Introduction
Although invertebrates comprise 80% of known species, the conservation status of < 1% is known (Collen et al., Reference Collen, Böhm, Kemp and Baillie2012). Amongst invertebrates the freshwater gastropods are most at risk: at least 51% of assessed species are considered threatened, with the potential threat level ranging from 33% (assuming no Data Deficient species are threatened) to 68% (assuming all Data Deficient species are threatened; Collen et al., Reference Collen, Böhm, Kemp and Baillie2012). Hotspots of freshwater gastropod diversity include the ancient lakes of Sulawesi, Indonesia and the Mekong basin (Strong et al., Reference Strong, Gargominy, Ponder and Bouchet2008). Despite the high diversity of freshwater molluscs in South-east Asia, research has mostly focussed on the zoonotic parasites they host, with little other emphasis beyond inventories of the molluscan hosts (e.g. Palmieri et al., Reference Palmieri, Sullivan and Ow-Yang1977; Burch & Lohachit, Reference Burch and Lohachit1983; Attwood, Reference Attwood2010).
Among the known parasite hosts are Ampullariidae, the largest freshwater snails in South-east Asia (Komalamisra et al., Reference Komalamisra, Nuamtanong and Dekumyoy2009; Hayes et al., Reference Hayes, Burks, Castro-Vazquez, Darby, Heras and Martín2015). Up to 12 ampullariid species of the genus Pila, and multiple synonyms, have been recorded from the region (Cowie, Reference Cowie2015). The confused taxonomy has complicated the understanding of species distributions, and other than a few important studies in the 1980s and 1990s (Keawjam, Reference Keawjam1986, Reference Keawjam1987a,Reference Keawjamb; Pagulayan & Remigio, Reference Pagulayan and Remigio1993), the current status of Pila in South-east Asia remains largely unknown. In contrast, the introduced South American confamilials, Pomacea spp., have been well studied because of their impacts in paddy fields (Isnaningsih & Marwoto, Reference Isnaningsih and Marwoto2011; Nghiem et al., Reference Nghiem, Soliman, Yeo, Tan, Evans and Mumford2013; Joshi et al., Reference Joshi, Cowie and Sebastian2017). There have been numerous anecdotal accounts of the decline of Pila species since the introduction of Pomacea spp. (e.g. Halwart, Reference Halwart1994; Marwoto et al., Reference Marwoto, Isnaningsih, Mujiono, Heryanto, Alfiah and Riena2011), but the neglect of Pila taxonomy has made it difficult to ascertain the status of the species. Although population trends of South-east Asian Pila species are unknown, seven species have been assessed as Least Concern (IUCN, 2017).
One such species is Pila scutata (Sri-Aroon & Richter, Reference Sri-Aroon and Richter2012), with which various synonyms have been associated (Low et al., Reference Low, Tan and Ng2013; Ng et al., Reference Ng, Tan and Low2014; Cowie, Reference Cowie2015). It is reported to be widely distributed across South-east Asia (Low et al., Reference Low, Tan and Ng2013), but some occurrences are unconfirmed and others are probably misidentifications. For example, Keawjam (Reference Keawjam1986) concluded from extensive surveys of Thailand ampullariids that previous records (Brandt, Reference Brandt1974) of P. scutata were Pila gracilis that lacked bands. Hayes et al. (Reference Hayes, Cowie and Thiengo2009b) reported on a previous misidentification of P. scutata (as Pila conica) from Viet Nam. Further south, the distribution of P. scutata in Peninsular Malaysia has not been mapped, but it has been reported to be common across the peninsula (van Benthem Jutting, Reference van Benthem Jutting1931; Berry, Reference Berry1974). In Singapore, P. scutata was formerly widespread and common in ponds and ditches (e.g. Ponniah, Reference Ponniah1962; Johnson, Reference Johnson and Chuang1973; H.E. Ng, pers. comm.), but by the early 2000s the species remained extant in only a small number of localities (Clements et al., Reference Clements, Koh, Lee, Meier and Li2006). It was also reportedly declining in Peninsular Malaysian rice fields (Joshi et al., Reference Joshi, Cowie and Sebastian2017).
Early records of P. scutata were from streams and rivers (e.g. de Morgan, Reference de Morgan1885, as Ampullaria wellesleyensis), but later surveys failed to find it in natural, undisturbed habitats (Tan et al., Reference Tan, Lee and Ng2013; D.S. Johnson, unpubl. manuscript, based on work in the 1960s and early 1970s). Records from the 1960s indicated that P. scutata was associated with anthropogenic habitats, such as fishponds, throughout the Malay peninsula (Johnson, Reference Johnson and Chuang1973). Up to the mid 20th century the species was a common food item for local communities, and for livestock feed (Ponniah, Reference Ponniah1962; Lim et al., Reference Lim, Yap, Krishnansamy, Ramachandran and Mansor1978). Its popularity as a protein source suggests that it was either naturally widespread (and abundant) or widely translocated by people, or both. This species has also been introduced to Pacific islands (Cowie, Reference Cowie1997, Reference Cowie and Barker2002; Smith, Reference Smith2003) purportedly as food, via the Philippines (Cowie, Reference Cowie1998; Tran et al., Reference Tran, Hayes and Cowie2008). The introduced population in Hawaii has very low mitochondrial DNA diversity (Tran et al., Reference Tran, Hayes and Cowie2008), as has been recorded for many non-native taxa that experience a genetic bottleneck during the introduction stage (Dlugosch & Parker, Reference Dlugosch and Parker2008).
