Non-alcoholic fatty liver disease (NAFLD), alternatively known as metabolic fatty liver disease, is associated with insulin resistance, dyslipidaemia, obesity and the metabolic syndrome( Reference Chalasani, Younossi and Lavine 1 ). In parallel with the obesity epidemic, NAFLD has become one of the most common causes of chronic liver disease in the world. Up to 30 % of adults are affected by NAFLD in America( Reference Williams, Stengel and Asike 2 ). A study from China reported that the prevalence of NAFLD was as high as 20 %( Reference Wong 3 ). Vitamin D deficiency (VDD) can be a result of inadequate or limited exposure to sunlight, improper diet or problems related to absorption of vitamin D. Certain diseases can impair vitamin D conversion into its active form. Some of these conditions include kidney disease, liver disease or some hereditary diseases. VDD usually results in bad mineralisation of bones, leading to bone softening, osteomalacia, rickets and osteoporosis. As VDD is a risk factor for developing these metabolic deficiencies and has been linked to disorders in bone metabolism, more recent studies have suggested that obesity and visceral obesity occur with VDD. The potential association between VDD and NAFLD has been examined in recent studies. Mohamed et al.( Reference Mohamed, Mahmoud and Ahmed 4 ) reported that VDD is an important risk factor for NAFLD, and Kong et al.( Reference Kong, Zhu and Bai 5 ) observed that VDD might up-regulate endogenous fatty acid synthesis in non-alcoholic steatohepatitis (NASH) through impaired enterohepatic circulation and that administration of 1,25-dihydroxyvitamin D3 corrected the NASH phenotypes in line with suppression of hepatic lipogenesis and inflammation. Rhee et al.( Reference Rhee, Kim and Park 6 ) reported that participants with higher serum 25-hydroxyvitamin D3 (25(OH)D3) showed a significantly reduced risk for NAFLD compared with low 25(OH)D3 groups, independent of obesity and the metabolic syndrome. Sharifi et al.( Reference Sharifi, Amani and Hajiani 7 ) and Targher et al.( Reference Targher, Scorletti and Mantovani 8 ) suggest that treatment of vitamin D3 deficiency may prevent or treat NAFLD. Bril et al.( Reference Bril, Maximos and Portillo-Sanchez 9 ) observed the plasma vitamin D levels of 239 patients who were divided into three groups; however, no positive association between vitamin D and NAFLD was observed. The inconsistency of these studies might arise from differences in the study populations, sample sizes and methods for diagnosing NAFLD.
We carried out a large-sample, cross-sectional survey to analyse the association between vitamin D levels and NAFLD in East China.
Methods
Survey on Prevalence in East China for Metabolic Diseases and Risk Factors-China study
East China comprises Shanghai and seven provinces (sixteen areas) with a population of approximately 395 million, accounting for 29·2 % of the people in China. People of Han Chinese ethnicity comprise 99·5 % of the population. Survey on Prevalence in East China for Metabolic Diseases and Risk Factors-China is a population-based, cross-sectional survey of the prevalence of metabolic diseases and risk factors in East China. The registration number of the trial is ChiCTR-ECS-14005052 (www.chictr.org). We used a stratified cluster sampling method. In every study site, data collection was performed by the same staff group from the Department of Endocrinology of the Shanghai Ninth People’s Hospital, which is affiliated to the Shanghai Jiao Tong University School of Medicine. The staff members were trained according to a standard protocol that familiarised them with the specific tools and methods used in the survey. The trained staff members used a questionnaire to collect information on demographic characteristics, medical history and lifestyle risk factors. From February to June 2014, this study was performed in an urban area in Shanghai and Jiangxi Province and in a rural area in Shanghai, Zhejiang and Jiangxi Province( Reference Wang, Kuang and Han 10 ). Adults aged 18 years and above who were Chinese citizens and had lived in their current residence for 6 months or longer were selected and invited to join our study. Individuals with severe communication problems, acute illness and unwillingness to participate were excluded from the study in the first exclusion phase. A total of 7200 participants remained after the first phase of exclusion. In the next step, we excluded participants who had missing laboratory results (n 183) and questionnaire data (n 112) and were younger than 18 years of age (n 6). Subsequently, 6899 subjects were enrolled. Further, subjects with history of excessive alcohol consumption and self-reported viral hepatitis were excluded. In addition, patients taking vitamin supplements and without vitamin D results were excluded. After these patients were excluded, 5066 patients were included into the final cohort of this study (Fig. 1 shows the protocol of the study design). The study protocol was approved by the Ethics Committee of the Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (approval number 2013(86)). The research was conducted according to the Declaration of Helsinki. All the participants provided their written informed consent before data collection.

