An unhealthy diet and physical inactivity together with a high BMI and smoking are thought to be the most important preventable determinants of cancer risk(1). Over the years many studies have been conducted assessing the association between nutrition and cancer incidence, most often investigating specific nutrients or food items. These analyses have several limitations, including the possible interaction or correlation among several nutrients. This makes it hard to investigate the true effect of the nutrient of interest. Moreover, the magnitude of the effect of individual nutrients or food items might be too small to detect.
Therefore, nowadays more studies focus on dietary patterns using a priori indices such as the Healthy Eating Index and the Healthy Diet Indicator based on dietary guidelines from the US Department of Agriculture and the WHO, respectively(Reference Guenther, Reedy and Krebs-Smith2, Reference Huijbregts, Feskens and Räsänen3). However, in previous studies(Reference McCullough, Feskanich and Rimm4–Reference Harnack, Nicodemus and Jacobs12) only a few indices(Reference Romaguera, Vergnaud and Peeters10–Reference Harnack, Nicodemus and Jacobs12) were significantly associated with cancer incidence, of which two indices were cancer-specific(Reference Romaguera, Vergnaud and Peeters10, Reference Cerhan, Potter and Gilmore11). Moreover, most indices were only associated significantly when non-dietary components, such as BMI and physical activity, were included in the score(Reference Cerhan, Potter and Gilmore11, Reference Harnack, Nicodemus and Jacobs12).
In 2006 the Dutch Health Council created Guidelines for a Healthy Diet for the Netherlands(13). These guidelines include one recommendation on physical activity and nine recommendations on diet (intake of fruit, vegetables, fibre, fish, saturated fatty acids, trans-fatty acids, consumption occasions with acidic drinks and foods, alcohol and salt). The recommendations were designed with a view to prevent deficiencies and chronic non-communicable diseases such as cancer.
Recently the Dutch Healthy Diet (DHD) index has been developed to measure adherence to the Dutch Guidelines for a Healthy Diet in a population(Reference van Lee, Geelen and van Huysduynen14). The DHD index was examined in association with micronutrient intake and is considered to rank participants according to their adherence to the Dutch Guidelines for a Healthy Diet.
Previous studies have shown lower hazard ratios for the association of diet and also fruit and vegetables with smoking-related cancer incidence compared with overall cancer incidence(Reference Romaguera, Vergnaud and Peeters10, Reference Boffetta, Couto and Wichmann15, Reference Couto, Boffetta and Lagiou16). Therefore, the aim of the present study was to investigate whether adherence to the Dutch Guidelines for a Healthy Diet using the DHD index is associated with a lower overall and smoking-related cancer risk within the European Prospective Investigation into Cancer and Nutrition–Netherlands (EPIC-NL).
Methods
The European Prospective Investigation into Cancer and Nutrition–Netherlands cohort
The EPIC-NL study consists of the two Dutch contributions to the EPIC study which were set up simultaneously between 1993 and 1997. The design and rationale of the EPIC-NL study have been described in detail elsewhere(Reference Beulens, Monninkhof and Verschuren17). In brief, the Prospect-EPIC study includes 17 357 women aged 49–70 years living in Utrecht and its vicinity who participated in the nationwide Dutch breast cancer screening programme. The MORGEN-EPIC cohort consists of 22 654 men and women aged 21–64 years selected from random samples of the Dutch population in three different towns (Doetinchem, Amsterdam and Maastricht). At baseline, a general questionnaire and an FFQ were administered and a physical examination was performed. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board of the University Medical Center Utrecht (Prospect) and the Medical Ethical Committee of TNO Nutrition and Food Research (MORGEN). Written informed consent was obtained from all participants.
From the total cohort (n 40 011), individuals who did not give permission for linkage with the municipal registry or cancer registry were excluded (n 2259). Additionally men and women with no information on dietary intake (n 173) or with implausibly high or low scores for total energy intake (those in the top 0·5 % and bottom 0·5 % of the ratio of reported energy intake to estimated energy requirement; n 346) were excluded. Furthermore, we excluded individuals with known cancer at baseline (n 1625). The final study population consisted of 35 608 men and women.
