Cr is an essential trace element and its beneficial effects on health are well documented in humans and other animals( Reference McCarty 1 , Reference Mertz 2 ) – for example, the element is an integral component of the glucose tolerance factor( Reference Lukaski 3 ) that influences the activity of insulin. Supplementation of the diet with trivalent Cr (Cr(III)) can be achieved using the salt CrCl3. Other sources of Cr such as low molecular weight organic Cr complexes such as picolinic acid and nicotinate salt forms( Reference Laschinsky, Kottwitz and Freund 4 – Reference Wang, Zhang and Russell 6 ) provide a myriad of benefits with higher organic bioavailability than the inorganic forms( Reference Goodman and Check 7 ) that are most often used as dietary supplements. Although Cr nanocomposites have even higher bioavailability than organic sources of Cr( Reference Eybe, Audinot and Udelhoven 8 ), their greater cost has inhibited widespread use. In humans and animal husbandry, there is a need to explore a cheap and convenient organic source of Cr for use in industrial applications.
In the south of China, summer temperatures can reach 35°C or more. Such high temperatures can cause heat stress to both humans and animals. It has been demonstrated that stress conditions increase the urinary excretion of Cr( Reference Anderson, Bryden and Polansky 9 ) and may exacerbate a marginal Cr deficiency( Reference Rubin, Miller and Ryan 10 ). It is also well known that stressful conditions may retard growth, depress food intake, change hormone release, increase disease susceptibility or lead to behavioural changes( Reference Xie, Tang and Lu 11 , Reference Toghyani, Shivazad and Gheisari 12 ). Some studies have reported that supplementation of the diet with Cr can alleviate the detrimental effects of heat stress( Reference Toghyani, Shivazad and Gheisari 12 , Reference Sahin, Ozbey and Onderci 13 ). Dietary Cr supplementation has also been shown to have a positive effect on growth performance and feed conversion ratio in poultry and dairy cows( Reference Leiva, Cooke and Aboin 14 , Reference Mirfendereski and Jahanian 15 ). Whether dietary supplementation with Cr might play similar roles against heat stress in humans and other model animals needs further study.
Bacillus subtilis is a probiotic bacterium that is widely used in diets of both humans and animals( Reference Khochamit, Siripornadulsil and Sukon 16 , Reference Park and Kim 17 ). Oral administration of B. subtilis can exert a range of beneficial effects including optimising the balance of intestinal microbiota, prevention and treatment of some diarrhoeal diseases, improvement of growth, enhancement of immune responses and reduction of serum cholesterol levels( Reference Jeong and Kim 18 – Reference Lei, Li and Wang 20 ). For these reasons, B. subtilis has attracted considerable attention as a potentially beneficial dietary supplement for human and animal health.
We thought that it might be worthwhile to explore whether the combined use of organic Cr and B. subtilis might have a greater effect on regulating body metabolism. To test this hypothesis, we grew Cr-enriched B. subtilis (CEBS), using certain concentrations of Cr, under the appropriate environmental conditions to enable the efficient conversion of inorganic Cr into organic forms. Thus, CEBS combines the virtues of B. subtilis and those of organic Cr and might induce an enhanced response to dietary supplementation. We tested CEBS using laboratory mice (Kunming strain), an experimental model in widespread use for such metabolic studies( Reference Li, Ding and Niu 21 , Reference Yang, Huang and Qin 22 ). The ultimate aim of our study was to determine whether CEBS supplementation could play a role in modulating body growth, lipid metabolism in healthy mice in summer conditions and might be of use for preventing heat stress-related metabolic diseases in humans and animals.