Fig. 1 Flow chart of sampling frame and participants selected from Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT)-China. Flow chart of SPECT-China. In total, with several exclusion criteria, 5066 participants were enrolled. NAFLD, non-alcoholic fatty liver disease; 25(OH)D3, 25-hydroxyvitamin D3.
Measurements
Body weight, height and blood pressure were measured using standard methods, as previously described( Reference Xu, Wang and He 11 ). Waist:hip ratio was calculated from the waist circumference measured on bare skin as the narrowest circumference between the 10th rib and the iliac crest and hip circumference at the widest point of the gluteal muscles. BMI was calculated as weight in kilograms divided by height in metres squared.
Serum sample collection and biochemical analysis
Venous blood samples were drawn after an overnight fast of at least 8 h. The blood samples for the plasma glucose test were collected into vacuum tubes with anticoagulant sodium fluoride and centrifuged immediately, within 1 h after collection. The blood samples were stored at −20°C after collection and shipped within 2–4 h of collection by air on dry ice to a central laboratory, which was certified by the College of American Pathologists. The glycated Hb (HbA1c) level was assessed by HPLC (MQ-2000PT; Shanghai HuiZhong Medical Technology Co. Ltd.). The plasma glucose and lipid profile including total cholesterol, TAG, HDL-cholesterol and LDL-cholesterol were measured using a Beckman Coulter AU 680 (Beckman Coulter) instrument. A chemiluminescence immunoassay (Siemens ADVIA Centaur XP; Siemens) was used to detect 25(OH)D3. Insulin resistance was estimated by calculating the homoeostatic model assessment for insulin resistance (HOMA-IR) index( Reference Matthews, Hosker and Rudenski 12 ), and the fasting insulin (FINS, pmol/l)×fasting glucose (mg/dl)/(22·5×6·965) levels were measured.
Non-alcoholic fatty liver disease evaluation
Liver ultrasonography (US) was performed to assess the degree of steatosis. The US examinations were performed by the same three operators by consensus. All imaging studies were performed in the fundamental brightness mode (B-mode). US findings were analysed based on criteria( Reference Hamaguchi, Kojima and Takeda 13 ) for US diagnosis in humans, according to the changes in liver echogenicity, classified into two patterns: normal, homogeneous liver parenchyma with medium-level echogenicity and a regular hepatic surface; and fatty liver, discrete coarse and heterogeneous parenchymal echogenicity and dotted, irregular or nodular hepatic liver surface.
Statistics
Statistical Package for the Social Sciences version 19.0 software was used for the analysis. All the analyses were two-sided; P<0·05 was considered to be statistically significant. All reported P values were two-sided. The general characteristics are summarised as means and standard deviations for continuous variables or as a number with proportion for categorical variables. To test for differences in characteristics between the participants with and without NAFLD, and among 25(OH)D quantiles, the Kruskal–Wallis test and ANOVA were used for continuous variables with skewed distribution and normal distribution, respectively.
OR and 95 % CI were calculated using logistic regression to determine the risk of NAFLD for each quartile of 25(OH)D. Model 1 controlled for age (continuous variable) and sex. Model 2 additionally controlled for smoking status, BMI, alanine aminotransferase (ALT) level, systolic blood pressure (SBP), TAG, HDL-cholesterol, LDL-cholesterol and diabetes.
Results
General characteristics of the study subjects
NAFLD was found in 2193 subjects (43·3 %). In the NAFLD group, the number of participants undergoing antihypertensive and hypolipemic treatment were 292 (13·32 %) and 18 (0·82 %), whereas in non-NAFLD group the numbers were 159 (5·53 %) and 7 (0·24 %), respectively. The subjects with NAFLD showed slightly lower vitamin D levels (40·05 (sd 9·83) v. 40·98 (sd 10·80) nmol/l) and more unfavourable metabolic profiles. The NAFLD group had higher HbA1c (5·48 (sd 0·95) v. 5·20 (sd 0·70) %) and elevated fasting plasma glucose (FPG) levels (5·83 (sd 1·50) v. 5·42 (sd 1·04) mmol/l). In addition, the NAFLD group showed significantly higher BMI (25·96 (sd 3·38) v. 22·62 (sd 2·95) kg/m2) and HOMA-IR levels (1·98 (sd 2·68) v. 1·26 (sd 1·30)) than did the non-NAFLD group. Further characteristics of the study subjects with and without NAFLD are presented in Table 1.