Exposure assessment
Daily nutritional intakes were obtained from a self-administered FFQ containing questions on the usual frequency of consumption of seventy-nine main food items during the year preceding enrolment. This questionnaire allows estimation of the average daily consumption of 178 foods. A registered dietitian checked the FFQ for inconsistencies, which were solved by contacting the participant. The validity of the FFQ was assessed against twelve monthly 24 h recalls over a 1-year period among 121 men and women(Reference Ocke, Bueno-de-Mesquita and Goddijn18, Reference Ocke, Bueno-de-Mesquita and Pols19). The FFQ was found to be reasonably valid for ranking individuals according to nutrient and food group intakes. Spearman correlations were good for fruit (r = 0·68 for men and r = 0·56 for women), alcohol (r = 0·74 for men and r = 0·87 for women) and dietary fibre intake (r = 0·61 for men and r = 0·74 for women); however, relatively low for vegetables (r = 0·38 for men and r = 0·31 for women) and fish (r = 0·32 for men and r = 0·37 for women).
Physical activity was assessed using the EPIC physical activity questionnaire(Reference Haftenberger, Schuit and Tormo20). Because there was no information on physical activity for 14 % of the participants, missing data were imputed using single linear regression modelling (SPSS MVA procedure).
The general questionnaire included questions on demographics, presence of chronic diseases and risk factors for chronic diseases. Smoking was categorized as never; former (quit smoking >20 years ago, quit 10–20 years ago, quit ≤10 years ago); current smoker (1–15 cigarettes/d, 16–25 cigarettes/d, >25 cigarettes/d); pipe or cigar smoker. Level of education was categorized as low (primary education up to those completing advanced elementary education), average (intermediate vocational education and higher general secondary education) or high (higher vocational education and university). BMI was calculated from the height and weight, which were measured during the physical examination.
Dutch Healthy Diet index
The DHD index is a continuous score with ten components that represent the ten Dutch Guidelines for a Healthy Diet of 2006 (Table 1)(Reference van Lee, Geelen and van Huysduynen14). The components physical activity, vegetables, fruit, fibre and fish are adequacy components and the scores for these components are calculated by dividing the reported intake or activity by the recommended minimum intake or activity level and subsequently multiplying this ratio by 10. The maximum score of 10 was assigned when the recommendation was met. Scoring of the physical activity level has been modified because physical activity in the EPIC-NL population was measured in a different way compared with the population in which the DHD index was developed. The development population filled out the Short Questionnaire to Assess Health-enhancing physical activity (SQUASH) which measures the number of activities per week, whereas in the EPIC-NL population the physical activity is measured in hours per week. The physical activity recommendation is set at 3·5 h of sports or cycling per week instead of five sessions of physical activity per week. For the components saturated fatty acids, trans-fatty acids, salt and alcohol, the guidelines say that they should be taken in moderation. Threshold values for these components were determined based on the 85th percentile of the 2 d average intake of a Dutch reference population(Reference Rossum, Fransen and Verkaik-Kloosterman21). The 85th percentile has also been used as cut-off value for other indices such as the Healthy Eating Index-2005(Reference Guenther, Reedy and Krebs-Smith2). The scores for the moderation components were calculated by dividing the difference between the reported intake and the maximum recommended intake by the difference between the threshold and maximum recommended intake and subsequently multiplying this ratio by 10. Finally the outcome is subtracted from 10. The maximum score of 10 was assigned when the recommendation was met. Salt intake from food products is available from the FFQ, but data on salt added during cooking and at the table are not available. The contribution of these sources is assumed to be on average 30 %(Reference van Lee, Geelen and van Huysduynen14). Therefore, the cut-off values for sodium are lowered by 30 %. The recommendation ‘maximum of seven consumption occasions with acidic drinks and foods per day’, which was included in the guidelines with the aim to prevent teeth erosion, has been left out because data on consumption occasions were not available from the FFQ. The scores for each component were summed into one score ranging from 0 to 90, with a higher score indicating better adherence to the Dutch Guidelines for a Healthy Diet.
Table 1 Components of the Dutch Guidelines for a Healthy Diet and the DHD index with cut-off values

DHD index, Dutch Healthy Diet index; en %, percentage of energy; NA, not applicable; M, men; W, women.
*Maximum of 100 g may be replaced by fruit juice.