Despite being considered indigenous to Singapore (Ng, Reference Ng1991; Chan, Reference Chan1996; Tan et al., Reference Tan, Clements and Chan2012), considering the near-exclusive occurrence of P. scutata in man-made or disturbed habitats, it is possible that its current distribution reflects past anthropogenic translocations. Such history would complicate efforts to resolve the taxonomy and biogeography of Pila in South-east Asia. With the apparent decline of Pila populations throughout the region, it is imperative that any anthropogenic influence be uncovered and considered when examining their biogeography. Thus, we aimed to document the current distribution of P. scutata in the Malay peninsula (specifically Peninsular Malaysia and Singapore) and to provide an assessment of the status of this cryptogenic species (assumed native, but possibly introduced) in Singapore.
Methods
Surveys and data collection
Records of P. scutata and other congeners from Singapore and Peninsular Malaysia (collectively referred to here as the Malay peninsula) were obtained from available literature (Supplementary Table 1). Specimens in the Zoological Reference Collection (ZRC) of the Lee Kong Chian Natural History Museum at the National University of Singapore were examined. Records and photographs of type material of P. scutata and its synonyms (following Cowie, Reference Cowie2015), and of other Pila species, were also obtained from the following collections: the Australian Museum, Sydney (AMS), the Academy of Natural Sciences, Philadelphia (ANSP), the Field Museum, Chicago (FMNH), the Forest Research Institute Malaysia, Kuala Lumpur, Malaysia (FRIM), the Natural History Museum, London (NHMUK), the Zoological Survey of India, National Zoological Collection India, Kolkata (NZSI), the Forschungsinstitut und Naturmuseum Senckenberg, Frankfurt (SMF), the University of Michigan Museum of Zoology, Ann Arbor (UMMZ), and the Zoologisches Museum Hamburg (ZMH).
Based on records from these sources, we surveyed known ampullariid localities in the Malay peninsula from August 2013 to March 2015. Opportunistic sampling was also carried out at other potentially suitable habitats, including water bodies around limestone outcrops, ditches, rice fields and ponds. Samples from one additional population in Singapore were obtained from Daniel J.J. Ng. Comparative material from Java in Indonesia was obtained from the collection of the Zoologisches Museum Berlin, Germany (the type locality of P. scutata is Pardana in Java; Mousson, 1848), and other material was collected from the Philippines and Viet Nam (regions where P. scutata has previously been recorded; Pagulayan & Remigio, Reference Pagulayan and Remigio1993; Jørgensen et al., Reference Jørgensen, Kristensen and Madsen2008).
DNA analysis
We extracted total genomic DNA from the foot tissue of at least 20 individuals of each P. scutata population, and at least one individual of other Pila species obtained. We added 900 µl CTAB buffer (0.1 M Tris pH 8; 1.4 M NaCl; 0.02 M EDTA; 20 g/l CTAB) and 20 µl Proteinase K (Invitrogen) to tubes containing the tissue samples. The samples were then incubated for 3 h at 55 °C. We extracted the DNA using 25 : 24 : 1 phenol : chloroform : isoamyl alcohol (Biozol), and precipitated with 100% ethanol. After washing the DNA pellets in 70% ethanol, we dissolved the pellets in 50 µL of water. Mitochondrial COI and 16S rRNA genes were amplified in polymerase chain reactions (PCR) with a total volume of 23–24 µl PCR reaction mixture (2.5 µl of BioReady rTaq 10 × buffer, 2 mM dNTPs, 1 µl each of 10 µM primers, 0.25 µl of BioReady rTaq DNA Polymerase (Bulldog Bio), and DNase-free sterile water), at 95 °C for 5 min, 34 cycles of 95 °C for 30 s, 45–48 °C for 30 s, and 72 °C for 30 s, and a final extension of 72 °C for 10 min. Amplicons of each gene were obtained using different pairs of primers depending on the quality of tissue obtained (Supplementary Table 2). The primer pairs LCO1490/HCO2198 and dg_LCO1490/dg_HCO2198 amplified approximately 600 base pairs (bp) in the COI barcode region, and mlCOintF/jgHCO2198 amplified a shorter fragment (313 bp) in the 3′ region of the COI. The latter pair was used in specimens that failed to amplify the standard barcode region. The size of 16S fragments amplified ranged from 320 to 476 bp. The PCR products were checked visually on a 1% agarose gel. Post PCR clean-ups were performed on successfully amplified products using SureClean reagent (Bioline Inc., London, UK) following the manufacturer's recommendations. The purified products were sequenced with BigDye Terminator reactions and analysed on the ABI PRISM 3130XL sequencer (Applied Biosystems, Foster City, USA) at the DNA Sequencing Laboratory of the National University of Singapore.
