Introduction
Lymphatic filariasis (LF) is a neglected tropical disease that causes debilitating, acute and chronic morbidities in affected individuals. It is caused by three mosquito-borne parasitic worms: Wuchereria bancrofti, which accounts for 90% of cases recorded globally (Ottesen, Reference Ottesen2006), Brugia malayi and Brugia timori accounting for the remaining 10%. LF is present in over 80 countries in the Americas, Asia, the Pacific and Africa (Molyneux et al., Reference Molyneux, Bradley, Hoerauf, Kyelem and Taylor2003). It is estimated that 120 million of the world's population is infected, while 40 million suffer from disabilities and psychological trauma due to stigmatization (Brady, Reference Brady2014; Ichimori et al., Reference Ichimori, King, Engels, Yajima, Mikhailov, Lammie and Ottesen2014; WHO, 2015). It is transmitted by mosquitoes belonging to the Aedes, Anopheles, Culex, Mansonia and Ochlerotatus genera (de Souza et al., Reference de Souza, Koudou, Kelly-Hope, Wilson, Bockarie and Boakye2012). In Africa, where W. bancrofti is the parasite responsible for LF, anophelines (Anopheles gambiae s.l. complex and Anopheles funestus) are the main vectors in rural areas across the continent while culicines (Culex quinquefasciatus) are the primary vectors in urban areas in eastern and southern parts of Africa (Pederson, Reference Pederson, Simonsen, Malecela, Michael and Mackenzie2008). More recently, Mansonia mosquitoes have been incriminated as potential vectors in rural Africa as well (Ughasi et al., Reference Ughasi, Bekard, Coulibaly, Adabie-Gomez, Gyapong, Appawu, Wilson and Boakye2012).
The Global Programme to Eliminate Lymphatic Filariasis (GPELF) was launched in 2000, with the primary goal to interrupt LF transmission through annual mass drug administration (MDA) with albendazole in combination with either ivermectin in Africa or diethylcarbamazine in areas outside Africa (Ottesen, Reference Ottesen2006). The GPELF has achieved great successes since its inception, with the elimination of LF in Cambodia, Cook Islands, Maldives, Niue, Sri Lanka, Togo and Vanuatu (Budge, Reference Budge, Dorkenoo, Sodahlon, Fasuyi and Mathieu2014; WHO, 2016, 2017). In addition, 13 countries are now in the post-elimination phase.
According to the WHO guidelines, transmission assessment surveys (TAS) support the decision to stop MDA (WHO, 2011) based on microfilariae (mf) prevalence <1% or antigen prevalence <2% (Stolk et al., Reference Stolk, Swaminathan, Oortmarssen, Das and Habbema2003). The tools available for transmission assessment include; immunochromatograhic test (ICT) [such as filarial test strip (FTS)], ELISA, polymerase chain reaction (PCR) and mf detection by microscopy (WHO, 2011). However, these tools require blood collection from large numbers of community volunteers. Further, the cross-reactivity of Loa loa and W. bancrofti reported by Wanji et al. (Reference Wanji, Amvongo-Adjia, Koudou, Njouendou, Ndongmo, Kengne-Ouafo, Datchoua-Poutcheu, Foyennso, Tayong, Fombad, Fischer, Enyong and Bockarie2015) in Cameroon, questions the reliability of ICT especially in parts of Central Africa where these parasites are co-endemic. Meanwhile, monitoring vectors for the presence of parasite DNA (xenomonitoring) is an important assessment tool for LF elimination programmes, with the advantage that it provides a real-time estimate of microfilaria in the community members (Okorie and de Souza, Reference Okorie and de Souza2016). Mosquito surveillance studies have shown to be useful in assessing the transmission of LF in the Pacific (Chanteau et al., Reference Chanteau, Luquiaud, Failloux and Williams1994; Burkot and Ichimori, Reference Burkot and Ichimori2002; Farid et al., Reference Farid, Morsy, Helmy, Ramzy, El Setouhy and Weil2007). Furthermore, studies in Africa show that xenomonitoring can provide valuable information to the LF elimination programme (Chanteau et al., Reference Chanteau, Luquiaud, Failloux and Williams1994; Boakye et al., Reference Boakye, Wilson, Appawu and Gyapong2004; Ughasi et al., Reference Ughasi, Bekard, Coulibaly, Adabie-Gomez, Gyapong, Appawu, Wilson and Boakye2012; de Souza et al., Reference de