Poor nutrition in the first 2 years of life has significant implications on child morbidity(Reference Lodge, Tan and Lau1,Reference Horta and Victora2) , mortality(Reference Sankar, Sinha and Chowdhury3,4) and cognitive development; additionally, poor nutrition increases the risk of chronic non-communicable diseases in adulthood(Reference Horta and Victora5,Reference Dewey and Begum6) . WHO recommends that, unless contraindicated, children should be exclusively breastfed for the first 6 months of life, followed by the introduction of complementary food alongside breastfeeding for up to 2 years or beyond(7). Although this recommendation is widely acceptable, breastfeeding rates remained mostly low despite continued efforts to improve breastfeeding practices(Reference Victora, Bahl and Barros8–Reference O’Dowd10). There are significant differences in breastfeeding indicators between low- and high-income countries. For example, studies have demonstrated an inverse association between breastfeeding at 6 months and the gross domestic product per person(Reference Victora, Bahl and Barros8,11) .
The period from 6 to 23 months after birth, which is the transition time from exclusive breastfeeding to consuming a wide range of family foods while breastfeeding continues, is an extremely important time during which the largest proportion of stunting occurs(Reference Aguayo12). During this period, children consume small amounts of foods, given their small gastric capacity; therefore, complementary foods need to have high nutrient density, and children need to be fed frequently to support optimal physical growth and brain development(Reference Dewey13). As a result, it has been suggested that meeting the nutritional needs of children aged 6–23 months can be challenging, not only in resource-poor settings but also in high-income settings, which has significant implications on their growth and development(Reference Dewey13,Reference Dewey14) . On the other hand, the early introduction of complementary foods (before the age of 4 or 6 months), as well as particular types of foods have been proposed to be related to obesity later in life(Reference Sun, Foskey and Allen15–Reference Wang, Wu and Xiong17).
In an effort to address the lack of standard indicators that assess feeding practices of infants and young children at national, regional and global levels, WHO published the infant and young child feeding (IYCF) indicators along with the technical details of their measurements and calculations(18,19) . These indicators, which address breastfeeding and complementary feeding, have allowed for meaningful comparisons between different countries and monitoring trends in IYCF practices over time. However, the ability of these indicators to predict anthropometric measurements and, in particular, stunting and underweight remain under intense debate.
WHO IYCF indicators have traditionally been obtained from demographic health surveys (DHS) in several low- and middle-income countries. Only a few high-income countries usually report on these indicators, making global comparisons difficult(Reference Victora, Bahl and Barros8). In Kuwait, as in other oil-rich Arab states in the Middle East, there is a lack of data on IYCF, and the global estimates of IYCF indicators in the Middle East and North Africa (MENA) region usually do not include data from these countries or include data from the Gulf Family Health Survey that was conducted more than a decade ago. The few reports that have published on IYCF practices in Kuwait(Reference Carballo, Khatoon and Maclean20) did not follow WHO IYCF indicators, hampering global comparison.
Furthermore, similar to other high-income countries, childhood obesity has become a major public health problem in Kuwait; more than 45 % of school children are either overweight or obese(21). Studying IYCF practices, such as exclusive breastfeeding during the first 6 months of life and complementary feeding, is a prerequisite to improve these practices, hence combating obesity and overweight at an early stage(Reference Sun, Foskey and Allen15–Reference Wang, Wu and Xiong17,Reference Horta, Loret de Mola and Victora22) . In 2014, the Kuwait Nutritional Surveillance System (KNSS) was reviewed to assess the feeding practices of infants and young children according to WHO IYCF indicators. Over 3 consecutive years (2015–2017), data have accumulated and provide a unique opportunity to document feeding practices for infants and young children. In this study, we aimed, first, to characterise the current IYCF practices in Kuwait and, second, to investigate the associations between WHO IYCF indicators and overweight/obesity, as well as stunting in Kuwaiti children aged 0–23 months.
Methods
Study participants
The KNSS collects data from Kuwaiti citizens using structured data collection forms through personal interviews conducted by trained data collectors who are permanently employed for this purpose. The overall objective of KNSS is to provide regular and updated information on the nutritional status of the Kuwaiti population (children and adults) and the influencing factors. The system also aims to provide nationwide information on nutritional status trends in all age groups by tracking nutritional status over time. The KNSS was reviewed to collect data on IYCF practices using the WHO guidelines(18,19) .
Children aged 0–23 months (<730 d of age) were recruited from health centres in all governorates of Kuwait at the time of their vaccination from January 2015 to December 2017. According to the vaccination schedule in Kuwait, children are vaccinated at birth; by the end of the 2nd, 3rd, 4th, 6th, 12th, 18th and 24th months after birth; and at the age of 3·5 years. Ideally, data should be collected through household surveys, a method that is probably not feasible in Kuwait. However, unlike other countries where vaccination coverage has been low, vaccination in Kuwait is free of charge and the coverage reaches almost 100 %. Thus, it is possible to assume that recruiting children from health centres will generate a representative sample of Kuwaiti children. The KNSS uses a list of health centres that are designated to be sentinel sites for surveillance in all governorates (provinces). No sampling method was used as every mother or child guardian attending the vaccination centres was invited to participate in data collection, and only less than 2 % refused to participate.