We visually inspected and trimmed sequences using Sequencher 4.6 (Genecodes, Ann Arbor, USA). The COI and 16S genes were aligned using MAFFT v. 7 (Katoh & Standley, Reference Katoh and Standley2013) with default settings. Aligned COI sequences were checked for translatability into amino acids and were gap free. DNA sequences were inspected using objective clustering in SpeciesIdentifier v. 1.7.9 (Meier et al., Reference Meier, Kwong, Vaidya and Ng2006). Objective clustering was performed using the SpeciesIdentifier (TaxonDNA 1.6.2; Meier et al., Reference Meier, Kwong, Vaidya and Ng2006) to determine molecular operational taxonomic units (mOTUs) at a range of thresholds (0–6%), to test stability of the results. In objective clustering sequences are grouped according to uncorrected pairwise distances (Meier et al., Reference Meier, Kwong, Vaidya and Ng2006, Reference Meier, Zhang and Ali2008; Srivathsan & Meier, Reference Srivathsan and Meier2012); i.e. members of a set of putative conspecific sequences have at least one match to a sequence in the set that falls within a given threshold distance. Previous studies have shown the intraspecific genetic distance for the majority of gastropods is < 2% for COI (Meier et al., Reference Meier, Zhang and Ali2008; Layton et al., Reference Layton, Martel and Hebert2014). Sequences were checked against the GenBank database (Benson et al., Reference Benson, Cavanaugh, Clark, Karsch-Mizrachi, Lipman, Ostell and Sayers2013) under default parameters in MegaBLAST (OMICtools, Le-Petit-Quevilly, France). Further analysis was not carried out because of the low variation within and among the populations (see Results). All sequences were deposited in GenBank (KY081728–KY081759, KY087525) and BOLD (SEAAM001–023).
Results
Historical distribution of P. scutata in the Malay peninsula
