Introduction
Ex situ management is increasingly used to prevent species extinctions (Seddon et al., Reference Seddon, Armstrong and Maloney2007; Redford et al., Reference Redford, Amato, Baillie, Beldomenico, Bennett and Clum2011), with 34 animals and 35 plants categorized as Extinct in the Wild and thus relying entirely on persistence of populations in captivity (IUCN, 2019). Successful case studies include the California condor Gymnogyps californianus in the Americas (Snyder & Snyder, Reference Snyder and Snyder2000) and crested ibis Nipponia nippon in Asia (Xi et al., Reference Xi, Lu, Zhang and Fujihara2002). The hope that these examples engender, coupled with the dire situation facing an increasing number of species in the wild (Butchart et al., Reference Butchart, Walpole, Collen, van Strien, Scharlemann and Almond2010), manifests in recommendations for captive breeding in the IUCN Red List accounts of 2,199 threatened or Near Threatened species (IUCN Conservation Planning Specialist Group, 2019). However, zoos can conserve only a small proportion of threatened species (Balmford et al., Reference Balmford, Mace and Leader-Williams1996), and the familiarity of successful case studies masks the fact that ex situ management is difficult, risky, time-consuming and financially costly, and can increase the risk of extinction for wild populations by removing individuals from the wild to create founder populations (Snyder et al., Reference Snyder, Derrickson, Beissinger, Wiley, Smith, Toone and Miller1996). A comprehensive global evaluation of conservation reintroduction, reinforcement or replacement case studies found that 72% of those with a known outcome succeeded and 28% failed; not eliminating the cause of decline was the greatest cause of failure (Bubac et al., Reference Bubac, Johnson, Fox and Cullingham2019). Faced with data that indicate a rapidly declining population, conservation managers must make a timely, informed decision regarding whether or not to proceed with ex situ management (McGowan et al., Reference McGowan, Traylor-Holzer and Leus2017). A failure to act quickly on available evidence of species declines can lead to extinctions (Martin et al., Reference Martin, Nally, Burbidge, Arnall, Garnett and Hayward2012), but making the wrong decision can also increase the risk of extinction (Snyder et al., Reference Snyder, Derrickson, Beissinger, Wiley, Smith, Toone and Miller1996).
Management of species occurs along a continuum of states ranging from free-ranging self-sustaining wild populations to species that exist only in captivity (Redford et al., Reference Redford, Amato, Baillie, Beldomenico, Bennett and Clum2011). Lines between states are increasingly blurred as a result of the increase in ex situ management (Redford et al., Reference Redford, Jensen and Breheny2012) and the variety of ex situ management regimes from small cages to extensive semi-natural environments. We follow the intuitive definition in IUCN/SSC (2014), which defines ex situ as conditions under which ‘the individuals are maintained in artificial conditions under different selection pressures than those in natural conditions in a natural habitat’. Duration of time in ex situ conditions can vary from short, for example the temporary removal of a population during predator control, to indefinite, for species for which there is no hope of reintroduction in the foreseeable future. Ex situ management has a range of purposes, including conservation (through establishing insurance populations, breeding for reintroduction, head-starting and research), education and restocking for sport (Fischer & Lindenmayer, Reference Fischer and Lindenmayer2000; Harding et al., Reference Harding, Griffiths and Pavajeau2016). Here we consider only captive breeding for preventing extinctions of species (i.e. the complete loss of all individuals of the species), for which we use the term ex situ conservation. The ultimate goal of ex situ conservation in this context is the reinforcement or reintroduction of wild populations, which distinguishes it from capture solely to establish captive populations for display, education, farming or keeping as pets. For this reason, individuals maintained and bred for ex situ conservation should be theoretically capable of producing offspring that can survive in the wild. Ideally, where possible, a parallel programme of in situ conservation should ensure that populations are maintained in the wild. However, ex situ conservation can inadvertently compromise source populations by harvesting too many individuals from free-ranging populations (increasing rates of decline), or distracting decision makers such as governments and funders to the detriment of in situ conservation (Snyder et al., Reference Snyder, Derrickson, Beissinger, Wiley, Smith, Toone and Miller1996). The IUCN guidelines suggest that success of in situ conservation should not be unduly jeopardized by ex situ conservation (IUCN/SSC, 2013, 2014) unless conditions in the wild are so hostile that the ex situ conservation plan requires that the entire wild population is taken into captivity (McCleery et al., Reference McCleery, Hostetler and Oli2014).