Souza, Sesay, Moore, Ansumana, Narh, Kollie, Rebollo, Koudou, Korama, Bolay, Bocckarie and Boakye2014). The traditional methods for xenomonitoring include dissecting vectors for the different developmental stages of the worm. However, as microfilariae (mf) prevalence in human populations becomes low due to MDA, the time and cost involved in processing such large numbers remain a challenge (Burkot and Ichimori, Reference Burkot and Ichimori2002). In this instance, molecular xenomonitoring, a method which allows for processing samples within a shorter time with high precision will improve sample processing (Derua et al., Reference Derua, Rumisha, Batengana, Max, Stanley, Kisinza and Mboera2017). Additionally, targeting epidemiologically relevant mosquitoes, i.e. older mosquitoes (blood fed, gravid or parous mosquitoes) will further enhance the chances of detecting parasite DNA (Springer et al., Reference Springer, Hoekman, Johnson, Duffy, Hufft, Barnett and Beard2016). Gravid traps have shown to be useful for such purposes (Mboera et al., Reference Mboera, Takken, Mdira and Pickett2000). The Centres for Disease Control and Prevention (CDC) gravid traps are routinely used for surveying culicine populations (Williams and Gingrich, Reference Williams and Gingrich2007; Facchinelli et al., Reference Facchinelli, Koenraadt, Fanello, Kijchalao, Valerio, Jones, Scott and della Torre2008), however, the attractive ovoposition substrate is not effective in collecting anophelines. Recent work by Lindh et al. (Reference Lindh, Dugassa, Lindsay and Fillinger2016) point to the potential of the Anopheles gravid trap (AGT) (OviART) in collecting gravid Anopheles mosquitoes. Other mosquito collection tools for monitoring mosquito populations include indoor and outdoor collection methods (e.g. the human landing catches, pyrethrum spray catches (PSC), aspiration of resting mosquitoes, exit traps (ET), barrier nets, box gravid trap (BOX), CDC-light trap (LIT), and the BioGents-sentinel trap). The traps exploit different mosquito behaviours, such as feeding and resting, and habitats with varying sensitivities.
The aim of our study was to assess the efficiency of the AGT for xenomonitoring purposes in two LF endemic areas in Ghana – the coastal Western region and the Northern region. The study evaluated the mosquito composition, density and physiological state of mosquitoes collected from this trap compared with five other collection methods, as well as W. bancrofti and Plasmodium spp. DNA positivity in the mosquitoes collected.
Materials and methods
Study sites
Our study was conducted in villages across the Western Region (Akonu, Agyan and Asemko) and Northern Region (Dugli and Sekyerekura) of Ghana (Fig. 1). Akonu and Agyan are neighbouring communities located in the Nzema East district while Asemko is in the Ahanta West district. Dugli and Sekyerekura are neighbouring communities in the Bamboi district. The major vectors of LF in the Northern savanna region are An. gambiae, Anopheles arabiensis and Anopheles funestus while in the coastal areas, the predominant vectors are A. gambiae s.s, Anopheles melas and An. funestus (de Souza et al., Reference de Souza, Kelly-Hope, Lawson, Wilson and Boakye2010). The Northern region is characterized by a rainy season which occurs between May and September and a dry season between December and April with temperatures as high as 40 °C. The Western region has a wet season that spans from April to November and a dry season from December to March.
Fig. 1. Map of Ghana showing the locations of study villages sampled.
Epidemiological survey
An epidemiological survey was conducted to determine the LF prevalence in Agyan (May 2016), Akonu and Asemko (January 2017), Dugli and Sekyerekura (October 2017). Finger-prick blood was collected from volunteers aged 16 years and above and tested using the FTS (Alere™) to detect circulating filarial antigen. Positive individuals were then followed up for the presence of microfilariae through the collection of blood between 10 pm and 2 am. This coincides with the peak time of mf in peripheral blood. Blood samples were screened by microscopy using the counting chamber technique described by Agbolade and Akinboye (Reference Agbolade and Akinboye2005).
