In the preface to his seminal book on oats, Welch( Reference Welch 1 ) states that ‘Oats have been likened to Cinderella, an attractive and productive servant, wholesome and dependable, almost thriving on neglect and disinterest, but overshadowed by more assertive if less attractive step-sisters. (O)ats remains a uniquely versatile crop and, like Cinderella, combines an air of robust romance with a powerful potential’. This truism will be expanded upon here and the case made for the future oats with respect to their place in agriculture and human nutrition as well as the consequences for oat breeding and the agrifood chain.
Oats (Avena sativa L.) have a long history of use as a nutritious foodstuff, with records suggesting that oats in a cultivated form have been in use since the Bronze Age( Reference Baum 2 ). They are a palatable and nutritious foodstuff, mainly supplying carbohydrate in the form of starch and with reasonably high lipid levels (Table 1). Furthermore, oats contain small quantities of several of the B vitamins, particularly thiamin, folic acid, biotin and pantothenic acid, and the protein contains a good balance of essential amino acids. Wholegrain products are significant contributors to our micronutrient intake, and oats are a good source of Mn, Mg, Se and Fe, as well as Ca, Zn and Cu( Reference Welch 1 ). In addition, oats are by far the predominant source of the soluble fibre β-glucan relative to other grains. Indeed, β-glucan (oat and barley) is the basis of the Food and Drug Administration (USA)( 3 )- and Joint Health Claims Initiative (UK)( 4 )-approved health claims highlighting this component's (and therefore oats’) ability to reduce blood cholesterol levels and the risk of CHD incidence (see Box 1, Stewart et al. ( Reference Stewart, Kennedy and Pavel 5 ) in this Supplement). The underpinning basis and extent of the impact of β-glucan on CHD are dealt with by Thies et al. ( Reference Thies, Masson and Boffetta 6 ) and the consequences of processing on oat nutritive and health-beneficial values by Decker et al. ( Reference Decker, Rose and Stewart 7 ).

* Data taken from Welch( Reference Welch 1 ) and the US Department of Agriculture Nutrient Database( 56 ).
† The 100 and 40 g columns aid in interpretation with respect to labelling; normally 100 g is used as a standard amount against which nutritional content labelling is used and 40 g is a common suggested serving amount for oatmeal.
‡ 14·0 μg/100 g – content is variable depending on soil and fertiliser regimen.
The fundamentals of oat biology with respect to grain biology and nutrient and health-beneficial component localisation are outwith the scope of this brief review of oat cultivation, agriculture and breeding, but are admirably covered by Miller & Fulcher( Reference Miller, Fulcher, Webster and Wood 8 ). Briefly, oats can be divided into two classes – hulled, comprising a groat enclosed in a hull, and naked, where the hull is lightly attached but lost during threshing – with the latter (hull-free oats) deemed nutritionally superior to the former. The hull is largely composed of non-nutritive or putatively anti-nutritive components such as hemicellulose, cellulose and lignin( Reference Thompson, Mustafa and McKinnon 9 ), and the increased nutritive value of the naked oats is due to a proportional weight difference basis because of the absence of a hull.
Cultivation and production
Oat is a crop produced on a global scale, but not at the level exhibited by what can be considered as staple crops, such as maize, rice, wheat, barley and millet (Fig. 1). The latest complete set of figures from The Food and Agriculture Organization Corporate Statistical Database (faostat.fao.org) indicate that in 2012 the global oat production was 19·6 megatonnes( 10 ). The US Department of Agriculture( 11 ) posted a preliminary figure of about 23·6 megatonnes for the 2013/2014 global oat production, a 10·6 % increase over the 2012/2013 harvest. These production data indicate a significant reduction on the global total of 46·9 megatonnes in 1961 and are indicative of a progressive decrease in global production (Fig. 2). However, this trend is perhaps a composite of several factors: the lack of development of oats into multiple products (being addressed only now) combined with the dominance of wheat and barley. Furthermore, the greater approval of the aforementioned health claims( Reference Stewart, Kennedy and Pavel 5 ) has slowly worked its way down the production and product development chain and has seen a slow evolution of products developed that have seen an upswing in food-related utilisation. Indeed, Strychar( Reference Strychar, Webster and Wood 12 ) has reported that feed (animal) use of oats had declined from 90 to 70 % of global production with the commensurate upswing in food and industrial utilisation.