Data collection
Table 1 includes the details of the core and optional indicators that we report in this study as per the WHO approach(18,19) . This approach provides a clear guidance for data collection and calculation of IYCF indicators with an example of the questionnaire that should be adapted. According to this approach, except for the two indicators, ‘early initiation of breastfeeding’ and ‘children ever breastfed’, all indicators were based on the respondents’ current status at the time of interview. In other words, mothers were not asked when they started or stopped a particular feeding practice; rather, they were asked to report their practices during the day and night before the interview. Following this approach, early initiation of breastfeeding was based on history recall from mothers of children aged 0–23 months who were put to the breast within 1 h of birth. Exclusive breastfeeding was defined considering infants 0–5 months (<183 d) who were breastfed and received no liquids (except oral rehydration solution and/or vitamins/mineral supplements) or complementary foods during the last 24 h. Predominant breastfeeding was defined as exclusive breastfeeding but allowing for water, water-based drinks and fruit juice but not non-breast milk, solid/semisolid or soft food. Early introduction of solid/semisolid or soft foods was defined as having received solid/semisolid or soft foods during the last 24 h before the age of 6 months. Bottle feeding was defined considering children aged 0–23 months who drank anything, including breast milk, from a bottle with a nipple during the previous day. Continued breastfeeding at 1 and 2 years was defined considering children aged 12–15 and 20–23 months, respectively, who received breast milk during the day and night before the interview. Minimum dietary diversity was defined among those aged 6–23 months who received foods from four or more food groups (out of seven food groups) during the previous day. These seven food groups are as follows: (1) grains, roots and tubers, (2) legumes and nuts, (3) dairy products (milk, yogurt and cheese), (4) flesh foods (meat, fish, poultry and liver/organ meats), (5) eggs, (6) vitamin A-rich fruits and vegetables and (7) other fruits and vegetables.
Table 1 WHO infant and young child feeding core and optional indicators(19) targeted by the Kuwait Nutritional Surveillance System (2015–2017)

* Food groups are: (1) grains, roots and tubers, (2) legumes and nuts, (3) dairy products (milk, yogurt and cheese), (4) flesh foods (meat, fish, poultry and liver/organ meats), (5) eggs, (6) vitamin A-rich fruits and vegetables and (7) other fruits and vegetables.
Unlike the DHS in other countries, the KNSS does not collect data on socioeconomic status, but data were collected on the type of delivery (normal vaginal or caesarean), place of birth (private or public hospital) and birth weight (BW), as reported by the mothers. The KNSS also collects data on gestational age (as reported by the mother), whether the mother attended antenatal care or received advice on breastfeeding immediately after delivery and, finally, whether the child had any illness that required medical consultation during the last 3 months. The weight of children was measured using a digital scale to the nearest 100 g, while the height of children was measured to the nearest 0·1 cm using a length board. To maintain accuracy, weighing scales were calibrated regularly using a well-known weight set. Data collectors were trained annually to measure the weight and height of children in a standardised manner.
Data were collected by face-to-face interviews with mothers or child guardians who came to vaccinate their children using a simplified data collection form that was developed in the Arabic language based on WHO IYCF indicators(18). The questionnaire was pilot-tested on 300 participants who were not included in the data analyses. The collected data from pilot testing were used to improve the clarity of questions and further train data collectors to reinforce data collection guidelines prepared for the KNSS. Although the KNSS is a public health survey, all data collection procedures have been reviewed by the Ethics Committee at the Ministry of Health and were granted ethical approval (No. 98). Each participant in the KNSS signed a written informed consent form before data collection was initiated.
Statistical methods
Data were analysed using Stata 12 (StataCorp 2011, release 12). All calculations were performed as per guidelines for both children 0–5 months (<183 d) and children 0–23 months (<730 d)(18). If the sample size was sufficient, the indicators were further disaggregated into small groups. The exact age of children, in days, was calculated by subtracting the date of birth from the date of interview. BMI was calculated as weight (kg)/height (m)2. To assess overweight/obesity, BMI-for-age z-scores were calculated using the WHO growth charts, and overweight was defined as ≥+2 sd and <+3 sd, while obesity was defined as ≥+3 sd(Reference de Onis and Lobstein23). Whether to use BMI-for-age or weight-for-length (WFL) to define overweight among infants and young children is still debated(Reference Aris, Rifas-Shiman and Li24–Reference Roy, Spivack and Faith26). Therefore, we calculated WFL z-scores, and overweight was defined as >+2 sd, while wasting was defined as <–2 sd. Length-for-age z-scores (LAZ) were calculated, and stunting was defined as <–2 sd. Similarly, weight-for-age z-scores were calculated, and underweight was defined as <–2 sd. The 95 % CIs were calculated for the key indicators using exact binomial distribution. The Mann–Whitney test was used to test the differences in z-scores. Logistic regression was used to calculate crude ORs and adjusted ORs (AORs) for the associations between WHO IYCF indicators and stunting (defined as length-for-age <–2 sd) or overweight. Two separate analyses were conducted, first while overweight was defined as BMI-for-age ≥+2 sd (Table 6), and second while overweight was defined as weight-for-length >+2 sd (Table 7). In the multivariable model, we adjusted for sex, BW, age and illness during the last 3 months. Statistical significance was tested using the chi-square test in univariable models, and the Wald test in multivariable models; factors with P < 0·05 were deemed statistically significant.