Mosquito collection methods
The AGT was designed and described by Dugassa et al. (Reference Dugassa, Lindh, Oyieke, Mukabana, Lindsay and Fillinger2013). It was made of a rectangular basin measuring 45 cm × 33 cm × 11.5 cm (length × width × height), with a 4 cm hole on the side and 6 L rectangular basin. An open plastic tube (collection chamber) was inserted into the hole and the other opening of the tube was sealed with fibre glass netting to prevent trapped mosquitoes from escaping. The tube was placed and secured halfway into an aluminium collapsible pipe. The flexible tube was connected to a 12 V fan that provided suction on the water surface. The efficiency of AGT was compared with two gravid traps; BOX-the Box gravid trap (by BioQuip, Rancho Dominguez, CA) and CDC-gravid trap (Model 1712 from John W. Hock Company), and LIT (by UPL limited) for outdoor collections. The AGT was also evaluated against the BGS-BioGents-sentinel, ET-Exit traps, IRC-Indoor resting collection and the PSC-pyrethrum spray catches which were used to collect mosquitoes indoor. The BGS (BioGents) was baited with the Anopheles odour lure. The LIT was baited with cotton wool soaked in 1-Octen-3-ol. The IRC were done using a battery-powered aspirator (Vazquez-Prokopec et al., Reference Vazquez-Prokopec, Spillmann, Zaidenberg, Kitron and Gürtler2009), with the interior ceiling, walls and any hanging clothing aspirated. The AGT, BOX, CDC, ET, LIT and BGS were set up at 6 pm and removed the following morning at 6 am. PSCs were conducted between 5:30 am and 6 am in selected houses in the Western region. Whereas, the IRCs were conducted from 5:30 am to 8:30 am in all households in the villages in the Northern region. Water from larval habitats was used in all the gravid traps. Only valid collection nights (nights where all the traps worked through the night) were used in comparisons. The data collected on days where batteries of one or more of the traps failed were excluded.
Western region mosquito collection
The AGT was compared with CDC, BOX, LIT, ET and PSC in the three Western region villages. Mosquito collections were conducted in March and May 2017 with a total of 26 collection nights each for AGT, BOX, CDC and LIT; 18 for ET and 37 for PSC in 13 consecutive nights. The four outdoor collection methods (AGT, CDC, BOX and LIT) were rotated among selected locations within the densely populated sections of the villages and larval habitats. The two indoor methods (ET and PSC) were done in randomly selected rooms with at least one sleeper.
Northern region mosquito collection
The AGT was compared with the BOX, BGS and IRC in the Northern region. Mosquito collections were conducted in October 2017 with 12 collection nights for each trap type. The three outdoor traps (AGT, BOX, BGS) were rotated among selected locations within each village. Each location consisted of a family compound and IRCs were conducted in all rooms which were occupied by a sleeper the night before (the number of rooms per compound ranged 1–8). The mean number of mosquitoes per room is presented for each location (Fig. 5).
Mosquito processing
The collected mosquitoes were identified morphologically using keys by Gillies and Coetzee (Reference Gillies and Coetzee1987). Mosquitoes collected from the Western region were scored based on abdominal status – fed, unfed or gravid. A proportion of the mosquitoes collected from the Western region were dissected and ovaries removed and dried to determine parity based on ovary tracheation as described by Detinova (Reference Detinova1962). Mosquito legs were used for molecular identification of members of the An. gambiae s.l. complex, based on restriction fragment length polymorphism described by Fanello et al. (Reference Fanello, Santolamazza and Della Torre2002). Mosquitoes from the Northern region were only identified using morphological features.
