more subjects to determine whether it is true that subjects with a higher BMI have an increase in urinary Ca with a weekly dose of 1250 μg/month.
The fact that there were no differences in 25(OH)D responses between the dose groups was not surprising because the total doses were very similar. Total daily doses (calculated from the actual analysed content of the supplements) for the three groups averaged to 70·1, 44·1 and 103 μg/d for the 50 μg/d, 250 μg/week and 1250 μg/month groups. In recent studies conducted in Antarctica on vitamin D3 supplementation (50 μg/d or 250 μg/week), we found that the efficacy of the supplement was influenced by BMI(Reference Zwart, Mehta and Ploutz-Snyder11) in that the greater the BMI, the smaller the change in serum 25(OH)D. Given the results from that study and the present study, people with a higher BMI may be likely to take larger doses of vitamin D to increase their vitamin D status, and they are also more likely to have an acute increase in urinary Ca.
Increased urinary Ca excretion is a potential adverse effect of high-dose vitamin D supplementation. Transient elevations in 24 h urinary Ca may not have clinical relevance to healthy individuals, but they are relevant to populations at risk for renal stones. It is worth noting that urinary Ca excretion of >250 mg/d (>6·24 mmol/d) is associated with increased renal stone risk (relative to the general population)(Reference Pak, Skurla and Harvey14). In light of the greater acute urinary Ca response in subjects with a higher BMI, their potential for increased renal stone risk with a long-term intermittent high-dose vitamin D supplementation seems clear, and this should be studied further. The present study illustrates the importance of collecting urine for 24 h multiple times when evaluating hypercalciuria risk with intermittent vitamin D supplementation. Despite the fact that hypercalciuria is a primary concern in vitamin D treatment, in the vast majority of studies of vitamin D supplementation, urine was not collected at all(Reference Tau, Ciriani and Scaiola15–Reference Sanders, Stuart and Williamson18), it was collected only once(Reference Kimball, Burton and O'Connor19) or before or after a long period of supplementation(Reference Lips, Binkley and Pfeifer20, Reference Leaf, Korets and Taylor21), or it was collected only as spot urine samples(Reference Eisner, Thavaseelan and Sheth22), perhaps to avoid the inherent difficulties in having subjects collect complete 24 h urine samples. Other studies have shown modest increases in urinary Ca excretion. After 16 weeks of a weekly dose of 8400 IU (210 μg) vitamin D3, urinary Ca excretion tended (P= 0·08) to increase(Reference Lips, Binkley and Pfeifer20), but it is not known whether urinary Ca excretion was elevated within the first 3 weeks of supplementation. In another study, 1250 μg/week ergocalciferol was administered for 8 weeks. After 8 weeks, the mean urinary Ca excretion was not different from baseline, although eleven of the twenty-nine subjects had an increase >20 mg/d(Reference Leaf, Korets and Taylor21).
One of the limitations of the present study is that we did not collect dietary intake information (other than vitamin D). Beyond this, the results of the present study should be considered preliminary because of our relatively small number of subjects; nonetheless, the relationships identified raise concern for some populations and warrant further study.
Conclusion
Taking fewer supplements in higher doses conferred no advantage with regard to circulating vitamin D. We found no overt signs of chronic vitamin D toxicity during the study. The data that we report here document a relationship between high-dose vitamin D supplementation and urinary Ca excretion in a subset of individuals. For remote populations, such as space travellers, Antarctic winter crews, submariners and others whose access to medical facilities is limited, the convenience of less frequent supplementation does not seem worth any acute increased risk of hypercalciuria. Given the limited direct evidence of a relationship between vitamin D supplementation and renal stone incidence, it seems that further research on appropriate and safe amounts of supplemental vitamin D for patients at risk for renal stones is warranted.
Acknowledgements
The authors are indebted to the participants for their time and efforts in completing the study. We also thank the National Aeronautics and Space Administration (NASA) Nutritional Biochemistry Laboratory for their efforts in the implementation and sample processing for the study. We thank Jane Krauhs for editorial assistance. The present study was funded by the NASA Flight Analogs Project of NASA's Human Research Program. All authors had input to the design of the study. S. R. Z. and S. M. S. oversaw the data collection, management and statistical analysis. S. R. Z., M. K. and S. M. I. analysed the data. All authors interpreted the results of the experiments. S. R. Z. and S. M. S. drafted the manuscript. All authors edited and revised the manuscript, and approved the final version of the manuscript. The authors declare that they have no conflicts of interest.