Outcome assessment
The outcomes of interest were overall cancer incidence and smoking-related cancer incidence. The choice of smoking-related cancers was based on previous publications(22, Reference Secretan, Straif and Baan23); we consider the same cancers smoking-related as other recent EPIC studies(Reference Romaguera, Vergnaud and Peeters10, Reference Berentzen, Beulens and Hoevenaar-Blom24). The smoking-related cancers considered include those of the upper aero-digestive tract, lung, liver, colon, rectum, stomach, kidney, pancreas and bladder.
Cancer cases were identified by annual linkage to the Dutch Cancer Registry, which holds a standard computerized register of cancer patients since 1990. This database was directly linked to the EPIC-NL cohort with patients’ names after obtaining their consent. Prevalent cases of cancer, which were excluded before analysis, were identified through linkage with the cancer registry (events occurring before study entry) as well as self-report in the baseline general questionnaire. Information on vital status was obtained through linkage with the municipal registries. Follow-up was complete until December 2007.
Statistical analysis
Baseline characteristics are presented across tertiles of the DHD index as means with standard deviations or as percentages. The DHD index was evaluated in tertiles as well as per 20-point increment in score. We used Cox regression modelling to estimate the hazard ratio (HR) and 95 % confidence interval of the associations between the DHD index and overall and smoking-related cancer. Furthermore we investigated the associations of the individual components of the DHD index with overall cancer (in tertiles and per 2-point increase). The duration of follow-up was calculated as the interval between the date of study entry and the date of cancer diagnosis, death, loss to follow-up or 1 January 2008, whichever came first. The estimates were adjusted for potential confounders; sex, age, smoking status and intensity of smoking, educational level, energy intake and BMI. The estimate for the association of a separate component of the DHD index with overall cancer incidence was additionally adjusted for the other components of the DHD index. All analyses were stratified for cohort by including the SAS STRATA statement in the Cox model (Prospect-EPIC or MORGEN-EPIC). Interactions of the DHD index with sex and BMI were tested by including an interaction term into the model. The analyses were repeated excluding the physical activity score from the DHD index to investigate the association of the DHD index with only dietary components. Furthermore, we repeated the analysis excluding alcohol from the DHD index because the highest score of 10 was assigned to participants with a moderate alcohol intake (20 g ethanol/d for men and 10 g ethanol/d for women), while a moderate alcohol intake might have a harmful effect on several types of cancer. In order to investigate if the association varies by smoking status we stratified the analysis by smoking status of the participants at baseline. In sensitivity analyses, we excluded cases that occurred during the first two years of follow-up. We also separately excluded energy intake and BMI from the model to explore the role of energy intake and BMI in the association between the DHD index and cancer incidence. The P for trend was calculated by modelling the tertiles of adherence to the guidelines as a continuous variable. Two-sided P values below 0·05 were considered to be statistically significant. All statistical analyses were conducted using the statistical software package SAS version 9·2.
Results
During an average follow-up of 12·7 years, 3027 cancer incidences were documented of which 1015 were smoking-related. Table 2 provides the baseline characteristics of the participants across tertiles of the DHD index. The average DHD index score ranged from 38·7 in the lowest tertile to 63·7 among participants with the best adherence to the guidelines in the highest tertile. Compared with participants in the lowest tertile, those in the highest tertile were more often women, older, higher educated, and less often smokers. They also had higher energy-adjusted vitamin and mineral intakes and a lower total energy intake.
Table 2 Baseline characteristics of the 35 608 EPIC-NL participants across tertiles of the DHD index
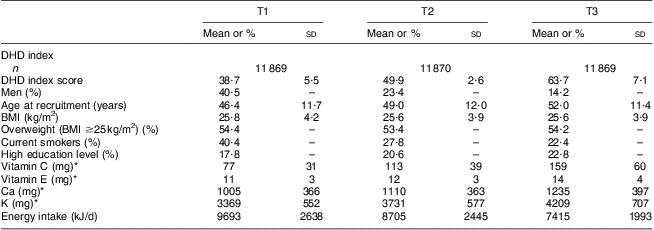
EPIC-NL, European Prospective Investigation into Cancer and Nutrition–Netherlands; DHD index, Dutch Healthy Diet index; T, tertile.
*Micronutrients per average energy intake.