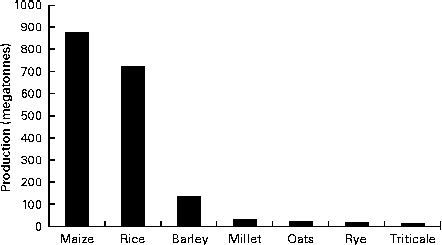
Fig. 1 Global production levels of the main cereal crops (values are for the 2010 production figures)( 10 ).
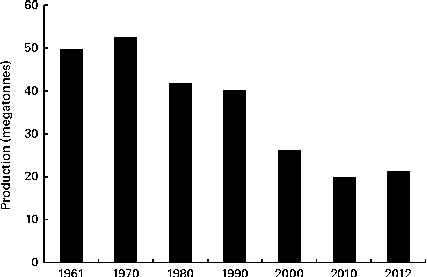
Fig. 2 Global production of oats from 1961 to 2010( 10 ).
It is clear from the figures that there are favoured regions for growth for many crops, and oats are no exception. Oats tend to be grown in temperate regions (Fig. 3) with reasonably high rainfall levels and generally in areas that have moderate temperatures: mid to north Europe, the Russian Federation, the USA and Canada comprise the major production regions (Fig. 4 and Table 2 with references therein). Optimum growth conditions are conditions of moderate temperature and long day length. In general, they can tolerate wet weather and acidic soils more effectively than other cereals such as wheat. In addition, and again in comparison with other food cereals, they are relatively resistant to foliar diseases, while requiring comparatively fewer pesticide and fertiliser inputs( Reference Givens, Davies and Laverick 13 ).

Fig. 3 Regionalised oat production for the years 1961 (■) and 2010 (![]() )(
10
).
)(
10
).

Fig. 4 Top twenty countries for oat production in 2010( 10 ).
Table 2 Influence of environment factors on beneficial oat components

MW, molecular weight; N, fertiliser nitrogen.
With respect to nutritive and health-beneficial values, there has been a significant level of study into the key factors that affect diversity and content. In essence, there are several drivers for this: cultivars (genetic diversity); soil; climate; agronomy. Changes in nutritive and health-beneficial values as a different cultivar (a cultivated variety of a plant that has been created or selected intentionally and maintained through cultivation) is assessed are well established. A study carried out by Doehlert et al. ( Reference Doehlert, McMullen and Hammond 14 ) of twelve oat varieties showed that, even within this limited sample set of cultivated germplasm, the variation in grain yield and starch, oil, protein and β-glucan contents was significant at 31, 6, 34, 56 and 33 %, respectively. Other studies( Reference Leonova, Shelenga and Hamberg 15 – Reference Lazaridou and Biliaderis 27 ) have focused more on specific components.
Leonova et al. ( Reference Leonova, Shelenga and Hamberg 15 ) focused on oil content and diversity and reported that, although wild (uncultivated) oat species exhibited higher total oil (% dry seed weight) and 18 : 1 fatty acid contents, the levels of the beneficial PUFA 18 : 2 (linoleic acid) and 18 : 3 (linolenic acid) were enhanced (as a % of the total lipids) in cultivated oats. This corroborates the finding of other similar and limited studies( Reference Frey and Hammond 16 , Reference Sahasrabudhe 17 ). More recently, a more extensive study( Reference Sahasrabudhe 17 ) using 917 oat accessions, mainly representing A. sativa but also including other Avena species (122 accessions) and cultivated oat replicated at different environments, has also confirmed the elevated levels of linoleic and α-linolenic acids in the cultivated lines. Furthermore, this study found that environment significantly affected only total oil content and not the composition, which is in contrast to the findings of others( Reference Welch 19 – Reference Saastamoinen, Kumpulainen and Nummela 21 ), who reported significant variation in composition. It is worth noting that in these latter studies the geographical variation was much broader than that utilised by Dhanda( Reference Dhanda 18 ).
The impact of oat genetic diversity is also evident for the health-beneficial component β-glucan, which is currently the main impetus behind much of the breeding effort in oats for human consumption. Several studies( Reference Lee, Horsley and Manthey 22 – Reference Newell, Asoro and Scott 26 ) have been undertaken in this respect and have identified that the existing range of β-glucan content generally falls within 2·5–8·3 % (dry grain). In addition to total β-glucan content, there is increasing interest in β-glucan polydispersity, essentially the relative distribution of polymer molar masses and degrees of polymerisation, with research pointing to biological efficacy and benefits deriving from the higher-molecular-weight components. In fact, molecular weight polydispersity is significant and has been reported to cover the range 65–3100 kDa( Reference Lazaridou and Biliaderis 27 ).