Results
Description of the study group
Over the 3-year study period, data were collected on 5839 Kuwaiti children (0–23 months), of whom 50·2 % were males. The mother was the source of information on the child in 87·6 % of interviews. The characteristics of the study group are shown in Table 2. The majority of Kuwaiti mothers had been to antenatal care clinics either in the private sector alone (57·2 %), the public sector alone (13·5 %) or both (28·6 %), and only 0·8 % had not been to antenatal care when pregnant with the index child. The majority of Kuwaiti children (79·9 %) were born in private hospitals, while 18·4 % were born in public hospitals. Approximately one-third were born by caesarean section (CS), which was significantly more common in public hospitals compared to private hospitals (42·5 v. 28·5 %; P < 0·001). We did not collect data on the clinical indications for CS to elucidate the underlying reasons for this difference between private and public hospitals. The majority of mothers or guardians (94·8 %) reported a recalled BW of their children; the mean (sd) value of BW was 2939·97 (584·51) g. More than 22·0 % of the children had a low BW (<2500 g), and 1·6 % had a high BW (≥4000 g).
Table 2 Characteristics of 5839 children (0–23 months) recruited in the Kuwait Nutritional Surveillance System, 2015–2017

* The question was asked only if the mother was the source of information (n 5118), and an answer was missing for fourteen mothers.
† Same remark as the previous question, and an answer was missing for fifteen mothers.
‡ Others included those who had their deliveries abroad; there were also three missing values.
§ An answer was missing for six mothers.
‖ As reported by mothers or guardians; 305 could not remember the birth weight of their children.
Infant and young child feeding practices
The core and optional indicators for feeding infants and young Kuwaiti children over the 3-year period are shown in Table 3. Only 39·1 % of mothers initiated breastfeeding within the first hour of birth or recovery from a CS. This was significantly lower among mothers who delivered by CS compared to those who delivered by normal delivery (29·6 v. 43·8 %, respectively; P < 0·001). The majority of Kuwaiti children (0–23 months) were ever breastfed (89·9 %); this indicator showed a significant variation among different governorates (P < 0·001). Exclusive breastfeeding (as defined by the WHO) was 9·5 % (95 % CI 7·7, 11·4 %) among infants in the 0–3 months age group, and 8·0 % (95 % CI 7·0, 9·1 %) among infants in the 0–5 months age group. Our findings showed that during the day and night before the interview, 84·2 % of infants aged 0–5 months were fed formula milk. As a result, both predominant breastfeeding and age-appropriate breastfeeding were low: 10·3 % (95 % CI 9·2, 11·6) and 7·4 % (95 % CI 6·7, 8·9), respectively.
Table 3 Infant and young child feeding practices for 5839 children (0–23 months) recruited in the Kuwait Nutritional Surveillance System, 2015–2017

CS, caesarean section.
* The question was asked only if the mother was the source of information (n 5118), and we also excluded women who delivered outside the country; an answer was missing for nine mothers.
† The same remark as the previous question; missing for 106 mothers.
The introduction of solid/semisolid or soft foods was 69·0 % (95 % CI 66·3, 71·5) in infants aged 6–8 months. The early introduction of solid/semisolid or soft foods among infants aged 0–5 months was low (6·3 %). The proportion of children 6–23 months who received complementary feeding from at least four of the food groups listed by the WHO (minimum dietary diversity) was 41·6 % (95 % CI 39·9, 43·3), while the consumption of eggs and flesh foods in this age group was 22·1 and 41·0 %, respectively.