Two Pila species are known from Singapore, P. scutata and Pila ampullacea, with the latter species recorded as a single population that is now considered extirpated (Ng et al., Reference Ng, Tan and Low2014). Pila scutata, however, was widely distributed prior to the 1990s, and found in fishponds and reservoirs throughout Singapore (Fig. 1; Supplementary Table 1). In Peninsular Malaysia P. scutata was similarly recorded from human-disturbed habitats such as artificial ponds and paddy fields (Fig. 1; Supplementary Table 1). These records of P. scutata from the peninsula were from the north, close to the border with Thailand (e.g. Kangar, Perlis, ANSP388826: Sow-Yan Chan, pers. comm.; Machang, Kelantan: Lim et al., Reference Lim, Yap, Krishnansamy, Ramachandran and Mansor1978; Boo Liat Lim, pers. comm.), and along the western coast of the peninsula. The southernmost record for the peninsula was from a stream in central Johor (ZRC.MOL.6955), c. 80 km from Singapore.
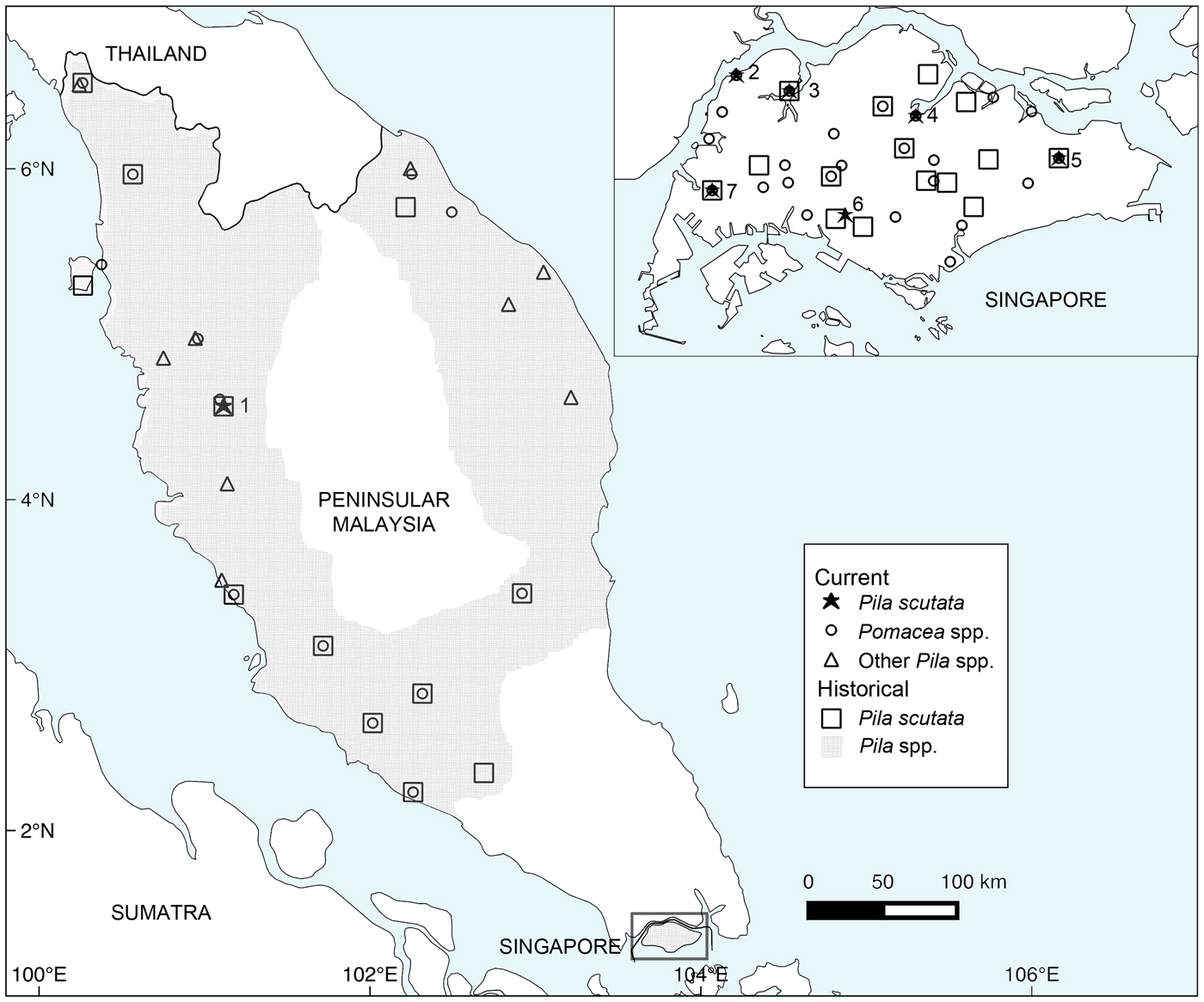
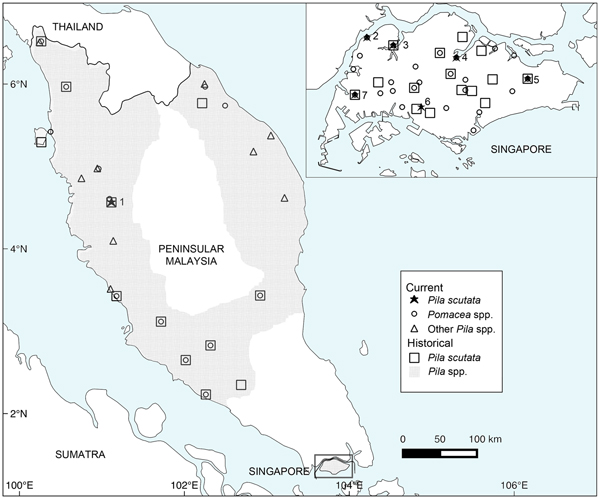
Fig. 1. Historical and current distribution of Pila scutata and other ampullariids in the Malay Peninsula (Peninsular Malaysia and Singapore). Sites with extant P. scutata populations: 1, Ipoh; 2, Sarimbun; 3, Kranji; 4, Seletar; 5, Tampines; 6, Pandan; 7, Jurong. Records within a 10 km radius in Peninsular Malaysia, and within a 1 km radius in Singapore were treated as single localities.
Current distribution of P. scutata in the Malay peninsula
We collected P. scutata from six localities in Singapore: Kranji, Tampines, Sarimbun, Seletar, Pandan and Jurong (Fig. 1, Plate 1). Pomacea spp. were syntopic with P. scutata at four of these sites (Kranji, Tampines, Sarimbun, Seletar). Only one population of P. scutata was found in Peninsular Malaysia, in Ipoh (Fig. 1, Plate 1). In contrast, Pomacea spp. were found at multiple sites where Pila spp. were previously recorded, although not at the Ipoh site where P. scutata was found (Fig. 1).

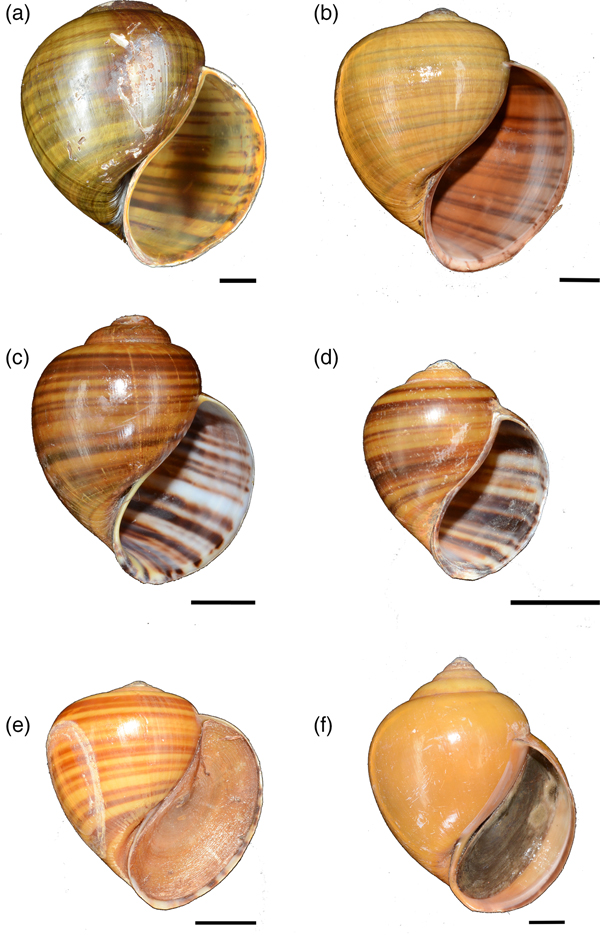
Plate 1 Pila scutata from South-east Asia: (a) Bogor, Java, Indonesia (ZMB106543), (b) Neuva Ecija, Philippines (ZRC.MOL.7018), (c) Ipoh, Malaysia (ZRC.MOL.6961), (d) Pandan, Singapore (ZRC.MOL.7062). Scale bars = 10 mm. Photographs by T.H. Ng.