Table 1 Demographic and general characteristics of the study participantsFootnote * (Mean values and standard deviations for continuous variables)

NAFLD, non-alcoholic fatty liver disease; WHR, waist:hip ratio; FPG, fasting plasma glucose; HbA1c, glycated Hb; TC, total cholesterol; FINS, fasting insulin; HOMA-IR, homoeostasis model assessment-insulin resistance; ALT, alanine aminotransferase; AST, aspartate transaminase; 25(OH)D, 25-hydroxyvitamin D.
* The Mann–Whitney U and Student’s t test was used for skewed and normal distribution, respectively.
The prevalence of different vitamin D status in the normal and non-alcoholic fatty liver disease groups
The average of 25(OH)D3 levels in our study was 40·58 nmol/l, which did not reach adequate 25(OH)D3 levels (50 nmol/l), and 84·56 % of the subjects showed a 25(OH)D3 level <50 nmol/l, which indicated vitamin D insufficiency; the percentage of vitamin D insufficiency in the normal group and the NAFLD group was similar (82·91 v. 86·73 %). Fig. 2 shows that VDD in men is not as severe as that in women (78·05 v. 87·55 %); however, men with NAFLD showed a lower than normal vitamin D level (84·87 v. 70·05 %), and this finding was not observed in women (88·02 v. 87·35 %).

Fig. 2 Prevalence of vitamin D (Vit D) insufficiency in different participants. Prevalence of serum Vit D insufficiency among total participants, the normal group and the non-alcoholic fatty liver disease (NAFLD) group. These values were also diagraph analysed in male and female groups. Vit D insufficiency was defined as levels <50 nmol/l (![]() ), and Vit D sufficiency was defined as levels >50 nmol/l (
), and Vit D sufficiency was defined as levels >50 nmol/l (![]() ).
).
Correlations between vitamin D and the prevalence of non-alcoholic fatty liver disease
To more effectively reveal the relationship between vitamin D and NAFLD, we analysed the association between serum vitamin D levels and the prevalence of NAFLD in men and women (Fig. 3). We divided the subjects into four groups according to their vitamin D level quartiles. We observed a significantly higher prevalence of NAFLD in men with lower vitamin D (63·1 v. 38·8 %), whereas the association in women was not obvious (38·9 v. 37·8 %).

Fig. 3 The prevalence of non-alcoholic fatty liver disease (NAFLD) according to 25-hydroxyvitamin D (25(OH)D) quartiles (Q) in men and women. 25(OH)D Q were divided into Q1: <33·33 nmol/l, Q2: 33·33–38·87 nmol/l, Q3: 38·87–45·68 nmol/l, Q4: >45·68 nmol/l. ![]() , Non-NAFLD;
, Non-NAFLD; ![]() , NAFLD.
, NAFLD.
The metabolic and biochemical parameters with different vitamin D levels of the subjects
The subjects were divided into four groups according to the quartiles of vitamin D levels in men and women (Table 2). With an increased vitamin D level, some metabolic variables such as BMI and lipid profile were ameliorated significantly in men, whereas other variables including FPG and HbA1c did not improve. In women, the BMI and FPG showed no significance among the different vitamin D groups, and only the TAG level was ameliorated with ascending vitamin D levels.
Table 2 The characteristics of the study sample by 25-hydroxyvitamin D (25(OH)D) quartiles (Q) in men and womenFootnote * (Mean values and standard deviations for continuous variables)
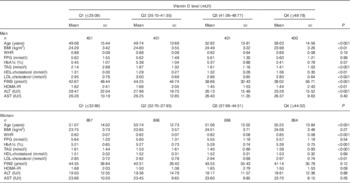
WHR, waist:hip ratio; FPG, fasting plasma glucose; HbA1c, glycated Hb; FINS, fasting insulin; HOMA-IR, homoeostasis model assessment-insulin resistance; ALT, alanine aminotransferase; AST, aspartate transaminase.
* The Kruskal–Wallis test and ANOVA were used for continuous variables with skewed distribution and normal distribution, respectively.