Table 3 presents the results of the association between the DHD index and risk of overall and smoking-related cancer. There was no association between the DHD index and overall cancer (tertile 3 v. tertile 1) in either the crude (HR = 0·98; 95 % CI 0·90, 1·08) or multivariable model (HR = 0·97; 95 % CI 0·88, 1·07). Only taking smoking-related cancers into account resulted in lower hazard ratios and a significant crude association (HR = 0·82; 95 % CI 0·70, 0·96) which attenuated to non-significance after adjustment for confounders (HR = 0·89; 95 % CI 0·76, 1·06). A 20-point increment in the DHD index in relation to cancer showed similar results. The hazard ratios did not materially change when the score for physical activity or alcohol was excluded from the DHD index (Table 4). Also when stratifying the association between the DHD index and overall and smoking-related cancer risk by smoking status the conclusions did not change (Table 5). Hazard ratios were higher among never smokers than among current smokers, but were also not statistically significant; furthermore, the 95 % confidence intervals overlapped.
Table 3 HR and 95 % CI for the association between the DHD index (across tertiles of the DHD index) and total and smoking-related cancer incidence among 35 608 EPIC-NL participants
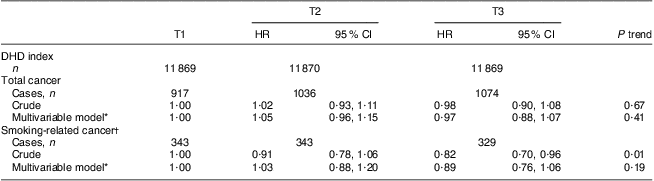
HR, hazard ratio; DHD index, Dutch Healthy Diet index; EPIC-NL, European Prospective Investigation into Cancer and Nutrition–Netherlands; T, tertile.
*Multivariable model includes adjustment for sex, age, energy intake, education, smoking status and intensity, and BMI.
†Smoking-related cancers: upper aero-digestive tract, lung, liver, colon, rectum, stomach, kidney, pancreas and bladder cancer.
Table 4 HR and 95 % CI for the association between the DHD index (per 20-point increase) and total and smoking-related cancer incidence among 35 608 EPIC-NL participants
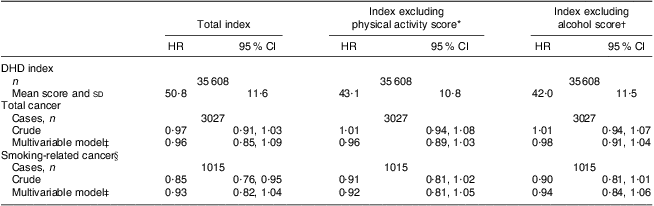
HR, hazard ratio; DHD index, Dutch Healthy Diet index; EPIC-NL, European Prospective Investigation into Cancer and Nutrition–Netherlands.
*Extra adjustment for physical activity, categorized according to the Cambridge Physical Activity Index.
†Extra adjustments for ethanol intake and alcohol drinking (never, quit, <1 drink/week, current).
‡Multivariable model includes adjustment for sex, age, energy intake, education, smoking status and intensity, and BMI.
§Smoking-related cancers: upper aero-digestive tract, lung, liver, colon, rectum, stomach, kidney, pancreas and bladder cancer.
Table 5 HR and 95 % CI for the association between the DHD index (per 20-point increase) and total and smoking-related cancer incidence among 35 608 EPIC-NL participants stratified for baseline smoking status
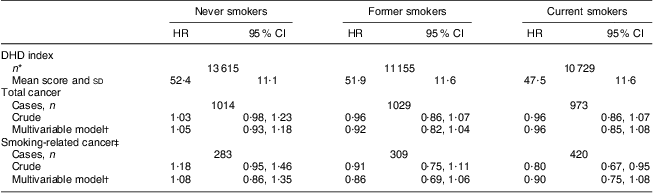
DHD index, Dutch Healthy Diet-index; EPIC-NL, European Prospective Investigation into Cancer and Nutrition–Netherlands.
*n does not add up to 35 608 due to 109 participants with missing smoking status at baseline.
†Multivariable model includes adjustment for sex, age, energy intake, education, smoking status and intensity, and BMI.
‡Smoking-related cancers: upper aero-digestive tract, lung, liver, colon, rectum, stomach, kidney, pancreas and bladder cancer.
Table 6 shows the mutually adjusted hazard ratios for overall cancer incidence in relation to the individual components of the DHD index. None of the associations was significant. Sensitivity analysis excluding energy intake or BMI from the multivariable model to explore the role of these factors did not change the results; neither did excluding the first two years of cancer incidences (data not shown). No interaction between adherence to the guidelines and sex or BMI was found (P value 0·85 and 0·84, respectively).