Wuchereria bancrofti and Plasmodium detection in mosquitoes
The anopheline mosquitoes that were in good condition (not damaged) were screened for W. bancrofti and Plasmodium spp. DNA, in pools of up to 5 mosquitoes based on trap type, species and location. The heads and thoraces were screened separately from the abdomens. Genomic DNA (gDNA) was extracted from the mosquito pools, using the Livak extraction method (Livak, Reference Livak1984). To determine the presence of W. bancrofti DNA, the ITS1 gene in the 18S and 5.8S subunits of the rRNA from filarial worms was amplified as described by Jiménez et al. (Reference Jiménez, González, Carranza, Bailo, Pérez-Ayala, Muro, Pérez-Arellano and Gárate2011). For the identification of Plasmodium spp. DNA, the COX-1 gene was amplified in a single step PCR as described by Echeverry et al. (Reference Echeverry, Deason, Makuru, Davidson, Xiao, Niedbalski, Yu, Stevenson, Bugoro, Aparaimo, Reuben, Cooper, Burkot, Russell, Collins and Lobo2017).
Data analysis
Morphological and molecular identification showed no differences in species composition between villages of the same region, therefore, data were combined for statistical analysis using SPSS (IBM24). To evaluate the trapping efficiency of AGT, a Bonferroni posthoc analysis of variance (ANOVA) was performed to compare the mean number of Anopheles mosquitoes caught by each method, per night.
Trapping efficiency was also evaluated by comparing an estimated mean number of Anopheles that were likely to have taken a blood meal in their lifetime since these are the target population for xenomonitoring. The proportion of mosquitoes that were unfed but parous, blood fed and gravid mosquitoes collected in each trap was estimated and multiplied by the mean catch per collection/night to get the estimated number that had previously taken a blood meal.
The prevalence of W. bancrofti and Plasmodium spp. DNA in mosquitoes was estimated using the PoolScreen software 2.0 (Katholi et al., Reference Katholi, Toe, Merriweather and Unnasch1995) and the maximum likelihood estimates reported with 95% confidence interval (CI).
Results
LF infection prevalence
Filarial antigen prevalence ranged 12.2–27.9% in the study villages and microfilaria prevalence ranged 1.5–3.8% (Table 1).
Table 1. Estimated CFA and mf prevalence in the five villages

Mosquito composition
Western region
A total of 1417 mosquitoes was collected in the Western region. Morphological identification of collected mosquitoes showed that 4.6% were Aedes spp, 36.8% were A. gambiae s.l., 58.4% were Culex spp and 0.2% were Mansonia spp. There was no significant difference in the species composition across the three villages. The largest proportion of mosquitoes caught using the outdoor collection methods (AGT, BOX, CDC and LIT) were Culex whereas, the largest proportion of the total catch in ET and PSC were Anopheles (Fig. 2). Molecular characterization of the 442 An. gambiae s.l. collected from the villages in the Western region, show that 44.0% were An. gambiae s.s., 43.8% were An. melas, while 12.1% did not amplify. Whereas in the Northern region, 55.1% of 78 Anopheles were identified as An. gambiae s.s. however, 44.9% did not amplify with the An. gambiae complex primers used (primers included; An. arabiensis, An. gambiae and An. melas). These proportions did not differ between the indoor and outdoor collection methods (χ 2 = 0.16, P = 0.7).