Ex situ conservation can buy time for conservation managers to address causes of decline by eliminating introduced predators, restoring habitat or enacting legislative changes that create conditions for species to survive in a wild state (Andrew et al., Reference Andrew, Cogger, Driscoll, Flakus, Harlow and Maple2018), but it must begin when there are sufficient wild individuals to establish a captive population. Species whose extinction might otherwise have been prevented, such as a number of reptiles and a bat (Christmas Island pipistrelle Pipistrellus murrayi) endemic to Christmas Island, and the po‘ouli Melamprosops phaeosoma, were lost because plans for ex situ conservation were not enacted until too few individuals remained (VanderWerf et al., Reference VanderWerf, Groombridge, Fretz and Swinnerton2006; Martin et al., Reference Martin, Nally, Burbidge, Arnall, Garnett and Hayward2012; Andrew et al., Reference Andrew, Cogger, Driscoll, Flakus, Harlow and Maple2018). Although most risks emanating from ex situ conservation can be mitigated, this is costly and time-consuming and none can be prevented entirely. However, avoidance of decisions about ex situ conservation because of perceptions of risk, fear of failure and fear of being perceived to have made the wrong decision is itself a decision to do nothing (Brook et al., Reference Brook, Dudley, Mahood, Polet, Williams and Duckworth2014) and can lead to extinctions (Woinarski et al., Reference Woinarski, Garnett, Legge and Lindenmayer2017).
IUCN has developed guidelines to help conservation managers determine how and when ex situ management should be used in conservation (IUCN/SSC, 2014). The guidelines provide a logical five-step decision-making process that finishes with a call to make a decision that is informed by the information gathered in the preceding four steps and ‘weighing the potential conservation benefit to the species against the likelihood of success and overall costs and risks of not only the proposed ex situ programme, but also alternative conservation actions or inaction’ (IUCN/SSC, 2014; McGowan et al., Reference McGowan, Traylor-Holzer and Leus2017). Step 1 is a review of the status of the species, in Step 2 the role(s) that ex situ management could play in the conservation of the species are defined, Step 3 is an evaluation of the precise nature of the desired ex situ population to meet identified role(s), and in Step 4 resources, expertise, feasibility and risks are appraised (McGowan et al., Reference McGowan, Traylor-Holzer and Leus2017). There is no method proposed for the critical fifth step, which is to make the decision on whether to initiate captive breeding. Because captive breeding for conservation can be risky and controversial, decision-making should be conducted using a method that enables wide participation in the process so that practitioners have ownership of the results rather than feeling disempowered by top-down systems of management (Black et al., Reference Black, Groombridge and Jones2011). Hidden value judgments can be revealed and managed by following a transparent process that documents why and how decisions were made, with uncertainty acknowledged and quantified where possible so that it can be incorporated into the decision-making process (Game et al., Reference Game, Kareiva and Possingham2013). However, without the aid of tools, people struggle to quantify risk in decision-making processes (Redford & Taber, Reference Redford and Taber2000). The tool used most commonly for structured decision analysis in conservation is the decision tree (Maguire, Reference Maguire1986; Gregory et al., Reference Gregory, Failing, Harstone, Long, McDaniels and Ohlson2012; Panfylova et al., Reference Panfylova, Ewen and Armstrong2019). Decision trees have been used to choose between ex situ conservation and other options to varying degrees of success, but when a species is under imminent threat of extinction in the wild the chance of success for any conservation plan is low (Regan et al., Reference Regan, Ben-Haim, Langford, Wilson, Lundberg, Andelman and Burgman2005).
Here, we apply the IUCN Guidelines (IUCN/SSC, 2014) to the South-east Asian subspecies of Bengal florican Houbaropsis bengalensis blandini, a Critically Endangered bustard now restricted to Cambodia (BirdLife International, 2018b). Although IUCN/SSC (2014) calls for dissemination of information regarding use of the guidelines, we have not found any peer reviewed articles that explicitly document their use. Since the mid 2000s (the date of the first reliable population data), the Bengal florican has experienced a decline of > 10% per annum (Gray et al., Reference Gray, Collar, Davidson, Dolman, Evans and Fox2009; Packman et al., Reference Packman, Showler, Collar, Son, Mahood and Handschuh2013b; Mahood et al., Reference Mahood, Hong, Son, Sum and Garnett2019) as a result of habitat loss, agricultural intensification, hunting, and predation by free-ranging domestic dogs (Packman et al., Reference Packman, Gray, Collar, Evans, van Zalinge and Son2013a; Ibbett et al., Reference Ibbett, Lay, Phlai, Song, Hong, Mahood and Milner-Gulland2019). In 2018 the population was estimated to be 138 (95% CI 119–156) birds at four sites where display activity still occurred, and one site (Koup Preah Buong Trea) where a few additional birds remain (Mahood et al., Reference Mahood, Hong, Son, Sum and Garnett2019). Intensive in situ conservation efforts stabilized population trends at one site, Stoung-Chikreang Bengal Florican Conservation Area (Mahood et al., Reference Mahood, Hong, Son, Sum and Garnett2019), but that population is now threatened by a newly-constructed power line that could lead to local extinction (Mahood et al., Reference Mahood, Silva, Dolman and Burnside2016). Given the dire situation facing the species there have been calls from within Cambodia to consider captive management to prevent its global extinction (M. Meyerhoff, pers. comm., 2018). Mindful of the need to make a timely decision on whether to proceed with captive management, but aware of the potential risks of making the wrong decision, we use the IUCN Guidelines to make a decision about ex situ conservation of the Bengal florican. Instructions for using the guidelines are available (McGowan et al., Reference McGowan, Traylor-Holzer and Leus2017), but not tools for each step or a worked example. We developed simple tables to evaluate the issues for consideration under Steps 3 and 4, and a decision tree for Steps 2 and 5. We use a demographic model developed by Dolman et al. (Reference Dolman, Collar, Scotland and Burnside2015) to inform Step 3 and explore the probability of success of ex situ conservation (Addison et al., Reference Addison, Rumpff, Bau, Carey, Chee and Jarrad2013). We show how these tools enabled us to make a decision about ex situ conservation of the Bengal florican and hope that the decision tree will assist other conservation managers facing similar predicaments.