Associations between infant and young child feeding practices and stunting
The prevalence of stunting, wasting, underweight, overweight and obesity among children 0–23 months is displayed in Table 4. The prevalence of stunting was 7·5 %, while that of wasting was 2·4 %. Using BMI-for-age, the prevalence of overweight was 6·5 %, while that of obesity was 1·6 %. The prevalence of overweight was 8·7 % using WFL, which is very similar to the prevalence estimated by BMI-for-age. The prevalence of underweight (weight-for-age) was 4·3 %, while that of concurrent stunting and overweight/obesity was 0·9 %. The associations between IYCF indicators and stunting are shown in Table 5. Although LAZ was significantly lower in the children of mothers who did not initiate early breastfeeding, the odds of stunting was not significantly different between the children of mothers who initiated breastfeeding early and those of mothers who did not (Table 5). Exclusive breastfeeding was significantly associated with stunting in the multivariable analysis, with children who were exclusively breastfed having higher odds of stunted growth (AOR 1·71; 95 % CI 1·08, 2·70; P = 0·021). Breastfeeding indicators were calculated based on whether the child was breastfed yesterday; hence they targeted infants and children at different age groups. Therefore, probably the most important indicator in this analysis is age-appropriate breastfeeding because it includes all children aged 0–23 months. Age-appropriate breastfeeding was more common in children with stunting in multivariable analysis (AOR 1·44; 95 % CI 1·01, 2·06; P = 0·046). On the other hand, the introduction of solid/semisolid or soft foods was inversely associated with stunting in univariable and multivariable analyses (OR 0·57; 95 % CI 0·34, 0·97; P = 0·037 and AOR 0·52; 95 % CI 0·30, 0·90; P = 0·021). Similarly, there was a borderline significant negative association between achieving the minimum dietary diversity and stunting in the univariable analysis (OR 0·71; 95 % CI 0·50, 1·00; P = 0·053), but this was non-significant in the multivariable analysis (AOR 0·74; 95 % CI 0·52, 1·07; P = 0·108).
Table 4 Prevalence of stunting, wasting, underweight, overweight and obesity among 5839 children (0–23 months) recruited in the Kuwait Nutritional Surveillance System, 2015–2017
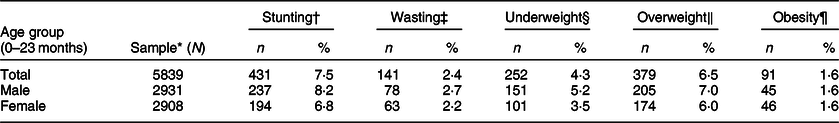
* Numbers may vary due to excluding children with biologically implausible z-scores (ninety-five children with length-for-age z-score either <–6 or >6; forty-eight children with weight-for-length z-score either <–5 or >5; thirty children with weight-for-age z-score either <–6 or >5; and forty-five children with BMI-for-age z-score either <–5 or >5).
† Stunting: length-for-age <–2 sd.
‡ Wasting: weight-for-length <–2 sd.
§ Underweight: weight-for-age <–2 sd.
‖ Overweight: BMI-for-age >+2 sd to <+3 sd.
¶ Obesity: BMI-for-age >+3 sd.
Table 5 Associations between infant and young child feeding indicators and stunting, Kuwait Nutritional Surveillance System, 2015–2017
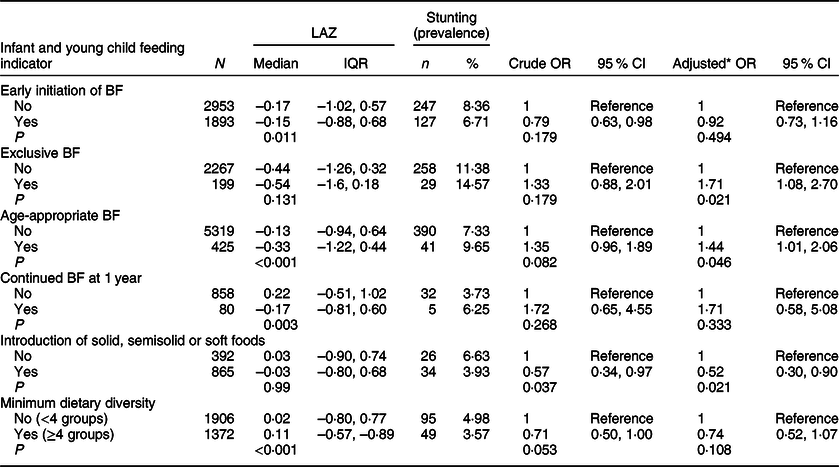
BF, breastfeeding; IQR, interquartile range (Q1, Q3); LAZ, length-for-age z-score.
* Adjusted for sex, age, birth weight and illness during the last 3 months.