Methods
Generation of chromium-enriched Bacillus subtilis
B. subtilis was provided by the Institute of Animal Husbandry and Veterinary Medicine, Anhui Academy of Agricultural Sciences in China. We grew the cells in medium containing CrCl3·6H2O (Cr concentration 30 μg/ml). After culture for 36 h, the cells were harvested when the live B. subtilis reached 1·0×109 colony-forming unit (CFU)/ml. Subsequently, cell-free culture supernatant (CFCS) was prepared by centrifuging 50 ml of the culture medium for 15 min at 3000 g and sterilising by filtration through a 0·45-μm membrane filter (Millipore Corporation). The precipitate from the centrifugation step was also collected. The Cr concentrations of the medium, CFCS and precipitate were 30 μg/ml, 1·2 μg/ml and 1438·3 μg/g, respectively. The Cr in the CFCS was mainly in the inorganic form, whereas in the precipitate it was mainly in the organic form. The rate of conversion to organic Cr was 96 %. In order to determine the Cr form, we extracted proteins and nucleic acids from the CEBS and measured the concentration of Cr bound to these molecules. The analysis indicated that 90·86 % of total organic Cr was bound to proteins, whereas 6·37 % of Cr was bound to nucleic acids. We concluded that the Cr in CEBS was mainly in the organic form.
Animals and groups
A total of ninety-six male mice (Kunming strain) with an initial live average body mass of 11·5 g were obtained from Anhui Provincial Hospital, Anhui, China. Mice were quarantined for a minimum of 5 d before testing. All animals were asymptomatic and were released from quarantine before the start of the study. Mice were housed in clean cages. The feed duration was 30 d from 5 July to 4 August. The ninety-six mice were divided randomly into four groups, each with three replicates of eight animals: group I was fed common basal feedstuff and given clean water (the concentration of Cr was 0·06 μg/ml); group II received basal feedstuff and water supplemented with CEBS (0·30 μg Cr/ml, 107 CFU/ml live B. subtilis); group III received basal feedstuff and water supplemented with 1·537 μg/ml CrCl3·6H2O (0·30 μg Cr/ml); and group IV received basal feedstuff and water supplemented with 107 CFU/ml B. subtilis. Supplementation with CEBS and normal B. subtilis was through drinking water by adding cells suspended in the fermented medium. The use of animals for this research was approved by the Institution of Animal Science and Welfare of Anhui Province (number: IASWAP2014070518). The temperature of the mouse facility was 33±3°C and the relative humidity was 55–60 %.
Growth performance and sample collection
Initial body weight and feed consumption were recorded. After 15 and 30 d, the body weights of the mice were obtained after an overnight fast. The change in body weight (average daily gain (ADG)) in relation to food intake (ratio of feed:gain (F:G)) was calculated as follows:
![]() $$\eqalignno{ & {\rm ADG}\,{=}\,{\rm body}\,{\rm increase}\,({\rm g})\!/\!{\rm number}\,{\rm of}\,{\rm days}, \cr & {\rm F}\colon\!{\rm G}\,{=}\,{\rm mass}\,{\rm of}\,{\rm food}\,{\rm intake}\left( {\rm g} \right)\!/{\rm body}\,{\rm increase}\,({\rm g}). $$
$$\eqalignno{ & {\rm ADG}\,{=}\,{\rm body}\,{\rm increase}\,({\rm g})\!/\!{\rm number}\,{\rm of}\,{\rm days}, \cr & {\rm F}\colon\!{\rm G}\,{=}\,{\rm mass}\,{\rm of}\,{\rm food}\,{\rm intake}\left( {\rm g} \right)\!/{\rm body}\,{\rm increase}\,({\rm g}). $$
After 30 d, ten mice from each treatment group were selected, fasted for 12 h and then tissues and blood samples were harvested under general halothane anaesthesia. All the blood samples were collected in 2·0-ml sterile heparinised tubes for plasma biochemical assays (described below). The heart, liver, kidney, spleen and skeletal muscles were collected with excess fat and veins carefully trimmed away; these tissues were frozen in liquid N2 and stored at −80°C until analysis.
These mice were killed, and their caeca were removed under aseptic conditions. The tissues were stored in sterile plastic tubes in boxes packed with ice and were immediately sent to our laboratory for plate counting of micro-organisms( Reference Mountzouris, Tsirtsikos and Kalamara 23 ) (eosin methylene blue agar for Escherichia coli, de Man, Rogosa and Sharpe agar for Lactobacillus, Staphylococcus plate-count agar for Staphylococcus and blood liver broth agar for Bifidobacterium using the pour plate method; the assays were repeated three times).