The environment and agronomic systems can have significant effects on the nutritive and health-beneficial values of oats, and abiotic (non-biological) stress factors, such as temperature, drought/flooding and fertilisation, have been assessed for their impact (Table 2). In general, these stressors are combined under ‘environment’, despite fertilisation being an agronomic process, and the impact is assessed as such. For example, Doehlert et al. ( Reference Doehlert, McMullen and Hammond 14 ) reported that oat yield and starch content were strongly influenced by environment, whereas protein and β-glucan contents were equally susceptible to genetic and environmental influence. Interestingly, Andersson & Börjesdotter( Reference Andersson and Börjesdotter 28 ) reported a greater effect of environment on the molecular weight of β-glucan (71 %) than on its content (42 %). It is worth stressing that these environmental effects do translate through to specific and quantifiable nutritional consequences. A study carried out by Dickin et al. ( Reference Dickin, Steele and Frost 29 ) on the impact of genotype, environment and agronomic management on β-glucan in naked barley grain (a sister species with appreciable β-glucan contents) highlighted that all these factors affected β-glucan content. Also, part of this study was a porridge intervention trial employing lines exhibiting variation in β-glucan content (5·8 v. 4·6 g/100 g (dry material)) to highlight the impact of β-glucan content variation on human metabolism. In this trial, the higher-β-glucan porridge was found to significantly reduce the total glucose released as well as blunt the peak glucose, a phenomenon associated with oxidative stress, inflammation responses and degenerative diseases such as endothelial dysfunction( Reference Ceriello, Esposito and Piconi 30 ).
A study carried out in Russia utilising a range of varieties from Germany, the USA, Russia, Canada, Sweden and other countries highlighted some broad environmental effects with oat yields being identified as susceptible to high temperature and drought( Reference Tamm 31 ). Interestingly, Frey( Reference Frey 32 ) reported that the modern oat varieties were more tolerant to drought stress than older ones. Most studies on environmental effects on oats have targeted specific (β-glucan, oil and protein) or gross (yield, lodging disease resistance) components. As has been mentioned above, β-glucan content and polydispersity have been shown to be environmentally influenced, and such studies have been extended to other beneficial components (Table 2). Unsurprisingly, protein content is responsive to fertiliser N addition, with total protein content being optimised by eliminating supraoptimal applied N. Interestingly, the nutritive value with respect to protein amino-acid content did differ between naked and husked oats in response to increasing N inputs( Reference Givens, Davies and Laverick 13 ).
Oat, similar to any other crop, is a host for attack by plant pathogens, and this attack invariably reduces crop yield and affects composition and therefore the accessibility of high-quality oat for human consumption. Of the viral pathogens, barley yellow dwarf virus (also known as red leaf) can cause significant crop and yield losses( Reference Frey 32 ); crown rust( Reference Jin, Domier and Kolb 33 ) (Puccinia coronata) and powdery mildew( Reference Jackson, Obert and Avant 34 , Reference Roderick, Jones and Sebesta 35 ) (Blumeria graminis) are the prevalent fungal diseases in North America and the cooler, humid regions of Europe, respectively.
However, it is the fungal infection of oats (and cereals) caused by species of the Fusarium genus that is of most concern to oat consumption and human health and well-being. These fungi are well reported to produce the mycotoxin trichothecenes, such as deoxynivalenol, nivalenol, T-2 and HT-2, and some other toxins such as zearalenone and fumonisins B1 and B2, and the specific production of these toxins depends upon where the oat is grown and hence the localised fungal strain population( 36 , Reference Edwards, Imathiu and Ray 37 ). The trichothecenes T-2 and HT-2 are the ones commonly reported in infected oats; the physiological consequences of consuming these either directly on the oat (products) or via animals fed with spoiled oat feed are acute, with chronic toxicity potentially leading to induced apoptosis in the immune system and fetal tissues( Reference Li, Wang and Beier 38 ). A combined approach of targeting resistance in new varieties, particularly using modern genetic/genomic approaches, and adherence to good agricultural practices should see the current-day levels of these deleterious components dramatically reduced.