Methods
Development of tools
We first conducted a review of the status of the Bengal florican (Mahood et al., Reference Mahood, Hong, Son, Sum and Garnett2019), following Step 1 of the guidelines (IUCN/SSC, 2014). We created a decision tree that combined Step 2 (identification of the potential role of ex situ conservation in the conservation of the species) with the part of Step 4 that evaluates risk to the wild population from ex situ conservation (Fig. 1). We created a table to evaluate the practical considerations associated with ex situ conservation of the species (Table 1: Step 3), and a similar table to evaluate practical risks (Table 2: Step 4). The practical risks were separated from risks to the wild population because they can be mitigated, unlike biological risks for which mitigation is often difficult or impossible.

Fig. 1 Steps 2 (pale grey boxes), 4 (white boxes) and 5 (dark grey boxes): decision tree to help conservation managers determine the kind of ex situ management required, and consider biological risks associated with ex situ management. Dashed arrows indicate the consensus of the stakeholder meeting for the Bengal floricon Houbaropsis bengalensis blandini.
Table 1 Step 3: evaluation of biological and practical considerations associated with ex situ conservation of the Bengal florican Houbaropsis bengalensis blandini.

1ACCB, Angkor Centre for Conservation of Biodiversity; WCS, Wildlife Conservation Society.
Table 2 Step 4: evaluation of logistical and practical risks associated with ex situ conservation of the Bengal florican.
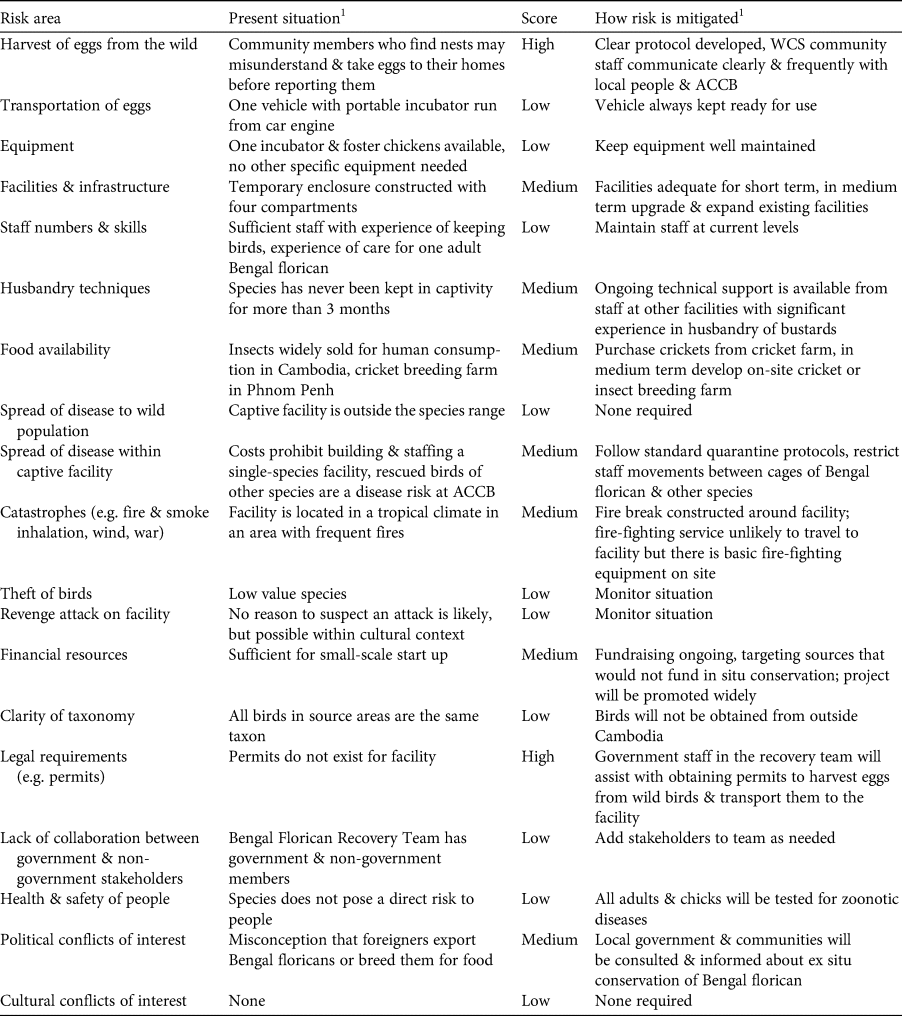
1ACCB, Angkor Centre for Conservation of Biodiversity; WCS, Wildlife Conservation Society.