Associations between infant and young child feeding practices and overweight
The associations between IYCF indicators and overweight (based on BMI-for-age; the term overweight includes obesity) are depicted in Table 6. Of all IYCF indicators, only age-appropriate breastfeeding was inversely associated with overweight in the univariable and multivariable analyses (OR 0·57; 95 % CI 0·36, 0·89; P = 0·013 and AOR 0·62; 95 % CI 0·39, 0·98; P = 0·043). The odds of overweight were significantly lower in children who were breastfed according to this indicator compared to children who were not. Early introduction of solid/semisolid or soft foods before the age of 6 months was positively associated with overweight (OR 2·24; 95 % CI 1·27, 3·96; P = 0·005 and AOR 2·36; 95 % CI 1·83, 6·18; P < 0·001). We repeated the above analysis while defining overweight based on WFL and present the results in Table 7. Similar to the previous analysis, age-appropriate breastfeeding was inversely associated with overweight in univariable and multivariable analyses (OR 0·55; 95 % CI 0·36, 0·86; P = 0·008 and AOR 0·58; 95 % CI 0·37, 0·92; P = 0·021). In this analysis (Table 7), continued breastfeeding at 1 year was also inversly associated with overweight, with the odds of overweight being significantly lower among those who were breastfed at 1 year (OR 0·27; 95 % CI 0·08, 1·88; P = 0·030 and AOR 0·29; 95 % CI 0·09, 0·94; P = 0·040). Finally, none of the IYCF indicators showed a significant association with wasting. Continued breastfeeding at 1 year was more common among children who were underweight (AOR 5·36; 95 % CI 2·65, 10·86; P < 0·001), while achieving minimum dietary diversity was inversely associated with underweight (AOR 0·26; 95 % CI 0·16, 0·45; P < 0·001) (data not shown).
Table 6 Association between infant and young child feeding indicators and overweight (including obesity based on BMI-for-age): data from the Kuwait Nutritional Surveillance System, 2015–2017
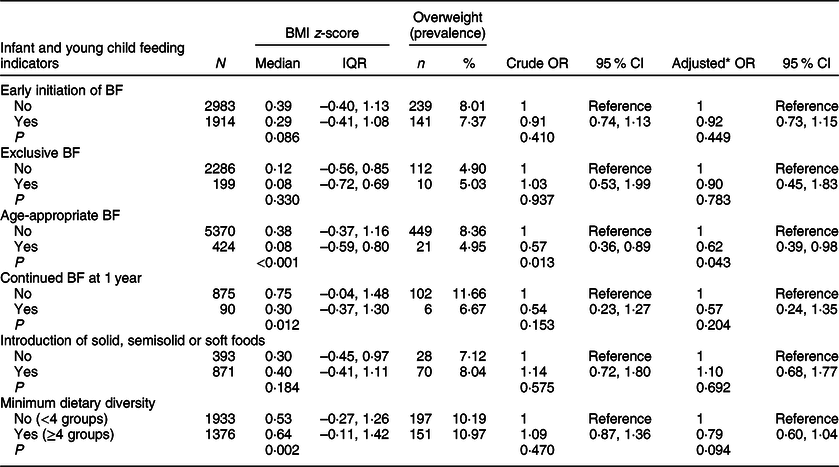
BF, breastfeeding; IQR, interquartile range (Q1, Q3).
* Adjusted for sex, age, birth weight and illness during the last 3 months.
Table 7 Associations between infant and young child feeding indicators and overweight (based on weight-for-length): data from the Kuwait Nutritional Surveillance System, 2015–2017
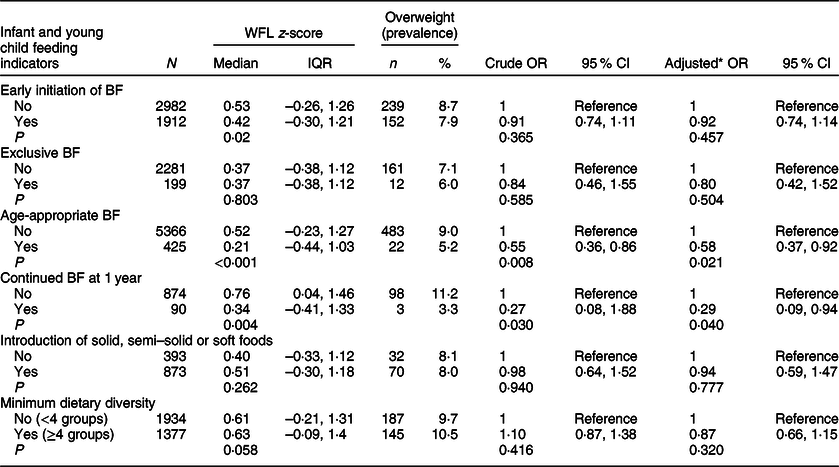
BF, breastfeeding; WFL, weight-for-length; IQR, interquartile range (Q1, Q3).
* Adjusted for sex, age, birth weight and illness during the last 3 months.
Discussion
This study aimed to assess IYCF practices in Kuwait as per the WHO indicators and to investigate the associations between IYCF indicators and anthropometric outcomes. WHO IYCF indicators have been rarely reported from high-income countries(Reference Victora, Bahl and Barros8) such as the oil-rich countries in the Middle East. We used data from the KNSS (2015–2017), which was reviewed to collect data on IYCF practices as per the WHO approach. Our findings showed that only a minority of Kuwaiti mothers initiated breastfeeding on time, and only a minority exclusively breastfed their infants during the first 6 months of life. Our results also showed that age-appropriate breastfeeding was inversely associated with overweight, while early introduction of solid/semisolid or soft food before the age of 6 months was more common in children who were overweight.