Oat breeding: cultivars for climate adaptation and pathogen resistance
The production and availability of oats are, as in all crops, affected by agricultural practices and climatic variation( Reference Ziska, Bunce and Shimono 39 ), which in turn may affect plant diseases( Reference Chakraborty, Tiedemann and Teng 40 ). If agriculture is to provide sufficient food for a growing population, crops providing a significant complement of the human requirement for nutrition and energy, such as oats, must adapt to climate change( 41 ). These changes will include shifts to mid-latitudes and adaptations to broader temperature ranges, more frequent weather extremes, and longer growing seasons, a shift to sustainable agronomic practices using nutrient-use-efficient varieties (reduced synthetic fertiliser use), greater stress and drought resistance, and elevated dew points. Successful adaptations for oats will require extensive understanding of the molecular mechanisms and pathways involved in, and affected by, abiotic stress factors and sustainable agricultural practices( Reference Ziska, Bunce and Shimono 39 – 41 ). An obvious approach to this understanding includes genetic modelling using tractable plant models such as Arabidopsis( Reference Chawade, Bräutigam and Lindlöf 42 ) or, more sensibly, an appropriate sister cereal model, such as barley, which has recently had its genome sequenced( 43 ). Furthermore, oat, as part of the cereal family, will benefit from the new initiatives such as Modelling European Agriculture with Climate Change for Food Security (http://www.macsur.eu/). This knowledge hub gathers the excellence of existing research in livestock, crop and trade science to describe how climate variability and change will affect regional farming systems and food production in Europe in the near and distant future and the associated risks and opportunities for European food security. Such synergistic programmes with modelling on a pan-European scale will invariably benefit future crop-breeding initiatives and effort.
As has been highlighted earlier, powdery mildew, crown and stem rust, and infections caused by species of the Fusarium genus are the predominant diseases that affect oats and can reduce crop yield by as much as 50 % among susceptible cultivars( Reference Jackson, Obert and Avant 34 – Reference Edwards, Imathiu and Ray 37 ), with, for example, crown and stem rust contributing to significant global reduction in yield and seed quality( Reference Frey 32 ). Barley yellow dwarf disease( Reference Jin, Domier and Kolb 33 ), which has aphids as its primary vector, is considered the most important viral disease of oats, and currently none of the oat cultivars is highly resistant to barley yellow dwarf virus. However, this is a focus in the oat-breeding community, and the identification of cultivars exhibiting degrees of resistance (while maintaining/increasing grain yield) is providing a foundation for further genetic research( Reference Sanz, Loarce and Fominaya 44 , Reference McCartney, Stonehouse and Rossnagel 45 ). Germplasm exchanges from specific oat cultivars since the late 1960s indicate a steady improvement in barley yellow dwarf virus tolerance, and this breeding effort has – following multiple collaborative efforts between universities and agriculture centres involving multiple generations of oat genomic clones of global origin, selective crossbreeding and the application of newer genome-mining technologies such as diversity array technology( Reference Tinker, Kilian and Wight 46 , Reference He and Bjørnstad 47 ) (see below) – produced cultivars that are more resistant to barley yellow dwarf virus and exhibit increased yields.
Oat breeding: state of the art and future plans
Modern-day oat breeding has progressed significantly as a result of the paradigm shift that has occurred in plant biology with the combined advances in molecular biological research, omics technologies, genome sequencing and bioinformatics. Perhaps because oats have (unfairly) been perceived as a relatively unimportant crop, they have not benefited from the level of research that has been put into wheat, rice, barley and even sorghum – but progress has been made using the approaches mentioned above. Our understanding of oats per se and also our knowledge of the rate at which yield, disease resistance, and compositional content and diversity are being manipulated have increased significantly( Reference Sanz, Loarce and Fominaya 44 – Reference He and Bjørnstad 47 ). MacKey( Reference MacKey 48 ), for example, highlighted that the yield potential of Swedish oat had remained virtually unchanged over the period from 1910 to 1960, whereupon it increased, with accompanying research effort yielding major increases in harvest index (the weight of a harvested product as a percentage of the total plant weight of a crop) and lodging resistance. This approach became the norm and, as the utility, nutritive value and health benefits (predominantly those attributable to β-glucan) became established, it became imperative that breeding needed to be able to manipulate, and ideally enhance, many of these traits simultaneously. Diverse germplasm has traditionally been used to modulate oat gross (e.g. yield) and specific (β-glucan) parameters. However, modern genetic analytical approaches such as simple sequence repeats( Reference Fu, Chong and Fetch 49 ), amplified fragment length polymorphisms( Reference Achleitner, Tinker and Zechner 50 ), restriction fragment length polymorphism( Reference Sanz, Loarce and Fominaya 44 ) and diversity array technology( Reference Tinker, Kilian and Wight 46 ) are being applied to oat per se either in isolation or in unison( Reference He and Bjørnstad 47 ) to maximise the identification of genetic diversity.