Chromium assay
A ZEEnit 700 P atomic absorption spectrometer (Analytik Jena) was used for assaying Cr levels in tissues. The analytical lines 357·869 nm for Cr were used; peak volume selected absorbance (PVSA, i.e., the integrated absorbance of the centre pixel (CP) only, or summated over three pixels around the line core (centre pixel plus the adjacent ones, CP71)) was used for signal evaluation, corresponding to a spectral interval of 2·3 pm (CP) for Cr at 428·972 nm. All the measurements were performed by the method described by Afridi et al.( Reference Afridi, Kazi and Talpur 24 ). Samples (0·1–0·2 g) of the heart, liver, kidney, spleen and skeletal muscles and 0·2 g feedstuff from all four groups were placed in beakers and digested by adding 10 ml of a nitric acid–perchloric acid (HNO3–HClO4) mixture. The mixture was heated on a sand bath until fumes appeared (the temperature was controlled at 200°C by monitoring the sand) and the solution had mostly evaporated. After cooling, 5 ml HNO3 was added, and the heating procedure was repeated at 180°C. The cooled remainder was made up to 10 ml with distilled water. Eight replicates were used for each group.
Determination of insulin receptor mRNA levels using quantitative real-time PCR
The following primers were used to amplify insulin receptor (IR): forward, 5′AACTCCCTGAAATGACAGTGAAGA3′, and reverse, 5′TGACTGAACACTAACCCGAACCT3′. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as the internal control and was amplified using the primers: forward, 5′CCATCTTCCAGGAGCGAGAT3′, and reverse, 5′AAACATGGGGGCATCAGC3′.
Total RNA was isolated from skeletal muscle using TRIZOL (Invitrogen) according to the manufacturer’s protocol. Dried RNA pellets were re-suspended in 40 μl diethylpyrocarbonate-treated water. The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm. The RNA was either used immediately or stored at −70°C before complementary DNA (cDNA) synthesis. First-strand cDNA was synthesised from 2 μg of total RNA using Oligo dT primers and SuperScript II Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. Synthesised cDNA was diluted to one-tenth concentration with sterile water and stored at −20°C before use.
Quantitative real-time PCR was performed on an ABI PRISM 7500 Detection System (Applied Biosystems). Amplification was performed using a 20-μl reaction mixture containing 10 μl 2×SYBR Green I PCR Master Mix (Toyobo), 1 μl of diluted cDNA, 1 μl each primer (10 μmol/l) and 7 μl of PCR-grade water. The amplification procedure for IR and GAPDH consisted of a 95°C step for 30 s, followed by 40 cycles of 95°C for 5 s, 60°C for 34 s and 72°C for 1 min and an extension step at 72°C for 7 min.
Western blotting
Total protein extracted from the muscles was harvested. The homogenate was centrifuged at 14 000 rpm for 20 min, and the supernatant containing the proteins was then collected. Total protein was estimated by the Bradford method. Using SDS-PAGE, proteins were separated after loading 120 μg/lane; the separated proteins were transferred onto a polyvinylidene fluoride membrane by overnight incubation with 5 % non-fat skimmed milk in Tris-buffered saline/0·1 % Tween 20 (TBST) buffer at 4°C. Non-specific binding sites were blocked. The membrane was washed 3×20 min times with TBST and then incubated with appropriate polyclonal primary antibodies: IRS-1 antibodies( Reference Liu, Han and Cao 25 ) (mouse monoclonal antibody, dilution 1:1000; Abcam Company) and GAPDH( Reference Sakellariou, Davis and Shi 26 ) (mouse monoclonal antibodies, dilution 1:1000; Abcam Company). The membrane was then washed 3×20 min with TBST and incubated with an anti-rabbit secondary antibody for 1 h at room temperature, washed and then incubated with the substrate for 1 min. The membrane was exposed to HyBlot film (Denvill) for 30 s in a dark room. The densities of the bands were analysed by ImageAlpha software. The GAPDH signal in each sample was used to normalise the insulin signal.