Identification of P. scutata and congeners from South-east Asia
Based on examination of Pila spp. collected for this study (with the exception of Pila from Java that was loaned from ZMB, and collected in 2015) from the known range of P. scutata, we identified P. scutata from Java, Indonesia (ZMB106543), and from Neuva Ecija, Philippines (ZRC.MOL.7018; Plate 1). From the states of Kelantan, Terengganu, Perak, Selangor and Perlis in Peninsular Malaysia three other congeners were identified (Plate 2): P. ampullacea, Pila angelica and P. gracilis. Ampullariids collected from Viet Nam were identified as Pila erythrochila, P. gracilis, and Pila virescens (Plate 2).
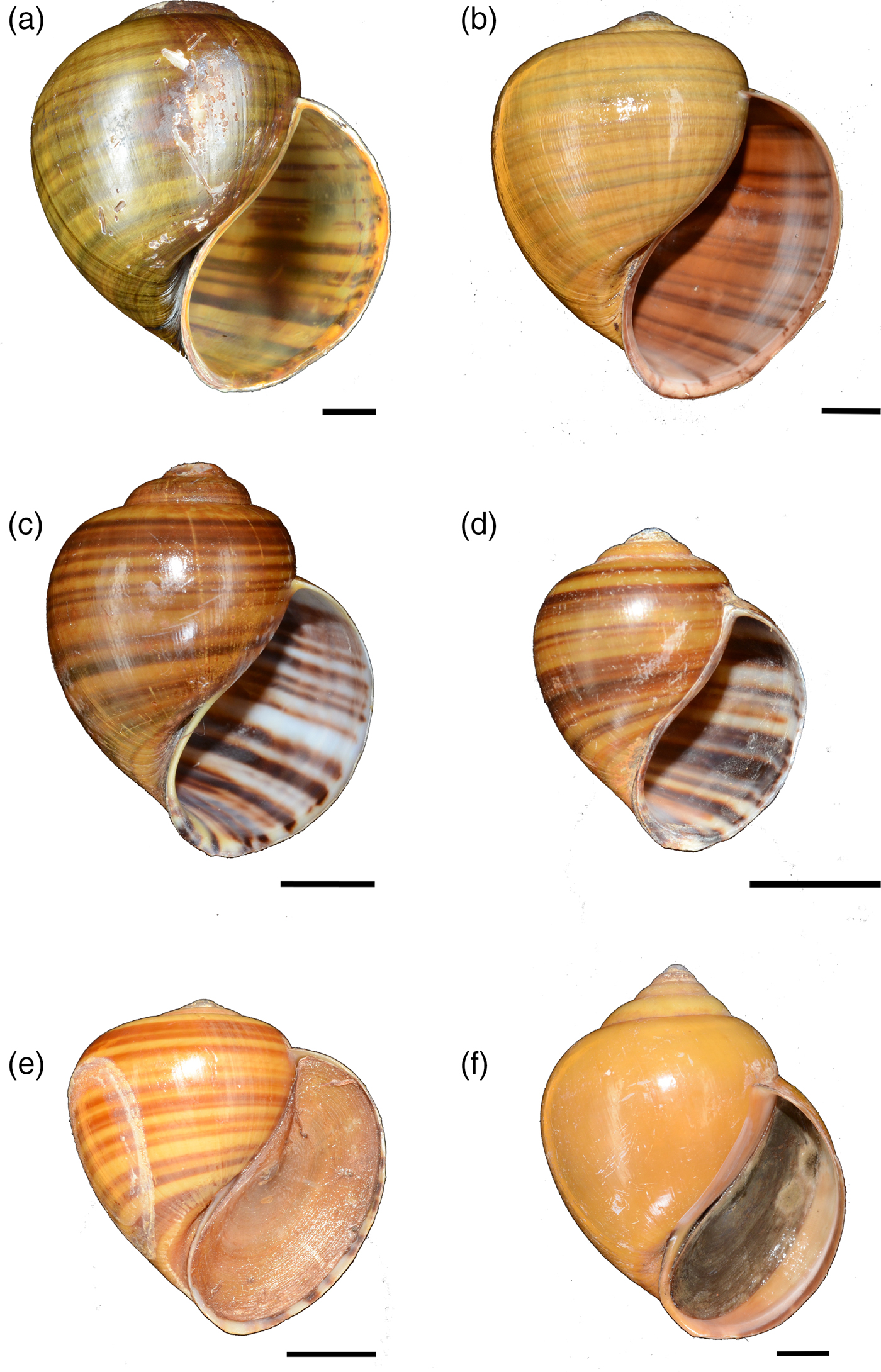
Plate 2 Other ampullariids recorded from Peninsular Malaysia and Viet Nam: (a) Pila ampullacea, Malaysia (ZRC.MOL.7001), (b) Pila angelica, Malaysia (ZRC.MOL.6971), (c) Pila gracilis, Malaysia (ZRC.MOL.6992), (d) P. gracilis, Viet Nam (ZRC.MOL.7006), (e) Pila erythrochila, Viet Nam (ZRC.MOL.7016), (f) Pila virescens, Viet Nam (ZRC.MOL.7014). Scale bars = 10 mm. Photographs by T.H. Ng.