Demographic modelling
To inform our evaluation of practical and biological risks to the wild and captive populations (Step 3), and evaluate whether ex situ management would increase the probability of persistence, we used a publicly available demographic model developed for evaluating the efficacy of captive breeding for the great Indian bustard Ardeotis nigriceps (Dolman et al., Reference Dolman, Collar, Scotland and Burnside2015). We retained the same parameters as Dolman et al. (Reference Dolman, Collar, Scotland and Burnside2015), who used data from a range of medium and large-sized bustard species, except where data existed for Bengal florican or the more similarly-sized little bustard Tetrax tetrax, or where variation was expected based on the smaller body size of the Bengal florican (Supplementary Tables 1 & 2). As these parameters include data from a number of bustard species that are larger and possibly slower to mature than the Bengal florican, they are likely to yield a conservative estimate of the chance of persistence (see Dolman et al., Reference Dolman, Collar, Scotland and Burnside2015, for detailed methodology; only key points are summarized here). Where we differ most from Dolman et al. (Reference Dolman, Collar, Scotland and Burnside2015) is in the nature of the situation with which we compare captive breeding. They compared the effect on the wild population of either pursuing a programme of captive breeding and subsequent release, or improving the efficacy of in situ conservation in the absence of captive breeding. In our case, with or without ex situ conservation the wild population continues to decline at an accelerated rate, even though since 2005 everything possible (within the constraints of relevant socio-economic and political factors) has been attempted to maintain it.
The model allowed us to evaluate the chance of persistence of a captive population under various strategies for collecting eggs from the wild, and the probability that individuals could be released within 50 years. We did not consider capture of adults from the wild because captive facilities that breed bustards advised against it. We did not use our model to evaluate the probability of persistence of the wild population because we had insufficient data to parameterize the model, but the current trajectory, based on data from 12 years of population monitoring, suggests that the Bengal florican will be functionally extinct by 2023 (Mahood et al., Reference Mahood, Hong, Son, Sum and Garnett2019). The captive breeding model assumes that we can collect a maximum of five or 10 eggs in the first year (the mean number of eggs found annually during 2014–2018 is 7.4; Wildlife Conservation Society, unpubl. data), but that this will decline by two eggs per year for 5 years as the wild population declines. The release model assumes that all released birds are < 1 year of age, to minimize behavioural adaptation to captivity (Inchausti & Bretagnolle, Reference Inchausti and Bretagnolle2005). Following Dolman et al. (Reference Dolman, Collar, Scotland and Burnside2015), releases do not occur until the captive population has reached 20 mature females so as not to jeopardize the persistence of the captive population (IUCN/SSC, 2013); we set the minimum group size of released birds to five because a minimum of four males plus one female is required for a Bengal florican exploded lek to function (Gray et al., Reference Gray, Collar, Davidson, Dolman, Evans and Fox2009). For captive management models, we considered four scenarios of performance quality to account for variation in demographic performance of captive management: full range, below average, above average and best possible, and accounted for the impacts of stochastic events (for parameters, see Supplementary Table 1; for methodological details, see Dolman et al., Reference Dolman, Collar, Scotland and Burnside2015). Outcomes of the captive breeding programme were assessed against the proportion of 1,000 model runs persisting by year 50, whether they provided surplus individuals for release (without compromising the maintenance of a captive population of > 20 mature females), and numbers of breeding age birds established in the wild, following Dolman et al. (Reference Dolman, Collar, Scotland and Burnside2015).
Assessment process
At a meeting held in April 2019 in Phnom Penh, Cambodia, all relevant stakeholders took part in the application of the IUCN guidelines for assessment of the potential role of ex situ management for the Bengal florican (all participants are co-authors here), including representatives from the relevant government ministries (Ministry of Environment and the Forestry Administration of Ministry of Agriculture, Forestry and Fisheries), the only NGO working on in situ conservation of the Bengal florican (Wildlife Conservation Society), and the captive facility that had expressed interest in ex situ management of the species (Angkor Centre for the Conservation of Biodiversity). The scope of the meeting and agenda were agreed in advance. Presentations were given to summarize the results of the status review (Step 1) and the demographic modelling described here. Participants worked through the first part of the decision tree to identify the role that ex situ management could play in conservation of the species (Step 2). Presentations were given on case studies of ex situ conservation successes and failures, facilities at Angkor Centre for the Conservation of Biodiversity, and ex situ conservation of bustards worldwide. Tables of practical considerations (Step 3: Table 1) and practical and logistical risks (Step 4: Table 2) were populated in advance with issues for consideration, using IUCN/SSC (2014). Participants were also invited to identify additional issues, which were then added to the tables. The whole group assessed the biological risks associated with ex situ management, using the decision tree (Steps 4 and 5) for the population as a whole, and for each of the four remaining subpopulations. Results were summarized by the facilitator. A basic plan for ex situ management was developed based on the outcome and outputs of the meeting (Step 5: Table 3).