Plasma hormone, glucose and lipid analyses
We centrifuged mouse blood at 3000 g for 10 min and collected the plasma. Mouse plasma insulin levels were determined using an ELISA kit (Nanjing Jiancheng Bioengineering Institute), and plasma glucose concentrations were measured by a colourimetric hexokinase glucose assay (Sigma Diagnostics). Plasma total cholesterol (TC), TAG, LDL-cholesterol and HDL-cholesterol concentrations were measured using the appropriate detection kits (Nanjing Jiancheng Bioengineering Institute).
Statistical analysis
Statistical analyses of the data were performed using SPSS 16.0 (SPSS Inc.). Data are presented as mean values with their standard errors. Differences between groups were compared using ANOVA. Differences between means were assessed by Tukey’s honestly significant difference test of post hoc multiple comparisons. Data on body weight, ADG, average daily feed intake (ADFI) and F:G was statistically processed as repeated measurements. A P value <0·05 was considered statistically significant.
Results
Growth performance
The growth performance of mice in the different treatments is shown in Fig. 1–4. Mice that received CEBS or normal B. subtilis had higher body weights and ADG after 15 d than control mice or those that received inorganic Cr supplementation (P<0·05). The index of F:G in CEBS- and B. subtilis-treated mice was significantly lower than those of the control (P<0·05) at 15 d.

Fig. 1 Effects of different treatments on mice body weight. The mice were treated with control, Cr-enriched Bacillus subtilis (CEBS), inorganic Cr and B. subtilis after 15 and 30 d. Values are means, with standard errors represented by vertical bars. Data of body weight were statistically processed as repeated measurements. Mean value was significantly different from that of the control group: * P<0·05, † P<0·05, †† P<0·01. ![]() , 15 d;
, 15 d; ![]() , 30 d.
, 30 d.

Fig. 2 Effects of different treatments on mice average daily gain (ADG). The mice were treated with control, Cr-enriched Bacillus subtilis (CEBS), inorganic Cr and B. subtilis after 15 and 30 d. Values are means, with standard errors represented by vertical bars. Data of ADG were statistically processed as repeated measurements. Mean value was significantly different from that of the control group: * P<0·05, † P<0·05, †† P<0·01. ![]() , 15 d;
, 15 d; ![]() , 30 d.
, 30 d.

Fig. 3 Effects of different treatments on mice average daily feed intake (ADFI). The mice were treated with control, Cr-enriched Bacillus subtilis (CEBS), inorganic Cr and B. subtilis after 15 and 30 d. Values are means, with standard errors represented by vertical bars. Data of ADFI were statistically processed as repeated measurements. There were no differences among groups between 15 and 30 d (P>0·05). ![]() , 15 d;
, 15 d; ![]() , 30 d.
, 30 d.

Fig. 4 Effects of different treatments on mice ratio of feed:gain (F:G). The mice were treated with control, Cr-enriched Bacillus subtilis (CEBS), inorganic Cr and B. subtilis after 15 and 30 d. Values are means, with standard errors represented by vertical bars. Data of ratio of F:G were statistically processed as repeated measurements. Mean value was significantly different from that of the control group: * P<0·05, † P<0·05, †† P<0·01. ![]() , 15 d;
, 15 d; ![]() , 30 d.
, 30 d.
After 30 d, final body weights and ADG in the CEBS group were significantly higher compared with the control and the inorganic Cr-supplemented groups (P<0·01). Mice in treatment group IV also had a higher final body weight and ADG than control mice (P<0·05). Over the entire feeding duration, the F:G in the CEBS group was the lowest (P<0·01). The F:G index of group IV was lower compared with the control and inorganic Cr groups (P<0·01). There were no differences in the ADFI among the four groups either after 15 or after 30 d.