Table 6 HR and 95 % CI for the associations between separate components of the DHD index and total cancer incidence among 35 608 EPIC-NL participants
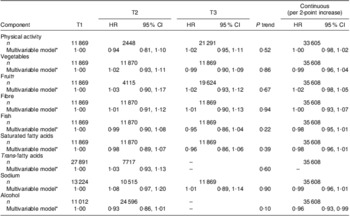
HR, hazard ratio; DHD index, Dutch Healthy Diet index; EPIC-NL, European Prospective Investigation into Cancer and Nutrition–Netherlands.
*Multivariable model includes adjustment for sex, age, energy intake, education, smoking status and intensity, and BMI, and furthermore mutually adjusted for each other.
†Maximum of 100 g may be replaced by fruit juice.
Discussion
In our cohort, greater adherence to the Dutch Guidelines for a Healthy Diet expressed in a DHD index score was not associated with a statistically significant reduction of overall or smoking-related cancer risk. Excluding the score for physical activity or alcohol from the total DHD index score did not change this conclusion. Furthermore, none of the individual components was associated with overall cancer risk.
The present study is the first one investigating the association of adherence to the DHD index with disease incidence. The results from the study provide no evidence that adherence to the Dutch Guidelines for a Healthy Diet, according to the DHD index, is associated with a reduced risk of overall cancer. Recently, the association between adherence to guidelines from the WHO and cancer incidence was also investigated in the EPIC-NL cohort and was also found to be not statistically significant(Reference Berentzen, Beulens and Hoevenaar-Blom24). Similar to our findings, other studies investigating adherence to national dietary guidelines and overall cancer in Germany and the USA (using several different indices) also did not find significant associations(Reference McCullough, Feskanich and Rimm4–Reference Fitzgerald, Dewar and Veugelers9). However, when non-dietary components, BMI and physical activity, were included in the score inverse associations were found(Reference Romaguera, Vergnaud and Peeters10–Reference Harnack, Nicodemus and Jacobs12). When a score for BMI was included in the DHD index the crude association with overall cancer also became statistically significant; however, this was attenuated to non-significance after adjustment for confounders (data not shown). The Mediterranean diet, on the other hand, has been found to be modestly associated with cancer incidence in a recent meta-analysis(Reference Sofi, Abbate and Gensini25) (HR = 0·94; 95 % CI 0·92, 0·96) and in the EPIC cohort (HR = 0·96; 95 % CI 0·95, 0·98)(Reference Couto, Boffetta and Lagiou16). However, the association in the Dutch part of the EPIC cohort, i.e. EPIC-NL, was borderline significant (HR = 0·96; 95 % CI 0·90, 1·01).
In another previous study in EPIC, participants with highest adherence to guidelines from the World Cancer Research Fund/American Institute for Cancer Research, which differed from the Dutch Guidelines for a Healthy Diet with respect to red and processed meat, energy-dense foods, BMI and breast-feeding, had an 18 % lower overall cancer risk (HR = 0·82; 95 % CI 0·75, 0·90)(Reference Romaguera, Vergnaud and Peeters10). This result did not change after excluding the non-dietary components (BMI and physical activity) from the score (risk estimates were not given). It is important to recognize that in contrast with the World Cancer Research Fund/American Institute for Cancer Research guidelines, the Dutch Guidelines for a Healthy Diet were not developed to prevent cancer but had a broader goal to prevent deficiency and chronic diseases. Whereas the World Cancer Research Fund/American Institute for Cancer Research only included components that are convincingly associated with cancer risk, the Dutch Guidelines for a Healthy Diet also include recommendations that have a weak association with cancer but a strong association with other diseases. For example, trans- and saturated fatty acids and fish intake are associated with CVD risk but only weakly associated with cancer(13, Reference Key, Schatzkin and Willett26). On the other hand, components such as vegetables and fruits which are also associated in the literature with a decreased cancer risk were not significantly associated with overall cancer risk in our separate component analysis.