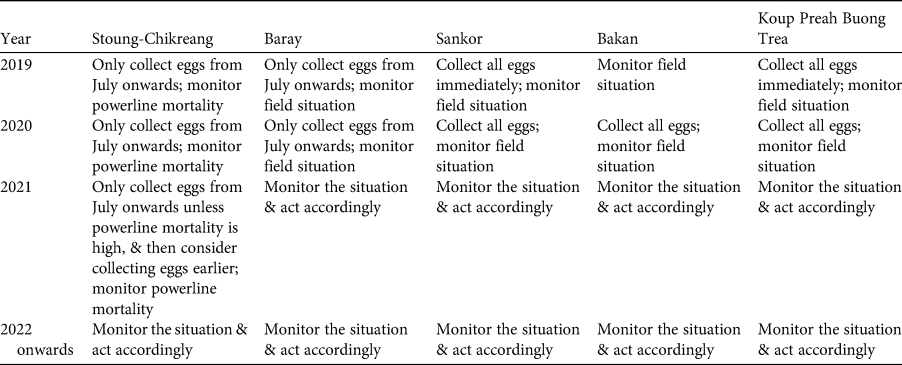
Table 3 Step 5: plan for harvest of eggs of the Bengal florican.
Results
Using the decision tree, the group decided to proceed with ex situ management of the Bengal florican, despite the risks, because of rapid, and potentially accelerating, declines in the wild population, the likelihood that threats could not be controlled in the wild before the taxon was rendered extinct, and the relatively high probability of establishing a captive population (Fig. 1). The demographic modelling indicated there is a 58% probability of a captive population persisting for 50 years under the above-average scenario of egg harvest rates of five per year for 5 years; this rises to 85% if harvest rates are 10 per year (Fig. 2). For the best possible scenario, probability of captive programme persistence is 89% even if egg harvest rates are only five per year for 5 years. The group considered that it was likely that harvest rates would be 5–10 eggs per year, but given the rate of decline of the wild population it was unlikely that harvest could continue beyond 5 years.

Fig. 2 Bengal florican captive demography for three scenarios of programme quality (1, full range; 2, above average; 3, best possible) and two rates of egg harvest (5 or 10 eggs/year, both for 5 years), without (top and middle rows) and with (bottom row) removal of birds from the captive population for reintroduction, with probability of extinction of the ex situ programme (PEP) after 50 years. The black line shows the geometric mean of model runs.
Under the below-average scenario, the captive population is never successfully established, and under the full-range scenario there is only a 19% chance of persistence of a captive population within 50 years (at egg harvest rates of five per year for 5 years). In this context, identification and mitigation of practical risks associated with ex situ conservation are critical to ensure that parameters match those in the above-average or best possible scenarios. Of the two risks that we rated high (Table 2), refusal of governmental permission to collect eggs was managed by beginning to request permits before the assessment, as a precautionary measure, given the time constraints, and to allow time to resubmit or modify the application if needed. Although at the time of writing, permits have been granted, this risk was rated high during the assessment because permission had not then been granted and ex situ conservation cannot begin without it (permission to harvest and transport eggs has since been granted). We rated risks associated with harvest of eggs from the wild as high because nests will have to be found by community members, and it is impossible to eliminate the possibility they will misunderstand instructions to leave eggs in the nest until ACCB staff arrive, and instead may take eggs to their homes, in an attempt to assist the project but inadvertently causing them to spoil. We also considered rating knowledge of husbandry techniques relevant to the species as high because the Bengal florican has rarely been kept in captivity, and captive breeding has never before been attempted. Good husbandry should aim to minimize adult mortality, because sensitivity analysis showed that probability of extirpation of the captive population was most heavily affected by changes in adult mortality (Supplementary Fig. 1). In our risk assessment we noted there is considerable global expertise in ex situ management and breeding of other bustard species on which we can draw; for instance the Asian houbara Chlamydotis macqueenii is bred extensively by the International Fund for Houbara Conservation, and the little bustard and great bustard Otis tarda are bred at Centro de Cría de Aves Esteparias in Spain. We have already begun receiving technical advice from the latter, which a staff member of the Angkor Centre for the Conservation of Biodiversity visited in early 2019 and again in early 2020 to learn about captive bustard husbandry. We plan to continue to expand our advisory network and formalize it through creating an advisory panel of relevant experts.