Infant and young child feeding practices
In our study, approximately 39 % of mothers initiated breastfeeding within 1 hour of birth, which is similar to estimates reported from various low-, middle-(Reference Victora, Bahl and Barros8,Reference Jones, Ickes and Smith27) and high-income countries(28). This estimate is also slightly lower than the world’s average for the early initiation of breastfeeding (42 %)(29). We have demonstrated that more than 31 % of Kuwaiti children were delivered by CS, which is one of the highest rates of CS in high-income countries(Reference Beake, Bick and Narracott30,Reference Ye, Betran and Guerrero Vela31) . In our study, early initiation of breastfeeding to babies delivered by CS was significantly lower compared to those delivered by normal vaginal delivery. A systematic review of fifty-three studies from thirty-three countries found breastfeeding initiation to be significantly lower after birth by CS compared to birth by normal vaginal delivery(Reference Prior, Santhakumaran and Gale32). The indications for CS, the type of anaesthesia and baby’s condition at birth can all contribute to the separation of the mother and the baby, impacting the timing of breastfeeding(Reference McFadden, Baker and Lavender33). It has been suggested that a successful breastfeeding after CS may require physical, psychological and emotional support, but only a limited number of interventions to improve breastfeeding among mothers who deliver by CS have been evaluated(Reference Beake, Bick and Narracott30). In a recent literature review, an intervention that included parent education and targeted breastfeeding support was found to increase the initiation and continuation of breastfeeding, but the quality of the studies was not adequate to draw a conclusion(Reference Beake, Bick and Narracott30). In our study, it is worth noting that all breastfeeding indicators, except for the early initiation of breastfeeding, were not significantly different between babies who were born by CS and those who were born by vaginal delivery. The fact that all deliveries occur in hospitals in Kuwait(34) shows the importance of efforts to improve early initiation of breastfeeding at the hospital level through initiatives such as baby-friendly hospitals. It is also worth noting that most of these deliveries occurred in private hospitals; therefore, efforts to improve early initiation of breastfeeding that do not include the private sector are unlikely to be productive. To date, very few hospitals (one public and one private) have been declared baby-friendly in Kuwait, and the impact of this initiative has never been formally evaluated or demonstrated in Kuwait. Mothers who initiated breastfeeding late were asked for the reason in an open question. Their answers were non-specific, such as the mother being tired or there was no breast milk, which reflects mothers’ poor knowledge and lack of support at the hospital level to encourage mothers to initiate breastfeeding early after delivery. Qualitative studies are recommended to investigate the underlying reasons for delays in the initiation of breastfeeding both in public and private hospitals.
As per the WHO approach, exclusive breastfeeding was defined so when babies were breastfed on the day or night preceding the interview and received no liquid or complementary foods. Based on this definition, only approximately 8 % of infants aged 0–5 months were exclusively breastfed. Furthermore, it has been suggested that this definition may overestimate exclusive breastfeeding, as some infants who are given liquids irregularly may not do so on the day before the interview(18,19) . In fact, more than 84 % of infants aged 0–5 months were fed formula milk on the day and night preceding the interview. Exclusive breastfeeding in our setting seemed to be substantially lower than in various low-(Reference Victora, Bahl and Barros8,Reference Jones, Ickes and Smith27) , middle-(Reference Victora, Bahl and Barros8) and high-income countries(28,35) . In our setting, exclusive breastfeeding seemed to be undermined by feeding infants formula milk rather than by early introduction of foods or drinks (only 6 % of infants aged 0–5 months received solid/semisolid or soft foods with or without breast milk). As a result, other breastfeeding indicators, such as age-appropriate breastfeeding and predominant breastfeeding, were also low. Efforts to improve breastfeeding in our setting should focus on reducing the use of formula milk in infants aged 0–5 months. Unlike low-income countries, the cost of formula milk is not a major financial burden for most Kuwaiti families(36), and it is unfortunate that formula milk is partially subsidised by the government. While the availability of subsidised infant formula milk through welfare programmes in the United Kingdom and the United States is thought to be an economic factor that may have contributed unintentionally to low breastfeeding practices among women in low‐income groups(Reference Jiang, Foster and Gibson-Davis37), it is not clear if this has an impact on breastfeeding in Kuwait. There are concerns about the quality and generalisability of the evidence demonstrating the effectiveness of interventions that presumably improve the indicators of breastfeeding(Reference Balogun, O’Sullivan and McFadden38). This highlights the need to locally test interventions that aim to overcome barriers to breastfeeding in Kuwait and other oil-rich countries in the Gulf region.