Caecal microflora
The caecal microflora in the different groups of mice was examined using the plate method (Table 1). Mice given CEBS or normal B. subtilis had lower numbers of E. coli and Staphylococcus compared with the control mice (P<0·05). There were no differences between the control and inorganic Cr-supplemented groups (P>0·05). The numbers of Lactobacillus and Bifidobacterium in the CEBS and normal B. subtilis groups increased significantly compared with the control and inorganic Cr-supplemented groups (P<0·05).
Table 1 Effects of different treatments on caecal microflora (log10 colony-forming units/g) (Mean values with their standard errors)
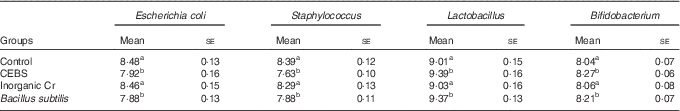
CEBS, Cr-enriched B. subtilis.
a,b Mean values within a column with unlike superscript letters were significantly different (P<0·05).
Tissue chromium concentrations
Cr concentrations in the heart, liver, spleen, kidney and skeletal muscles were measured (Table 2). The results indicated that mice fed inorganic Cr had significantly more Cr in these tissues compared with the control and B. subtilis supplementation groups (P<0·01). Mice in the CEBS group had the highest Cr concentrations in tissues, except for the spleen; for this organ, there was no significant difference with the inorganic Cr group.
Table 2 Effects of different treatments on tissue chromium content (Mean values with their standard errors)

ppb, Parts per billion; CEBS, Cr-enriched B. subtilis.
Aa,Bb,Cc Mean values within a column with unlike superscript letters were significantly different (P<0·01).
Insulin receptor expression
The level of IR mRNA in skeletal muscles was measured (Fig. 5). Compared with control mice, there was a significant increase in IR mRNA in mice treated with inorganic Cr and CEBS. The difference between control and B. subtilis groups was not significant. Similar results were found for IR protein levels (Fig. 6) – that is, mice treated with inorganic Cr or CEBS had higher levels of IR protein.

Fig. 5 Effects of different treatments on mRNA levels of insulin receptor (IR) in skeletal muscles. The mice were treated with control, Cr-enriched Bacillus subtilis (CEBS), inorganic Cr and B. subtilis. IR mRNA in skeletal muscles was measured by quantitative real-time PCR, and the ratio of the levels of IR mRNA:glyceraldehyde phosphate dehydrogenase (GAPDH) internal control was used for statistical comparison. Values are means, with standard errors are represented by vertical bars. Mean value was significantly different from that of the control group by one-way ANOVA followed by Tukey’s multiple comparison tests: * P<0·05, ** P<0·01.

Fig. 6 Effects of different treatments on protein levels of insulin receptor (IR) in skeletal muscles. Mice were treated with control, Cr-enriched Bacillus subtilis (CEBS), inorganic Cr and B. subtilis. IR protein in skeletal muscles was measured by Western blotting, and the ratio of the levels of IR protein:glyceraldehyde phosphate dehydrogenase (GAPDH) internal control was used for statistical comparison. Values are means, with standard errors are represented by vertical bars. Mean value was significantly different from that of the control group by one-way ANOVA followed by Tukey’s multiple comparison tests: * P<0·05, ** P<0·01.
Plasma insulin, glucose and lipids
Mice treated with inorganic Cr showed similar levels of plasma insulin as controls (P>0·05; Table 3), whereas mice treated with CEBS showed enhanced insulin levels (P<0·01) compared with controls. CEBS-treated mice also had the lowest plasma glucose levels (P<0·05). Mice given inorganic Cr or B. subtilis had lower plasma glucose concentrations than the controls (P<0·05). The levels of lipids (TC, TAG, LDL-cholesterol) in mice given inorganic Cr were significantly lower compared with the controls, but were still higher than those of the CEBS group (P<0·05). The concentrations of HDL-cholesterol in the plasma of mice in the CEBS and inorganic Cr groups were higher compared with the control mice (P<0·05), the former being higher in CEBS than inorganic Cr treatment (P<0·05). The ratio of TC:LDL-cholesterol (TC:LDL-cholesterol) was calculated, which indicated that mice supplemented with CEBS had the highest ratio of all (P<0·01). Mice supplied with inorganic Cr showed higher TC:LDL-cholesterol compared with the control and B. subtilis groups (P<0·01). The results for the ratio of TC:HDL-cholesterol (TC:HDL-cholesterol) was opposite of that of TC:LDL-cholesterol. The ratio of the CEBS group was the lowest of all treatments (P<0·01), and that for inorganic Cr was higher than that for CEBS but lower compared with the control and the B. subtilis treatments (P<0·01).