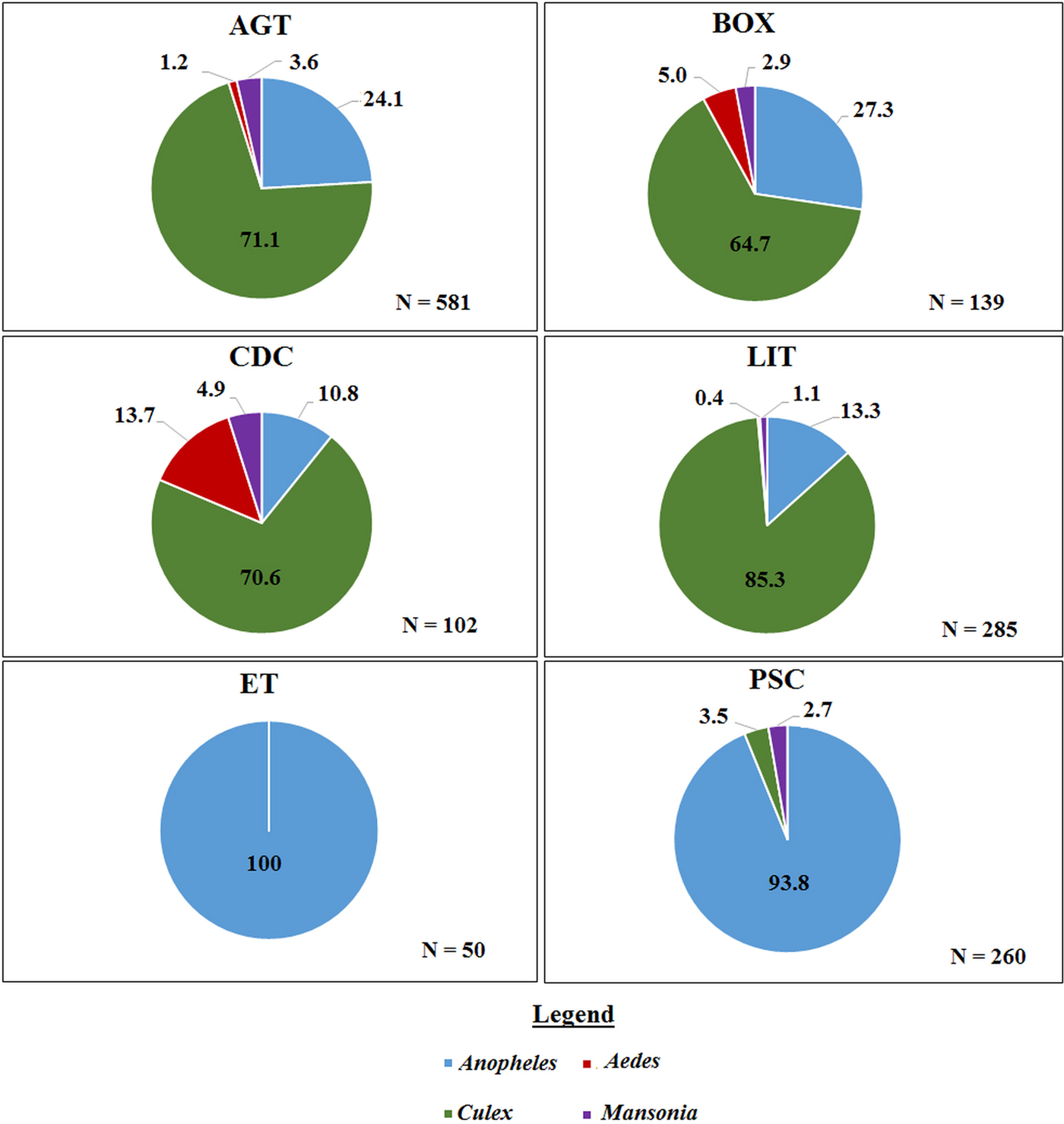
Fig. 2. The proportion of mosquito genera caught per trap type in the Western region. Where ‘N’ is the number of mosquitoes caught in each trap. AGT, Anopheles gravid trap; BOX, Box gravid trap; CDC, CDC gravid trap; ET, Exit trap; LIT, Light trap; PSC, Pyrethrum spray catch.
Northern region
A total of 771 mosquitoes was collected in the Northern region. Of the 555 mosquitoes collected indoors, 473 (85.2%) were An. gambiae sl, and 68 (12.2%) were An. funestus, 10 (1.8%) were other Anopheles spp. including Anopheles rufipes and Anopheles coustani and 4 (0.7%) were Culex spp (Fig. 3).