Overall, these approaches identified that, due to the limited initial selection of landraces (essentially traditional localised varieties), diversity has become limited and that if there are to be significant increases in, for example, the levels of β-glucan and vitamins, reassessment and integration (introgression) of the wild material into breeding programmes are necessary. Such an approach is already being undertaken on a significant scale for sister grass species Lolium perenne (perennial ryegrass), with the introgression of the entire genome of Festuca pratensis (meadow fescue) into L. perenne in overlapping chromosomal segments with the aim of crop improvement by tapping the F. pratensis untapped reservoir of genetic variation for a wide range of agronomically important traits( Reference King, Armstead and Harper 51 ). This approach, using a combination of modern genetic/genomic and high-throughput analytical approaches, makes this an achievable goal and one that can deliver on the development of oat for food uses as well as targeted utilisation in other sectors, such as cosmetics and food ingredients( Reference Weightman, Laverick and Maunsell 52 ).
Modern commercial oat breeding is a complex and structured system that combines the selection and assessment of the breeding material and progeny, normally at a restricted acreage level, with the commercial trialling over broader and diverse environments (Fig. 5). As with other crops, this is a rolling-and-staggered approach to ensure the continuous production of better and distinct varieties that match the current and projected market requirements. It is clear that going forward there will be multiple targets for oat breeding to address( Reference Newell, Asoro and Scott 26 , Reference Tanhuanpää, Manninen and Beattie 53 ). From the aspect of new product development, these targets will undoubtedly include the following:
-
(1) Oat β-glucan – building on the approved health claim.
-
(2) Nutritional components – good source of protein and unsaturated fatty acids.
-
(3) Desirable organoleptic properties – these are predominantly derived from the action of processing/cooking on the oat lipid content and vary with process/cooking conditions and lipid composition.

Fig. 5 A schematic description of the UK process of oat variety generation with approximations of the scale of effort involved. The parental cross schematic highlights the scale of plant lines needed to progress from the initial parental cross through generation to be multiplied up for assessment and ultimately for recommendation (RL) to farmers. NL (National Listing): this is a legal requirement for new varieties of the main agricultural and vegetable species that seeks to ensure that no new variety is marketed unless it is genuinely new and, for agricultural crops, an improvement in key characteristics on varieties already being sold. RL (Recommended Listing): this provides information on yield and quality performance, agronomic features and market options for recommended varieties to assist growers with variety selection. Varieties are generally trialled on an annual basis while they remain on the Recommended List. For oats in the UK, this is administered by the Home Grown Cereal Authority. RLT, Recommended Listing trials.
What does the future hold?
The advances now being made in oat biology as a consequence of technological advancements in other cereals, such as rice, wheat and barley, are exponential. The adoption of genotype by sequencing( Reference Luo, Wight and Zhou 54 ) holds major potential for identifying both the variation in and the genes underpinning the desirable nutritional and health-beneficial traits, meaning that these genome regions can then be used as molecular markers to accelerate trait enhancement in future germplasm. Furthermore, this approach can also be exploited along with transcriptome profiling that uses deep-sequencing technologies( Reference Wang, Gerstein and Snyder 55 ) to tease out the genetic drivers to the environmental response that we have already shown (Table 2) can impinge upon nutritional and health-beneficial contents. Alignment of this with high-throughput and detailed phenotyping systems, such as metabolomics, will ultimately lead to an accelerated development of new and tailored oat varieties.
The next step in the development of oats will be the elucidation of the genome sequence, and this is being actively pursued by a consortium of oat scientists across the world.
Acknowledgements
D. S. received an honorarium from Quaker Oats Company (a subsidiary of PepsiCo, Inc.) for attending a workshop in May 2012 to discuss the content of the supplement, and the James Hutton Institute received an unrestricted grant from the Quaker Oats Company. D. S. is part of the QUOATS Consortium (www.quoats.org), which is jointly sponsored by BBSRC, DEFRA, SG-RESAS, WAG, AHDB and industry partners. D. S. and G. M. acknowledge grant-in-aid and contract research funding from SG-RESAS for strategic research and MACSUR (www.macsur.eu) activities.
The authors’ contributions are as follows: D. S. prepared the first draft of the paper; G. M. provided input to the introduction and content for the tables. Both authors reviewed and commented on the paper.
This paper was published as part of a supplement to British Journal of Nutrition, publication of which was supported by an unrestricted educational grant from Quaker Oats Co. (a subsidiary of PepsiCo Inc.). The papers included in this supplement were invited by the Guest Editor and have undergone the standard journal formal review process. They may be cited.
The Guest Editor to this supplement is Roger Clemens. The Guest Editor declares no conflict of interest.