Table 3 Effects of different treatments on plasma insulin, glucose and lipids (Mean values with their standard errors)
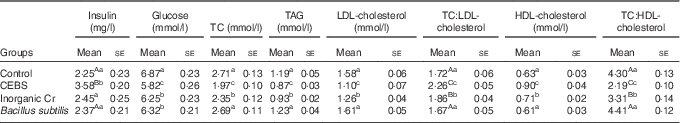
TC, total cholesterol; TC:LDL-cholesterol, ratio of TC:LDL-cholesterol; TC:HDL-cholesterol, ratio of TC:HDL-cholesterol; CEBS, Cr-enriched B. subtilis.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P<0·05).
Aa,Bb,Cc Mean values within a column with unlike superscript letters were significantly different (P<0·01).
Discussion
Although trivalent Cr (+3 oxidation state) is normally useable by organisms( Reference Schroeder 27 , Reference Offenbacher and Pi-Sunyer 28 ), under conditions of heat stress Cr is increasingly secreted from the body. Under stress conditions, supplementary Cr(III) may be necessary( Reference Moeini, Bahrami and Ghazi 29 , Reference Zhang, Weng and Wang 30 ). B. subtilis is often used as a probiotic and there are reports that it can be used to reduce heat stress in animals( Reference Deng, Dong and Tong 31 , Reference Moore, Globa and Pustovyy 32 ). Our experiments indicated that rates of growth were improved in mice given CEBS or normal B. subtilis supplements, whereas there was no improvement after inorganic Cr supplementation. These results indicate that supplementary inorganic Cr cannot improve growth performance. Mice given CEBS had higher average body weights and greater feed utilisation efficiency than controls. The F:G index of the CEBS group was the lowest over the entire feeding period, suggesting that this treatment was more efficient than B. subtilis alone in regulating body growth performance.
Probiotic supplements have been reported to modify the composition of the caecal microbiota( Reference Salazar, Binetti and Gueimonde 33 – Reference Shim, Ingale and Kim 35 ). Similarly, our results indicated that B. subtilis supplements could alter the bacterial flora in the caeca of treated mice, whereas inorganic Cr did not have a significant effect compared with the control. In this study, 0·3 μg/ml CrCl3 was added to the drinking water; this is an appropriate concentration for the treatment of animals( Reference Jiajun, Aiyun and Shanshan 36 , Reference Zha, Xu and Wang 37 ). The total Cr concentration in the CEBS treatment was 30 μg/ml; we therefore added a 1 % supplement to the water. The live B. subtilis reached 107 CFU/ml in the drinking water. Our results suggest that this supplementary dose of B. subtilis was suitable for the mice.
Previous studies have reported that Cr supplementation can increase the Cr content of tissues, although the results are varied( Reference Zha, Xu and Wang 37 – Reference Uyanik, Eren and Guclu 40 ). In our study, the Cr contents of the heart, liver, spleen, kidney and skeletal muscles were significantly increased with the addition of Cr in the inorganic form and as CEBS. In rats, the increase in tissue Cr following Cr supplementation is the greatest in the kidney, followed by the liver, with considerably smaller changes in the heart and skeletal muscle tissues( Reference Prescha, Krzysik and Zablocka-Slowinska 38 ). Our results showed that the supplemental Cr in CEBS had a significant influence on the Cr content of skeletal muscles in agreement with a previous report( Reference Zha, Xu and Wang 37 ). The effect of CEBS was greater than that for CrCl3 in the present experiment, indicating that CEBS had greater bioavailability as an organic Cr resource.