Although the recommendation that infants should be exclusively breastfed until the age of 6 months is widely acceptable, the age at which complementary food should be introduced (3–4 v. 6 months) remains under some debate(39,Reference Fewtrell, Bronsky and Campoy40) . It has been suggested that this recommendation should be applied to the population level rather than to individuals, and that mothers who are unwilling or unable to breastfeed should be supported(39–Reference Kramer and Kakuma41). Approximately 69 % of infants aged 6–8 months were fed solid/semisolid or soft foods during the day and night preceding the interview in our setting, which is similar to that reported from most countries, including the MENA region, but lower than that reported from Latin America and the Caribbean region(Reference White, Begin and Kumapley42). This also means that a considerable number of children aged 6–8 months did not receive solid/semisolid or soft foods on the day and night before the interview. As mentioned above, introducing solid/semisolid or soft foods before the recommended age was not common in our setting as only less than 10 % consumed solid/semisolid or soft foods at the age of 4–5 months (91–183 d). In some settings, half of the infants aged 4–5 months were reported to be already consuming solid/semisolid or soft foods(Reference White, Begin and Kumapley42,Reference Clayton, Li and Perrine43) .
More than 40 % of children aged 6–23 months achieved a minimum dietary diversity, which is higher than that reported from low-income countries(Reference Jones, Ickes and Smith27), or global estimates except for Latin America and the Caribbean region(Reference White, Begin and Kumapley42). However, a minimum dietary diversity was achieved only in less than 15 % of infants aged 6–11 months, which is the period facing the greatest challenge to meet nutritional requirements(Reference Dewey14,39) . Unlike low-income settings, Kuwait is an affluent country, and foods are subsidised for Kuwaiti families; thus, it is likely that cultural beliefs and poor knowledge of what constitutes an adequate diet for young children are the underlying reasons. Previously, it was demonstrated that diet diversity among infants and young children is poor even among rich families(Reference White, Begin and Kumapley42). The indicators of complementary feeding in Kuwait seem to be good compared with high-income countries, which is consistent with the previous observation that in countries where continued breastfeeding at 1 and 2 years is low, the indicators of complementary feeding are usually high(Reference White, Begin and Kumapley42).
Associations between infant and young child feeding practices and stunting
It has been suggested that the importance of WHO IYCF indicators should be judged on their ability to predict anthropometric outcomes. This is because they are not meant to be merely used to monitor IYCF practices, but also to identify populations at risk and to evaluate the impact of nutritional interventions(Reference Jones, Ickes and Smith27). We explored the associations between WHO IYCF indicators and anthropometric outcomes, including stunting, underweight and overweight. Our data suggest that breastfeeding indicators are more common in children aged 0–23 months with stunting. In particular, we found that age-appropriate breastfeeding and exclusive breastfeeding were significantly associated with stunting. Children who were appropriately breastfed according to their age had higher odds of stunted growth compared to those who were not. Similar to our findings, Jones et al.(Reference Jones, Ickes and Smith27) examined the associations between IYCF indicators and stunting in various low-income countries and reported a negative association between exclusive breastfeeding and LAZ, which was significant in two countries. In our study, there was no significant difference in LAZ between babies who were exclusively breastfed and those who were not (P = 0·131), but LAZ was significantly lower among children who were appropriately breastfed according to their age (P < 0·001). Introducing solid/semisolid or soft foods was inversely associated with stunting, which is similar to that reported in other studies(Reference Jones, Ickes and Smith27). Additionally, achieving a minimum dietary diversity was inversely associated with stunting (with borderline significance) in univariable analysis, but lost statistical significance in the multivariable analysis. This is similar to other studies that showed that the consumption of a minimally diverse diet at 6 months of age has been associated with greater LAZ at the age of 18 months(Reference Ruel44,Reference Mallard, Houghton and Filteau45) . In our analysis, except for fruits and vegetables, all other complementary foods were inversely associated with stunting, which is consistent with other studies that showed that children 6–23 months of age who consumed no animal-source foods had significantly higher odds of stunting(Reference Krasevec, An and Kumapley46).
In a study in Cambodia, using the DHS data, the authors reported that compliance with all of the age-specific WHO IYCF indicators was not associated with stunted growth(Reference Marriott, White and Hadden47). In another study that aimed to investigate the overall average effect of WHO IYCF indicators in fourteen low-income countries, children who were fed as per the IYCF indicators were significantly less likely to be underweight or stunted(Reference Marriott, White and Hadden48). These two studies combined breastfeeding and complementary feeding into one indicator, and thus their results cannot be directly compared with our findings. However, our data, in addition to other studies(Reference Jones, Ickes and Smith27), suggest that breastfeeding indicators are either not associated with stunting, or positively associated with stunting (i.e. negatively associated with LAZ). Although this could be a genuine association, it may be explained by the nature of cross-sectional data collection on WHO IYCF indicators, which makes it possible that the mothers of children with poor growth tend to breastfeed their children (reverse causality). On the other hand, the timely introduction of complementary foods and achieving a minimum dietary diversity seemed to be inversely associated with stunting. Growth during the first 2 years of life is also affected by prematurity and BW(Reference Aryastami, Shankar and Kusumawardani49,Reference Rahman, Howlader and Masud50) . In our data, BW was a strong predictor of stunting (P < 0·001), overweight (P = 0·018) and underweight (P < 0·001). Therefore, we recommend that studies looking at the associations between WHO IYCF indicators and anthropometric measurements at an early age adjust for prematurity and BW, or at least for one of these factors if strong collinearity is found between them.