COI and 16S from 128 P. scutata individuals were successfully sequenced. From Singapore, these included 20 each from Kranji, Tampines, Sarimbun and Pandan, six from Seletar, and one from Jurong; from Peninsular Malaysia, 20 from Ipoh; from the Philippines, 20 from Neuva Ecija; from Indonesia, one from Java. Among the other species, both COI and 16S were sequenced from P. ampullacea and P. gracilis from Peninsular Malaysia, whereas only COI was sequenced from P. angelica, and only 16S was sequenced from P. gracilis and P. virescens from Viet Nam (all one individual each).
Sequence clustering of COI using objective clustering (Meier et al., Reference Meier, Kwong, Vaidya and Ng2006) produced five mOTUs that were mostly congruent with the morphologically-identified species over 1–5% thresholds (Table 1). Across these thresholds, the numbers of clusters remained stable, with no specimens involved in mOTU reassignment between thresholds. Over the same threshold range P. scutata sequences separated into two mOTUs: a Javan specimen on its own, and the other 127 sequences as a single mOTU (Table 1). At 6% threshold, the Javan P. scutata sequence merged with the larger P. scutata cluster. Results for 16S were similar to those for COI; the Javan specimen remained a separate mOTU up to the 4% threshold. The mOTUs for COI for other Pila spp. remained stable across 1–6% thresholds, corresponding to the morphologically-identified species.
Table 1. Number of Pila species identified based on morphology compared to number of molecular operational taxonomic units (mOTU) at objective clustering thresholds between 1 and 6% (uncorrected pairwise distances) for COI and 16S genes.

SGP, Singapore; MYS, Malaysia; PHL, Philippines; IDN, Indonesia; VNM, Viet Nam.
All specimens that were identified as P. scutata based on morphology were matched to GenBank COI sequences of P. scutata collected from Hawaii (as Pila conica in Rawlings et al., Reference Rawlings, Hayes, Cowie and Collins2007; Tran et al., Reference Tran, Hayes and Cowie2008) with 100% identity, except for the Javan P. scutata, which was a 95% match (Supplementary Table 3). Other than P. scutata, only P. gracilis (Peninsular Malaysia and Viet Nam) and P. virescens (Viet Nam) had high matches (99–100% identity) to available sequences on GenBank (Supplementary Table 3).
Mitochondrial DNA diversity of P. scutata populations
All 107 P. scutata individuals from Singapore and Peninsular Malaysia shared a single COI haplotype (0% uncorrected pairwise distance). Pairwise distances were 0.78% between the Malay peninsula population and that of the Philippines, 0–0.7% between the Philippines population and individuals from Hawaii (Rawlings et al., Reference Rawlings, Hayes, Cowie and Collins2007; Tran et al., Reference Tran, Hayes and Cowie2008), and 5.4–7.1% between the Javan individual and all other P. scutata. For 16S, P. scutata from Singapore, Peninsular Malaysia, the Philippines, and Hawaii shared a single 16S haplotype (0% uncorrected pairwise distances), whereas the pairwise distances between the Javan individual and all other P. scutata were 4.7–4.8%.
Discussion
Historical and current distribution of P. scutata in the Malay peninsula
The first record of an ampullariid in Singapore (as Ampullaria sp.) by Traill (Reference Traill1847) lacks figures or specimens, preventing identification of the species. We found that museum and literature records corroborate historical accounts of P. scutata being formerly widespread and common in Singapore (Johnson, Reference Johnson and Chuang1973) and Peninsular Malaysia (Berry, Reference Berry1974). Yet in our exhaustive surveys we discovered only six populations in Singapore, and only one population in Peninsular Malaysia. Five of these populations overlapped with previous records (Supplementary Table 2), with only Sarimbun and Pandan being newly reported localities. All seven sites were at former village areas, which included plantations and ponds (Ponniah, Reference Ponniah1962; Yeap, Reference Yeap2015; National Archives of Singapore, 2016).
The decline of P. scutata in Singapore has been attributed to habitat loss and canalization of open country streams (Ng et al., Reference Ng, Chou and Lam1993; Chan, Reference Chan1996). Many previously known P. scutata localities no longer exist (Ng et al., Reference Ng, Tan and Low2014). A similar situation was encountered in Peninsular Malaysia, where many former sites have been modified or have disappeared altogether. Lack of habitat alone, however, does not completely explain the decline of P. scutata, as this resilient species was known to be common even in degraded habitats such as stocked fishponds and septic ponds (Ponniah, Reference Ponniah1962; Johnson, Reference Johnson and Chuang1973). Furthermore, some concrete canals around Singapore with banks that had recently been naturalized or softened for aesthetic and recreational reasons (Lee et al., Reference Lee, Soh, Kalyanaraman, Yeo, Wang and Lim2010) now resemble freshwater habitats of the past that were ideal for snails (Johnson, Reference Johnson and Chuang1973), and paddy fields where the species thrived are still present in rural areas of Peninsular Malaysia.