Demographic modelling indicates there is little chance that ex situ management will produce a sufficient number of Bengal floricans to reintroduce birds within 50 years (Fig. 3). Even under the best possible scenario with egg harvest rates of 10 per year for 5 years, there are unlikely to be sufficient birds to release for 20–30 years, by which time the species will almost certainly be extinct in the wild. Participants considered this information as they worked through the decision tree to assess biological risks associated with ex situ management (Fig. 1), but concluded that, given the relatively low risk of extirpation of a well-managed captive population and the very high risk of extirpation of the wild population, embarking on a programme of ex situ management was prudent (Fig. 3).

Fig. 3 Numbers of free-living adult female Bengal florican established by captive breeding and release (dark lines) or by a strategy of in situ conservation only (pale grey lines) alive in each programme year (1–50), under two scenarios of in situ conservation: current situation (solid lines), likely future situation (dashed lines). Per cent of model runs under which no birds were able to be released (failed to release) and probability of extinction of the ex situ programme (PEP) after 50 years are indicated.
Based on the assessment of risks described above, the participants re-examined Tables 1 & 2, and used them to develop a plan for ex situ management of the Bengal florican (Table 3), which is summarized here, with notes on what eventuated. It was hoped that as many eggs as possible would be harvested from the unprotected population at Sankor in the 2019 breeding season (April–September), but none were found. This population is declining rapidly and is likely to be extirpated within a year because the habitat has been transformed from grassland to rice (Table 3). At all sites, any eggs laid during July or later will be harvested, because these probably represent late or second breeding attempts that are likely to fail because of heavy rain and rising floodwater at this time. Mortality caused by the power transmission line (completed in May 2019) that bisects the stable, protected, population at Stoung-Chikreang Bengal Florican Conservation Area was monitored throughout the 2019 and 2020 breeding seasons. Based on relatively low levels of mortality it was decided to continue restricting egg harvest to the period after July in 2019. No eggs were found in 2020, although search effort was low because of COVID-19 related restrictions on fieldwork. It was decided not to take any adults from the wild because of the high risk of mortality, which was thought likely to lead to the entire ex situ conservation programme being closed by the government. Improvements to the in situ conservation programme at the protected population in Baray Bengal Florican Conservation Area were made in early 2019; this population will be monitored intensively, and if signs of decline are detected then a decision will be made on whether to take eggs. The tiny population at Bakan will be left in the wild because efforts to harvest eggs are best directed towards locations with more individuals and there is still potential for this population to persist because funding has been secured to confer legal protection to this site. In 2019, eggs were found only at Stoung-Chikreang Bengal Florican Conservation Area, where nine eggs were collected during July–September. Five eggs hatched but two chicks subsequently died of unknown causes. Five adult Bengal floricans (three males and two females) were confiscated from hunters in 2019 (possibly owing to increased awareness) and brought to the Angkor Centre for Conservation of Biodiversity, all survived to mid 2020 (at least) except for one female that had been missing a leg. Weekly carcass surveys recorded two Bengal florican mortalities (one male and one female) during May 2019–June 2020, so the 2019 egg collection plan was resumed in Stoung-Chikreang Bengal Florican Conservation Area in 2020. Samples will be taken from all captive individuals for molecular genotyping to determine relatedness so that we can design a captive breeding programme that maximizes fitness of offspring (Hogg et al., Reference Hogg, Wright, Morris, Lee, Ivy, Grueber and Belov2018).
Discussion
Conservation managers are increasingly forced into a situation in which they are ‘held in the pressured space between extinction (as a limit on numbers and time) and the fragile wild (as a limit on intervention). Fail to intervene, and the object is lost; intervene, and the object may also be lost, although in other ways’ (Reinert, Reference Reinert2013, p. 22). For many species, given the numbers of individuals available to be taken into captivity, and differences in selective pressure between captive and wild birds, it is inevitable that captive populations will differ from those in the wild (Frankham, Reference Frankham2008; Robert, Reference Robert2009) even with careful genetic management of the captive flock (Williams & Hoffman, Reference Williams and Hoffman2009; Witzenberger & Hochkirch, Reference Witzenberger and Hochkirch2011). Such changes in birds include reduced brain volume of captive-bred waterfowl compared with wild birds (Guay & Iwaniuk, Reference Guay and Iwaniuk2008), reduced vigilance (Carrete & Tella, Reference Carrete and Tella2015) and inappropriate behavioural responses to predators (Griffin et al., Reference Griffin, Blumstein and Evans2000).