The association between vitamin D and non-alcoholic fatty liver disease by logistic regression analyses
Table 3 shows the association between vitamin D and NAFLD according to our binary logistic regression analyses. In the multivariate-adjusted model 1, after adjustment for age and sex, the OR for NAFLD in the lowest compared with the highest quartile of serum vitamin D was 1·64 (95 % CI 1·39, 1·94). After further adjustment for the smoking status, BMI, ALT, SBP, TAG, HDL-cholesterol, LDL-cholesterol and diabetes, the risk for the prevalence of NAFLD decreased across the vitamin D quartiles, and the OR in the lowest compared with the highest quartile of serum vitamin D was 1·54 (95 % CI 1·26, 1·76). As the OR were attenuated, the gradually increasing trend with decreasing vitamin D showed significance.
Table 3 Associations of the vitamin D level with non-alcoholic fatty liver diseaseFootnote * (Odds ratios and 95 % confidence intervals)

25(OH)D, 25-hydroxyvitamin D; Ref. referent values; ALT, alanine aminotransferase; SBP, systolic blood pressure.
* Logistic regression analysis was used.
† Model 1 controls for age (continuous variable) and sex.
‡ Model 2 additionally controls for the smoking status, BMI, ALT, SBP, TAG, HDL-cholesterol, LDL-cholesterol and diabetes.
Discussion
In our study, we found that the NAFLD prevalence was 43·3 % in East China, which exceeded the prevalence that had previously been reported in China( Reference Ong and Younossi 14 ). The subjects with NAFLD showed slightly lower vitamin D levels and more unfavourable metabolic profiles including a higher HbA1c level as well as elevated FPG, BMI and HOMA-IR. The average 25(OH)D3 level in our study was 40·58 nmol/l, which did not reach the adequate 25(OH)D3 levels (50 nmol/l): 2·13 % of the subjects showed a 25(OH)D3 level <25 nmol/l, which indicated VDD, 82·43 % had a level <50 nmol/l and 1·05 % demonstrated a level above the 75 nmol/l target suggested by vitamin D experts( Reference Ross, Manson and Abrams 15 , Reference Holick, Binkley and Bischoff-Ferrari 16 ). FINS in the NAFLD group was apparently increased compared with the NAFLD group, which may be explained by the mechanism of the metabolic syndrome with insulin resistance. VDD and NAFLD have indirect and direct associations with obesity and a sedentary lifestyle, and therefore it is expected that VDD would co-exist with NAFLD. Some studies have explored the potentially causative relationship between VDD and NAFLD, and clinical trials have identified trends in the epidemiology of VDD and NAFLD. In a study of 262 patients referred to an endocrinology clinic, the relationship between NAFLD and VDD was confirmed, regardless of age, sex, TAG levels and insulin resistance( Reference Barchetta, Angelico and Del Ben 17 ). Targher et al.( Reference Targher, Bertolini and Scala 18 ) verified this point and confirmed the relationship between liver histology and vitamin D. In our study, we found that VDD had an independent relationship (OR 1·54; 95 % CI 1·26, 1·76) with NAFLD. These results are in accordance with the studies of Hao et al.( Reference Hao, Ma and Luo 19 ) and Lu et al.( Reference Lu, Pan and Hu 20 ) who concluded that high serum 25(OH)D3 levels were a protective factor against NAFLD after adjusting for risk factors. How does it work, and what is the mechanism? Kong et al.( Reference Kong, Zhu and Bai 5 ) suggested a novel mechanism for NASH development, by which VDD down-regulates ileal apical sodium-dependent bile acid cotransporter (iASBT) expression, resulting in a poor bile acid pool and elevation of hepatic lipogenesis and inflammation. In conclusion, vitamin D and bile acid sequestration may be explored as new strategies to treat or prevent NASH. The inverse association between serum vitamin D levels and the metabolic syndrome may also be frequently explained by the following. First, the answer might lie in the mechanism of the ‘two-hit’ hypothesis of the pathogenesis of NAFLD, which includes a number of metabolic pathways. VDD could positively affect these pathways, including hormonal, immunological and cellular differentiation mechanisms. Second, VDD could affect adipocytokines (i.e. adiponectin, resistin and omentin) and pro-inflammatory cytokines (i.e. TNF-α and IL-6), which are secreted by adipose tissue and have important roles in the formation of NAFLD( Reference Fantuzzi 21 – Reference Roth, Elfers and Figlewicz 23 ). Sharifi et al ( Reference Sharifi, Amani and Hajiani 24 ) has reported that improved vitamin D status led to amelioration in serum C-reactive protein (CRP) and malondialdehyde in patients with NAFLD, and vitamin D might be considered an adjunct therapy to attenuate systemic inflammation and lipid peroxidation with other treatments of NAFLD patients. We found that higher vitamin D levels exerted a protective effect on NAFLD, and we recommend higher daily intake of vitamin D.