In our data the hazard ratios for the non-significant association of adherence to the Dutch Guidelines for a Healthy Diet with smoking-related cancers were lower than for the non-significant association with overall cancer. Furthermore, the point estimates of the hazard ratios for never smokers were higher compared with those for current smokers, especially for the analysis with smoking-related cancer. This suggests that the association of the DHD index with cancer risk, if any, is stronger in never smokers. However, the hazard ratios did not reach statistical significance, maybe because of the reduced power in stratified analysis; furthermore, the 95 % confidence intervals overlapped. We also cannot rule out the possibility of residual confounding due to misclassification of smoking behaviour. On the other hand, slightly stronger associations have also been observed in other studies where dietary scores and fruit and vegetable intakes were more strongly associated with smoking-related cancers than overall cancer risk(Reference Romaguera, Vergnaud and Peeters10, Reference Boffetta, Couto and Wichmann15, Reference Couto, Boffetta and Lagiou16). This might be a consequence of many bioactive compounds in fruit and vegetables which are a large part of the score. For instance, the high antioxidant content of fruit and vegetables may reduce or prevent the oxidative damage caused by cigarette smoking and therefore has a stronger effect on smoking-related cancers(Reference Ros, Bueno-de-Mesquita and Kampman27).
Strengths of the present study are its large sample size and prospective design with over 12 years of follow-up. However, several limitations need to be addressed. The source of dietary information in our study was a single FFQ administered at baseline. The effect of participants changing their dietary patterns after baseline is uncertain. However, excluding participants from the analysis who are more likely to alter their dietary habits (i.e. participants who were diagnosed with cancer in the first two years of follow-up) did not change the results. The FFQ was validated against twelve monthly 24 h recalls in a population of 121 subjects(Reference Ocke, Bueno-de-Mesquita and Goddijn18, Reference Ocke, Bueno-de-Mesquita and Pols19). That study showed good validity for the dietary components fruit, alcohol and dietary fibre intake; however, lower relative validity for vegetables and fish which might have diluted the effect. Certain components of the original DHD index(Reference van Lee, Geelen and van Huysduynen14) had to be adapted slightly due to the differences in diet and physical activity measurement in our population compared with the population in which the index was developed. The scoring of the physical activity component was changed and we excluded the recommendation ‘maximum of seven consumption occasions with acidic drinks and foods per day’ because data on consumption occasions were not available from the FFQ. However, this recommendation was included in the Dutch Guidelines for a Healthy Diet with the aim to prevent teeth erosion, which is quite different from the aims of the other recommendations to prevent deficiency and chronic disease. Therefore, we think that excluding this recommendation from the score did not bias our results. Equal weights have been assigned to each component of the index even though the health effects may be different. Due to the power we grouped smoking-related cancer types together instead of assessing them individually; however, the disadvantage of this is that the associations with the DHD index might vary between the cancer types included in the cancer-related smoking group. Finally, we adjusted for all known confounders. However we cannot rule out the possibility of unknown or unmeasured confounding.
In summary, in this large, prospective cohort study participants with a higher adherence to the Dutch Guidelines for a Healthy Diet were not at lower risk of overall or smoking-related cancer. This does not exclude that other components not included in the DHD index may be associated with overall cancer risk. The present results need to be confirmed in future prospective studies.
Acknowledgements
Sources of funding: The EPIC-NL study was funded by the ‘Europe against Cancer’ Programme of the European Commission (SANCO), the Dutch Ministry of Health, the Dutch Cancer Society, the Netherlands Organisation for Health Research and Development (ZonMW) and the World Cancer Research Fund (WCRF). The analysis was also supported by a grant of the Dutch Research Council (NWO-ZonMW; grant no. 40-00812-98-10040). None of the study sponsors had a role in the study design, data collection, analysis or interpretation, report writing or the decision to submit the report for publication. Conflicts of interest: None declared. Authorship: A.M.M. and J.W.J.B. contributed equally to this work. E.A.S. conducted the statistical analyses and drafted the manuscript; A.M.M., J.W.J.B. and P.H.M.P. supervised data analysis and provided critical review of the manuscript; H.P.F., G.A.W., J.M.A.B., N.C.O.-M., J.H., Y.T. S. and H.B.B.-M. provided critical review of the manuscript; J.W.J.B., H.B.B.-M. and P.H.M.P. shared responsibility for data collection. All authors read and approved the final manuscript. Acknowledgements: The authors thank the National Cancer Registry for follow-up data.