The first questions about captive breeding are therefore philosophical: given the genetic, morphological and behavioural changes induced by captivity, conservation managers and those who support them must be satisfied that the birds that may eventually be reintroduced to the wild are approximate surrogates of the former wild populations, especially if they cannot be returned to their native range. These concepts are rarely considered explicitly in advance of ex situ conservation, but deeply-held opinions on what it means for an animal to be wild may be revealed at a stage when they can derail the process of ex situ conservation. For example, effective conservation of the California condor was delayed for several years because the prevailing ideology favoured a hands-off approach, until a change in management brought all remaining individuals into captivity and eventually reversed population declines through releases of captive-bred birds (Snyder & Snyder, Reference Snyder and Snyder2000). In another example, those operating captive management of the alalã (Hawaiian crow) Corvus hawaiiensis, which is extinct in the wild, have decided to teach the crows to behave in a similar way to the original forest dwelling alalã (although their habitat is different since the arrival of the feral pigs that caused them to go extinct), rather than training them to become a human commensal, as many wild populations of other crow species have done of their own volition (van Dooren, Reference van Dooren2016).
A different approach has been taken with captive-bred Asian houbaras, which have lower fecundity, a docile temperament and differences in migration behaviour compared to wild-bred birds (Villers et al., Reference Villers, Millon, Jiguet, Lett, Attié, Morales and Bretagnolle2010; Dolman et al., Reference Dolman, Collar and Burnside2018). These changes lead to lower mortality in captivity but cause higher mortality among released captive-bred individuals compared to wild birds, as is also the case with captive-reared great bustards (Burnside et al., Reference Burnside, Carter, Dawes, Waters, Lock, Goriup and Székely2012; Dolman et al., Reference Dolman, Collar and Burnside2018). Perhaps unsurprisingly, like most reintroduction attempts (Fischer & Lindenmayer, Reference Fischer and Lindenmayer2000; Bowkett, Reference Bowkett2009), no reintroduction of bustards has been completely successful (Dolman et al., Reference Dolman, Collar, Scotland and Burnside2015; Ashbrook et al., Reference Ashbrook, Taylor, Jane, Carter and Székely2016). There is a significant chance that any reintroduction of the Bengal florican will also fail to establish a self-sustaining wild population, even if the species persists in captivity. A major impediment may be lack of habitat. If the wild population is extirpated, protection of the remaining fragments of grassland on the Tonle Sap floodplain may become harder, so habitat of the kind that is currently associated with the species may not be available for reintroduction. Although this situation is relatively common for amphibians and reptiles, which are frequently maintained in captivity until suitable conditions exist in the wild for their release (Turtle Conservation Fund, 2002; Krajick, Reference Krajick2006; Zippel et al., Reference Zippel, Johnson, Gagliardo, Gibson, McFadden and Browne2011), it is relatively rare in birds (BirdLife International, 2018a). In general, birds are harder to maintain in captivity than herptiles, but easier than mammals. The Guam kingfisher Todiramphus cinnamominus is so far the only bird species Extinct in the Wild never likely to be returned to its native range, because the snakes that drove it extinct cannot be eradicated, although it may be introduced to a nearby island (Laws & Kesler, Reference Laws and Kesler2012). The little spotted kiwi Apteryx owenii has recently been reintroduced to predator-free sanctuaries on the New Zealand mainland; prior to this it persisted for decades only in captivity and on tiny offshore islands where it had never occurred naturally (Holzapfel et al., Reference Holzapfel, Robertson, McLennan, Sporle, Hackwell and Impey2008).
For these reasons, and because ex situ management is costly and risky, it can only be justified if less intrusive alternatives are unlikely to secure species persistence (Snyder et al., Reference Snyder, Derrickson, Beissinger, Wiley, Smith, Toone and Miller1996). By using the IUCN guidelines to assess the potential role of ex situ conservation in preventing the extinction of the Bengal florican, we were able to make a decision that comprehensively considered all of the risks, and we concluded that ex situ conservation should be attempted immediately. A similar process was used to evaluate the potential role of ex situ conservation for the South Australian subspecies of glossy black-cockatoo Calyptorhynchus lathami halmaturinus, which concluded that although technically feasible, captive management would be costly and the population would probably recover without it (Crowley et al., Reference Crowley, Pedler and Garnett1999). This proved correct: the population recovered from 195 individuals in 1995 to c. 356 individuals in 2014 without captive management (Morgan et al., Reference Morgan, Barth and Kinloch2015). In contrast, based on information available to us at the time, we concluded that we should proceed with captive management of the Bengal florican and begin egg harvest in 2019 because the wild population is likely to decline at an accelerated rate owing to new threats that are affecting the only stable population, and because the chance of establishing a captive population is relatively high if we draw on global bustard husbandry expertise to minimize adult mortality in captivity. We did not consider how best to manage the captive population to maximize genetic variation; this could have been integrated into the assessment process, but instead the breeding programme will be planned using molecular data obtained from any chicks that hatch from harvested eggs (Hogg et al., Reference Hogg, Wright, Morris, Lee, Ivy, Grueber and Belov2018).