These remaining suitable areas and newly-created habitats, however, are being rapidly colonized by South American Pomacea spp. Throughout the Malay peninsula, Pomacea spp. appear to have replaced P. scutata in areas where the latter were once common (Fig. 1). Thus, a plausible alternative hypothesis for the decline of P. scutata may be competition from the confamilial Pomacea spp. (but see Ng et al., Reference Ng, Chou and Lam1993; Chan, Reference Chan1996; Tan et al., Reference Tan, Clements and Chan2012). The decline in P. scutata in Singapore appears to have coincided with the introduction and spread of Pomacea spp. since the late 1980s (Ng et al., Reference Ng, Tan and Low2014). Pomacea spp. were first collected from a single reservoir in 1988, but are now found in most reservoirs and many canals and ponds (Ng et al., Reference Ng, Tan and Low2014). The possible displacement of native apple snail species has also been reported in other regions invaded by Pomacea spp. (e.g. Halwart, Reference Halwart1994; Thaewnon-ngiw et al., Reference Thaewnon-ngiw, Klinbunga, Phanwichien, Sangduen, Lauhachinda and Menasveta2004; Marwoto et al., Reference Marwoto, Isnaningsih, Mujiono, Heryanto, Alfiah and Riena2011). Efforts by collaborators from the Philippines and Indonesia to find comparative material of Pila for this study were largely unsuccessful as they encountered an overwhelming presence of Pomacea instead (Roberto Pagulayan & Ristiyanti M. Marwoto, pers. comms). Pomacea was also established at the same locality in the Philippines where P. scutata was obtained for this study.
It is uncertain if the extant populations of P. scutata in the Malay peninsula will survive in the long-term. Two of the localities with no Pomacea present, Pandan (Singapore) and Ipoh (Malaysia) have experienced recent habitat modification (THN, pers. obs.), and the third, in Jurong (Singapore) is connected by a canal to Tengeh Reservoir (Daniel J.J. Ng, pers. comm.), where Pomacea is established. The faster growth, higher reproductive capacity, and voracious appetite for aquatic macrophytes of Pomacea spp. (Cowie, Reference Cowie and Barker2002; Carlsson et al., Reference Carlsson, Bronmark and Hansson2004; Morrison & Hay, Reference Morrison and Hay2011; Chaichana & Sumpan, Reference Chaichana and Sumpan2014) are invasive traits that threaten the continued survival of P. scutata in the remaining four localities where it co-occurs with Pomacea (i.e. Kranji, Sarimbun, Tampines, Seletar).
Anthropogenic influences and taxonomic confusion
Another factor that may have contributed to the decline of P. scutata in the Malay peninsula is the unexpected lack of mitochondrial variation we found across all extant populations. Although the species is considered native to the Malay peninsula, the current low genetic diversity and lack of geographical structure may have stemmed from anthropogenic introduction, and a resulting founder effect. Although non-native species may overcome the limitations of genetic bottlenecks experienced during introduction (Roman & Darling, Reference Roman and Darling2007), low genetic diversity is known to affect the fitness of populations (Kinziger et al., Reference Kinziger, Nakamoto, Anderson and Harvey2011).
The low level of variation in the Malay peninsula P. scutata mitochondrial genes mirrors that of the introduced population in Hawaii (as P. conica in Tran et al., Reference Tran, Hayes and Cowie2008). Clustering analysis showed the Hawaiian populations to be closely linked to the Philippines population, strongly indicating the Philippines as the origin of P. scutata in Hawaii (as P. conica in Cowie, Reference Cowie1995; Tran et al., Reference Tran, Hayes and Cowie2008), with the Philippines population showing some slight variation (0.3–0.6% uncorrected pairwise distances). The extremely low mitochondrial diversity among Malay peninsula populations of P. scutata is thus not likely to be natural. By comparison, preliminary analysis of COI genes of P. gracilis from the Malay peninsula (four sites 60–250 km apart) showed that individuals from each population had distinct haplotypes.
Comparisons of genetic diversity between the native range and introduced localities, or among non-native regions, can uncover introduction pathways (Hayes et al., Reference Hayes, Joshi, Thiengo and Cowie2008; Wong et al., Reference Wong, Meier and Tan2010). Attempts to obtain comparative specimens from the type locality of P. scutata (i.e. Java) and other countries for this study were largely unsuccessful. It is thus impossible to draw definitive conclusions regarding the true native distribution of P. scutata, nor is it certain that the lack of genetic diversity in Malay peninsula P. scutata is the result of a historical genetic bottleneck experienced by a native population (rather than of a founder effect caused by anthropogenic introduction). To address such biogeographical questions it would be necessary first to conduct a comprehensive revision of the genus in South-east Asia with a wider range of samples from throughout the region, using multiple molecular markers, including nuclear genes (Meier, Reference Meier and Wheeler2008).
All extant populations of P. scutata identified in our study, however, were found in human-disturbed areas. The pre-modified natural inland waters of Singapore were probably too soft and acidic to sustain a high diversity of molluscs (Johnson, Reference Johnson1967; Yeo & Lim, Reference Yeo, Lim, Ng, Corlett and H.T.W. Tan2011). The historical absence from natural habitats, and invariably close association of P. scutata with human-modified habitats, supported by the lack of genetic diversity across the populations in this study, therefore strongly indicate that present-day populations of P. scutata in the Malay peninsula have been introduced.