Associations between infant and young child feeding practices and overweight
Overweight and obesity among children is increasing even in low-income countries. The link between WHO IYCF indicators and stunting or underweight has been described before, but studies investigating the link between WHO IYCF indicators and overweight or obesity are lacking. Of all WHO IYCF indicators, only age-appropriate breastfeeding was inversely associated with overweight in univariable and multivariable analyses (Table 6). This is of public health significance in Kuwait, where more than 45 % of school children are either overweight or obese(21). Our findings are consistent with the conclusion from a literature review of 113 studies conducted mostly in high-income countries, in which a longer period of breastfeeding was associated with a reduction in the odds of overweight and obesity(Reference Horta, Loret de Mola and Victora22). Efforts to tackle obesity in Kuwait should focus on early-life exposure, including breastfeeding and other IYCF practices. Some studies have suggested that early introduction of complementary foods (before the age of 4 or 6 months) may increase the risk of having a BMI that is higher than normal(Reference Sun, Foskey and Allen15–Reference Wang, Wu and Xiong17,Reference Pearce, Taylor and Langley-Evans51) , but other studies did not support this assertion(Reference Grote, Theurich and Luque52). In our data, only a small number of children in this age group received solid/semisolid or soft foods, and this was more common among children who were overweight in univariable and multivariable analyses.
Strengths and limitations
Our study provides insights into the WHO IYCF practices for a high-income country in the Middle East that are distinct from other neighbouring low-income countries in the region. Unlike previous studies that focused only on underweight, wasting and stunting, we also looked at the associations between WHO IYCF indicators and overweight. However, our data have several limitations, one of which is the cross-sectional data collection approach, which makes the inference about the temporal associations between WHO IYCF indicators and anthropometric measures unclear. It is possible that a mother may have changed the way she feeds her child if she felt his/her growth was unsatisfactory. The other limitation of this study is the lack of data on the socioeconomic status of participants. Additionally, because different WHO IYCF indicators target different age groups (e.g. exclusive breastfeeding target infants aged 0–5 months), the sample size differed. Also, we did not collect any data from those who refused to participate. We reported data on five core and four optional WHO IYCF indicators. Three core indicators (minimum meal frequency, minimum acceptable diet, and consumption of iron-rich or iron-fortified foods) were not covered for operational reasons. Finally, for several logistical reasons, the KNSS collects data only on Kuwaitis; thus, our findings cannot be extrapolated to non-Kuwaitis living in Kuwait. The latter comprises a diverse group of South East Asians, South Asians and Arabs; hence, describing the IYCF practices in this group may require separate studies that take into account the cultural differences.
Conclusion
We documented the IYCF practices according to the WHO IYCF indicators in Kuwait. We found that only a minority of Kuwaiti mothers initiated breastfeeding immediately after birth, and that less than one out of ten infants aged 0–5 months were exclusively breastfed. More than eight of every ten infants aged 0–5 months were fed formula milk. Efforts are needed to improve early initiation of breastfeeding in both private and public hospitals, and to discourage the use of infant formula milk. Our findings suggest that age-appropriate breastfeeding is inversely associated with overweight, which is of public health significance in Kuwait, where childhood obesity is a major public health issue. Policymakers should recognise the impact of suboptimal feeding practices on children in terms of child survival, educational potential and chronic non-communicable diseases later in life. Such an understanding will help to scale up interventions to improve IYCF practices in Kuwait based on locally generated evidence. Finally, our data suggest that the associations between different WHO IYCF indicators and stunting, as well as overweight is complex, which reflects the need for a better understanding of WHO IYCF indicators in both low- and high-income settings.
Acknowledgements
Acknowledgements: We would like to thank Dr Mona Alsumaie for her efforts during the review of KNSS. We also would like to thank Dr Reem Al-Sabah for comments on the first draft of this article. Financial support: KNSS is funded by the Kuwaiti government. This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: A.A.-T. supervised data collection, analysed the data and drafted the manuscript. N.A. supervised data collection, contributed to data interpretation and revised the manuscript with significant intellectual input. M.S.H. contributed to data interpretation and revised the manuscript with significant intellectual input. F.A. supervised data collection and data entry, contributed to data management and revised the manuscript with significant intellectual input. N.A.-D. supervised data collection and data entry, contributed to data management and revised the manuscript with significant intellectual input. M.S. supervised data collection and data entry, contributed to data management and revised the manuscript with significant intellectual input. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Ethics Committee at the Ministry of Health in Kuwait (No. 98). Written informed consent was obtained from all parents or guardians.