At the time of writing we received additional information on breeding parameters of captive little bustards from Centro de Cría de Aves Esteparias. Using these data we repeated the population modelling to inform our ex situ conservation programme. Demographic modelling indicated that the chance of persistence of a captive population was better than we had predicted at the time of the workshop under all scenarios except below-average. Our estimate of probability of persistence of a captive population for 50 years under an above-average scenario increased from 60 to 79% at egg harvest rates of five per year for 5 years, and from 83 to 97% if harvest rates are 10 per year. The probability of producing sufficient individuals for release was > 75% for above-average and best possible scenarios under egg harvest rates of at least five per year for 5 years, indicating that there was a much greater chance of releasing captive-bred Bengal floricans within 50 years than we had anticipated, but failure is still possible.
Although we used the same demographic model as Dolman et al. (Reference Dolman, Collar, Scotland and Burnside2015), and our model outputs are unsurprisingly similar, the conclusions that we reached are different. There are three reasons for this: (1) differences in data used to parameterize the model, (2) differences in characteristics of the counterfactual no ex situ scenario, and (3) local stakeholders led the assessment process. To parametrize the model we used data from the Bengal florican and little bustard, where it was available, in addition to the data from larger bustard species used by Dolman et al. (Reference Dolman, Collar, Scotland and Burnside2015). However, this had relatively little impact on model outputs; for instance, with egg harvest rates of five per year for 5 years, Dolman et al. (Reference Dolman, Collar, Scotland and Burnside2015) report probability of extinction over 50 years for the great Indian bustard under a best possible scenario of 17%, compared with 11% for Bengal florican (although we report this as an 89% probability of persistence); we interpret this as an indication that if ex situ management is done well it is likely to prevent the extinction of the species. These results are compared with a counterfactual scenario for the wild population in which no egg harvest takes place. We believe that we formulated a plausible future scenario for the wild population of the Bengal florican, given trends, threats and resources available for additional in situ conservation (Mahood et al., Reference Mahood, Hong, Son, Sum and Garnett2019). For instance, although we believe that we know how to manage areas under rice cultivation (such as Sankor) for the Bengal florican, we do not have the resources to do this at the scale that is necessary within the time available, and success is at least partially dependent on factors outside our control, such as the attitudes of farmers, market forces and government policy. In contrast, the counterfactual future scenario used by Dolman et al. (Reference Dolman, Collar, Scotland and Burnside2015) imagined a situation where in situ conservation was considerably more successful than has eventuated, although what was envisaged may have been possible at the time.
We aimed to use the IUCN guidelines (including model outputs and a decision tree) to support local stakeholders to make an informed, unbiased decision about ex situ management of the Bengal florican, and having made that decision, to identify risks that needed to be mitigated to maximize the chance of success. We do not want the Bengal florican to join the list of taxa that have been lost for want of a decision to evaluate ex situ conservation or initiate it in time (Woinarski et al., Reference Woinarski, Garnett, Legge and Lindenmayer2016). Demographic modelling indicates there is a reasonable chance that a captive population can be established and that in 20–30 years it should be large enough to consider reintroduction if habitat is available and threats have been mitigated. We acknowledge that such a population is likely to be small and based on a limited number of founders, so failure is possible at every step.
Our decision to attempt ex situ management of the Bengal florican was made based on a thorough evaluation of risks and resources, informed by demographic modelling. We considered both philosophical and practical issues using a decision tree. The process that we used is transparent and we hope that our decision tree will be helpful in other, similar situations. We will continue to do everything possible to prevent the loss of remaining wild populations of the Bengal florican, but we acknowledge that, if not managed properly, taking eggs and adults from the wild may accelerate declines. With hindsight, we should have considered ex situ management in 2012, when there were a number of small unprotected populations that had little chance of survival owing to financial and practical constraints on in situ conservation. Our results indicate that, with support for bustard husbandry techniques, there remains a reasonable chance of establishing a captive population. However, we anticipate that the Bengal florican may persist only in captivity for many years, and it may never be released in a situation that resembles its current wild state. We consider this is preferable to complete loss of the taxon, and captive or semi-wild individuals may serve an educational purpose. We urge conservation managers faced with rapidly declining species to evaluate comprehensively the potential role of ex situ management, rule it out whenever there is the possibility of successful conservation of wild populations, or pursue it rapidly where not.
Acknowledgements
We acknowledge the ongoing support of the Royal Government of Cambodia, in particular the General Department for Administration of Nature Conservation and Protection of the Ministry of Environment, and the Department of Wildlife and Biodiversity of the Forestry Administration, Ministry of Agriculture, Forestry and Fisheries. We thank Dolman et al. (Reference Dolman, Collar, Scotland and Burnside2015) for making the R code for their demographic model publicly available, and we encourage others to use it alongside the IUCN captive management guidelines (IUCN/SSC, 2014). We are grateful to two anonymous reviewers, whose comments improved this article significantly. SPM is a recipient of a Prestigious International Research Tuition Scholarship at Charles Darwin University. This research received no specific grant from any funding agency, or commercial or not-for-profit sectors.
Author contributions
Study design, modelling, writing: SPM; revision, editing: CH, MM, PF, PS, VS, STG.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards.