Skeletal muscles account for 80 % of insulin-stimulated glucose disposal in the body( Reference Baron, Brechtel and Wallace 41 , Reference Lang 42 ). Total RNA levels were increased in these studies by dietary supplementation with chromium picolinate. Therefore, we measured IR expression in skeletal muscles and found that total IR mRNA increased after 0·3 μg/ml inorganic Cr or after B. subtilis Cr supplementation. A previous study reported that 0·209 μg/ml Cr supplementation could increase insulin binding in cells and cause elevated IR mRNA levels in muscle cells in vivo ( Reference Qiao, Peng and Wang 43 ). Our Western blotting analysis supported these results as IR protein levels increased after inorganic Cr or B. subtilis Cr supplementation. The amount of IR protein has been shown to increase after chromium picolinate supplementation in diabetic animal models and human patients( Reference Wang, Zhang and Russell 6 , Reference Jain, Kahlon and Morehead 44 – Reference Chen, Chen and Liu 46 ). Diabetic patients tend to lose the ability to convert Cr into a form that might potentiate insulin action. In our experimental conditions in which mice were maintained at 33°C, the loss of Cr led to a deficiency. Therefore, supplemental Cr could play a crucial role in regulating IR expression.
The level of serum glucose is regulated mainly by insulin. Under normal conditions, body glucose content returns to a normal level after dietary restriction or stress via the action of the glucose tolerance factor. Cr plays a part in the insulin signalling auto-amplification mechanism by stimulating IR kinase activity( Reference Davis and Vincent 47 ). In the present study, we found that CEBS supplementation of drinking water increased the level of insulin and reduced plasma glucose levels. Likewise, supplementary inorganic Cr and B. subtilis reduced the plasma glucose levels and slightly increased insulin levels. Under heat stress conditions, supplemental Cr and B. subtilis might improve Cr metabolism and enhance the formation of glucose tolerance factor. TC in the body is regulated by the concentration of blood glucose and acetyl-CoA. An increase in fatty acids and a reduction in glucose mobilisation lead to a decrease in acetyl-CoA level, which regulates the synthesis of cholesterol. Our data suggested that the concentration of plasma TC and TAG were decreased in mice treated with inorganic Cr or B. subtilis. CEBS enhanced the metabolism of TC and TAG. HDL-cholesterol, synthesised mainly in the liver and the small intestine, plays an important part in eliminating serum cholesterol. The ratios of TC:HDL-cholesterol and TC:LDL-cholesterol are positively correlated with the incidence of CHD and atherosclerosis( Reference Hofman, Ott and Breteler 48 , Reference Walker, Register and Appt 49 ). We found that supplemental inorganic and B. subtilis Cr could reduce plasma LDL-cholesterol levels and TC:HDL-cholesterol and increase HDL-cholesterol levels and TC:LDL-cholesterol. These results led us to conclude that supplementation with Cr may enhance the metabolism of cholesterol under heat stress conditions.
In conclusion, feeding supplementary CEBS combined the benefits of Cr and probiotics and altered body growth and caecal microbiota, tissue Cr concentrations, IR expression and plasma biochemical profile in mice maintained under heat stress.
Acknowledgements
The work was sponsored by the fund of doctor startup project in Anhui Academy of Agricultural Sciences of China, grants from the Anhui Modern Agricultural Project for Pig Industry, the Anhui Swine Industry Technology System Project, the Anhui Finance Project for Animal Husbandry Development and the Anhui Academy of Agricultural Science and Technology Innovation Team Building Project (no.: 13C0405).
The contributions of the authors are as follows: J. Y. cultured and researched the CEBS, measured the mRNA and protein levels and wrote the paper. Y. X. fed the mice and recorded the growth data. K. Q. was involved in technical direction. W. Z. measured tissue Cr concentrations. D. W. measured the caecal microflora. C. W. analysed plasma hormones, glucose and lipids. The final manuscript was read and approved by all the authors.
The authors declare that they have no conflicts of interest.