Table 2 shows that BMI increased with the vitamin D quartiles, especially in women, but there was no significance, whereas in men the change was opposite and in accordance with other studies. The reason may be the testosterone levels in males, which may play an important role. Although this is a surprising outcome, it is a fact, explained in the study by Wang et al.( Reference Wang, Li and Han 25 , Reference Wang, Han and Li 26 ).
Another finding is that VDD in men is not as severe as that in women, but the NAFLD in men displayed an obviously lack of normal vitamin D levels, whereas this difference was not observed in women. We also observed a significantly higher prevalence of NAFLD in subjects with lower vitamin D levels among men, whereas this association in women was not obvious. This suggests that vitamin D supplements may ameliorate the fatty liver prevalence in men better than in women, but the mechanism still requires further exploration.
Limitations
Our study had some limitations. Although liver biopsy is regarded as the gold standard for detecting hepatic statuses( Reference Kobyliak and Abenavoli 27 ), liver biopsy was not feasible in the case of this population-based study. US is the most suitable diagnostic test of choice for NAFLD because it is non-invasive, safe, sensitive and specific in terms of identifying fatty infiltration. However, as this was judged by the subjective feeling of the operators, the occurrence may deviate, but our US examinations were performed by the same two operators by consensus so as to minimise the deviation.
In our study, we found that female patients with highest HbA1c and fasting glucose had the highest vitamin D levels, whereas in men the results showed no significance, which is contradictory to previous studies. Zhou & Ye( Reference Zhou and Ye 28 ) suggested that low levels of 25(OH)D is significantly associated with the occurrence of type 2 diabetes mellitus (T2DM) complicated with lower extremity arterial disease. Moreover, Lim et al.( Reference Lim, Kim and Choi 29 ) also reported that vitamin D metabolism may play a role in T2DM pathogenesis independently of known risk factors. We think this may be related to the age factor in the women group, with increase in age HbA1c levels elevated significantly and diabetes mellitus prevalence increased.
As HOMA index is usually used to calculate insulin resistance in subjects with the metabolic syndrome without diabetes, or in those with diabetes without therapy, the use of HOMA-IR may be debatable in diabetic individuals. However, many studies( Reference Mojiminiyi and Abdella 30 – Reference Sarafidis, Lasaridis and Nilsson 33 ) have also demonstrated that HOMA-IR is significantly associated with diabetes.
In our study, 5066 persons were enrolled, among them 539 participants had diabetes, and thirty-eight participants were using insulin treatment. In the sensitivity analyses, we have re-calculated the HOMA-IR among the participants excluding subjects with diabetes, and the results of comparison are in accordance with the former.
Conclusion
The vitamin D level in most of the population is insufficient, and we have demonstrated the relation between vitamin D levels and NAFLD. Low vitamin D levels conferred a significantly higher risk of developing NAFLD, especially in men in East China. This association remained significant even after adjusting for the BMI and some risk factors of NAFLD. Thus, it may be worthwhile to monitor serum vitamin D levels to screen for NAFLD, and vitamin D supplementation is recommended. The mechanisms underlying the association between vitamin D and NAFLD are still largely unclear. Further studies are necessary to uncover new mechanisms linking vitamin D and NAFLD.
Acknowledgements
The authors thank all the team members and the participants from Shanghai, Zhejiang Province and Jiangxi Province in the Survey on Prevalence in East China for Metabolic Diseases and Risk Factors-China study.
This study was supported, in part, by the National Natural Science Foundation of China (grant numbers 81270885, 81070677 and 81300653); Clinical Potential Subject Construction of Shanghai Jiao Tong University School of Medicine (grant number 2014); the Ministry of Science and Technology in China (grant number 2012CB524906); the Science and Technology Commission of Shanghai Municipality (grant number 14495810700); and funds for outstanding academic leaders in Shanghai (grant number 12XD1403100).
H.-L. Z., N.-J. W., B. H. and Q. L. collected the data and drafted the manuscript. Y. C., C.-F. Z., Y.-C. C., F.-Z. X., Z. C., C.-X. Z. and M. L. participated in the study design and performed the statistical analysis. Y.-L. L. conceived the study and participated in its design and coordination and helped to draft the manuscript. All the authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.