Further complicating matters, our results also suggest that the species thus far identified as P. scutata may be a species complex. Pila scutata of Java was separated from P. scutata of the Malay peninsula and the Philippines up to a threshold of > 4% in both COI and 16S. Intraspecific pairwise difference for the majority of gastropods is < 2% (Meier et al., Reference Meier, Zhang and Ali2008; Layton et al., Reference Layton, Martel and Hebert2014), although there are exceptions: e.g. a terrestrial snail, Cepaea nemoralis, has > 10% intraspecific divergence (Thomaz et al., Reference Thomaz, Guiller and Clarke1996). Interspecific relationships for some Pila species have been investigated previously (Hayes et al., Reference Hayes, Cowie and Thiengo2009b), but intra- and interspecific differences for the genus, especially in South-east Asia, are poorly known. We are unable to determine conclusively whether the Javan snails on the one hand and the Malay peninsula and Philippine snails on the other hand represent two distinct species or a single species with high intraspecific variation. Apple snails are notoriously difficult to distinguish by conchological characters (Hayes et al., Reference Hayes, Cowie, Thiengo and Strong2012), and possible hybridization sometimes confounds genetic analysis (Matsukura et al., Reference Matsukura, Okuda, Cazzaniga and Wada2013). Thus, a taxonomic revision of South-east Asian ampullariids is overdue (Hayes et al., Reference Hayes, Cowie, Jørgensen, Schultheiß, Albrecht and Thiengo2009a, Reference Hayes, Burks, Castro-Vazquez, Darby, Heras and Martín2015).
Consequences for conservation
Outreach campaigns in Peninsular Malaysia over the past 20 years successfully raised awareness of the threat of Pomacea among farmers, who actively remove apple snails from paddy fields but are unable to tell the genera apart (Joshi et al., Reference Joshi, Cowie and Sebastian2017). Older literature, including medically-related studies, identified P. scutata as the most common apple snail on the Malay peninsula (e.g. Berry, Reference Berry1974; Lim et al., Reference Lim, Yap, Krishnansamy, Ramachandran and Mansor1978). However, we found only one population of P. scutata, in Ipoh, compared to multiple populations of P. gracilis, along both the east (Kelantan, Terengganu) and west (Perak, Selangor) coasts of Peninsular Malaysia. Given the morphological similarity between different Pila spp., especially juveniles (Keawjam, Reference Keawjam1986), it is possible that some of the historical records of P. scutata in the Malay peninsula were of P. gracilis or juveniles of P. ampullacea (Plate 2). Many of the historical records could not be verified because of the lack of clear photographs and voucher specimens. The example here of poorly resolved taxonomy affecting conservation is not unique to ampullariids, it is symptomatic of a wider issue across understudied invertebrate groups in general (Collen et al., Reference Collen, Böhm, Kemp and Baillie2012), and requires more attention.
Conclusions
Our findings strongly suggest that present-day populations of P. scutata in the Malay peninsula were probably introduced by people. The questionable origins of the South-east Asian apple snail P. scutata in its presumed native range necessitates a reassessment of the species' current IUCN Red List status. These extant populations pose a dilemma, and the lack of genetic diversity is of concern in light of Pila decline throughout South-east Asia. Conservation management of P. scutata and its other South-east Asian congeners must therefore be better informed by greater taxonomic resolution, and more comprehensive investigations of their ecology, both in native and introduced ranges.
Acknowledgements
We thank Robert Cowie and an anonymous reviewer for their critiques, Y.M. Yong, T.S. Ng, N.L. Zulkipeli, R. Meier, W.H. Wong, A. Srivathsan and all staff and students of the Freshwater and Invasion Biology Laboratory (National University of Singapore), for field and laboratory assistance, S.-Y. Chan, J.K. Foon, B.L. Lim, R.M. Marwoto, R.C. Pagulayan, S. Ambu, M. Reid, P. Callomon, A. Lawless, J. Gerber, N. Badruddin, J. Ablett, B. Tripathy, T. Lee, A.N. van der Bijl, J. Goud, B. Hausdorf, R. Janssen, T. von Rintelen and C. Zorn for information or material, and access to photographs of museum material, and the National Parks Board, Singapore, PUB Singapore's National Water Agency (formerly Public Utilities Board), Ministry of Defence (Singapore), and Economic Development Board (Malaysia) for research permits and access to sampling sites. We acknowledge financial support from the Department of Biological Sciences, National University of Singapore, the National Research Foundation and the Economic Development Board (SPORE, COY-15-EWI-RCFSA/N197-1), Wildlife Reserves Singapore Ah Meng Memorial Conservation Fund (NUS grant R-154-000-617-720), Malacological Society of Australasia Mollusc Research Grant 2013, and the Conchological Society of Great Britain and Ireland Research Grant 2013.
Author contributions
Study conception and design: THN, SKT, HHT and DCJY; data collection and analyses: THN, SKT, AA, VTD, RCJ and WYW; reagents, materials and analysis tools: AA, HHT and DCJY; writing: THN, SKT, AA, VTD, RCJ, WYW, HHT and DCJY.
Conflicts of interest
None.
Ethical standards
All animal handling procedures were in accordance with the ethical standards of the Institutional Animal Care and Use Committee of the National University of Singapore and comply with the journal's Code of Conduct.