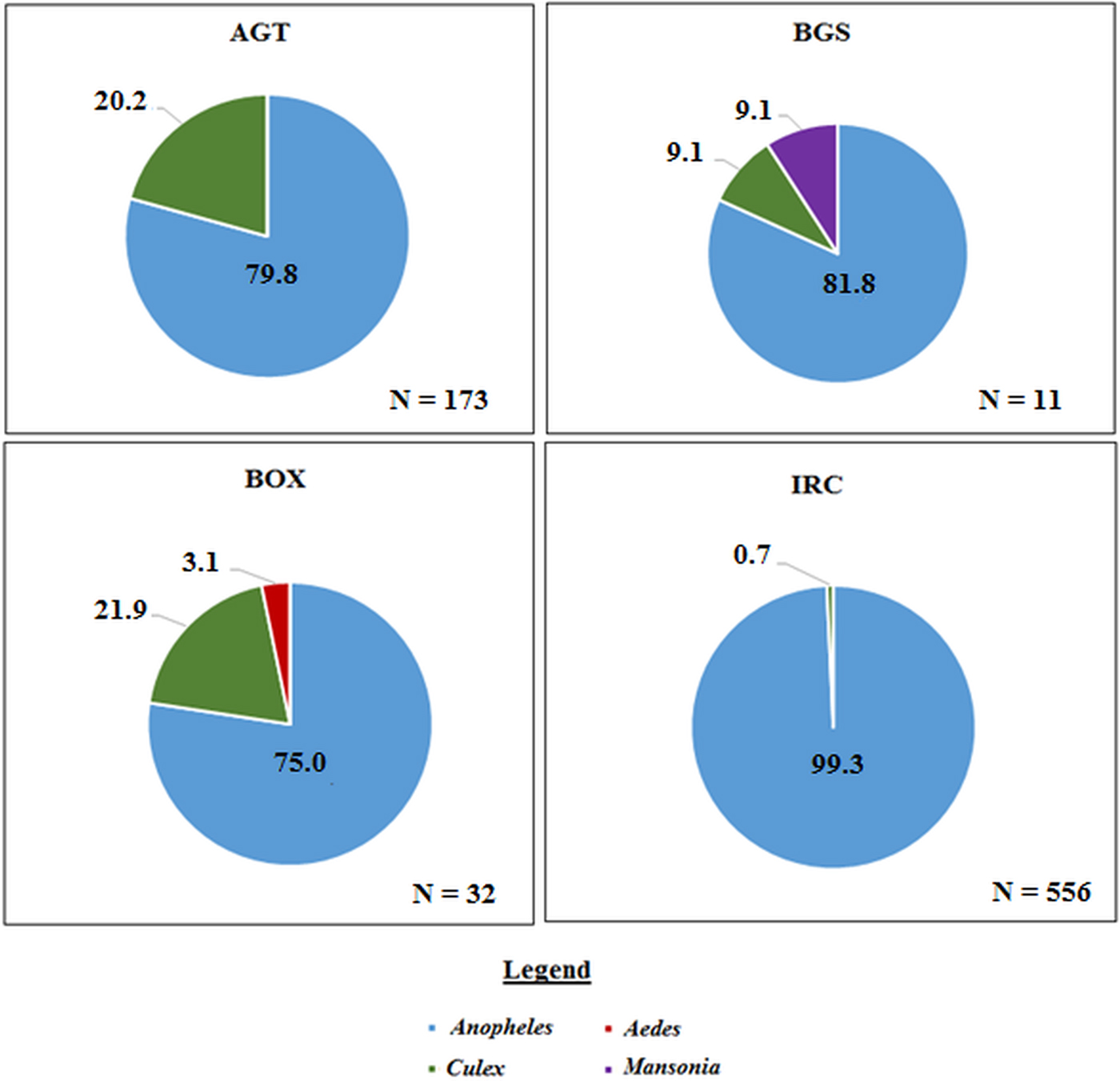
Fig. 3. The proportion of mosquito genera caught per collection method in the Northern region. Where ‘N’ is the number of mosquitoes caught in each trap. AGT, Anopheles gravid trap; BGS, BG sentinel trap; BOX, Box gravid trap; IRC, indoor resting collection.
Comparison of the trapping efficiency by mosquito composition and density
In the Western region, AGT caught the highest mean number of anophelines per collection night (4.62, 95% CI 0.49–8.74) followed by PSC (3.05, 95% CI 1.85–4.26) and the least being CDC (0.15, 95% CI 0.01–0.30). Pairwise comparisons show that the differences in the mean catch per collection night of anophelines was only significant for AGT when compared with CDC, F (5, 153) = 2.959, P = 0.014 (Fig. 4).
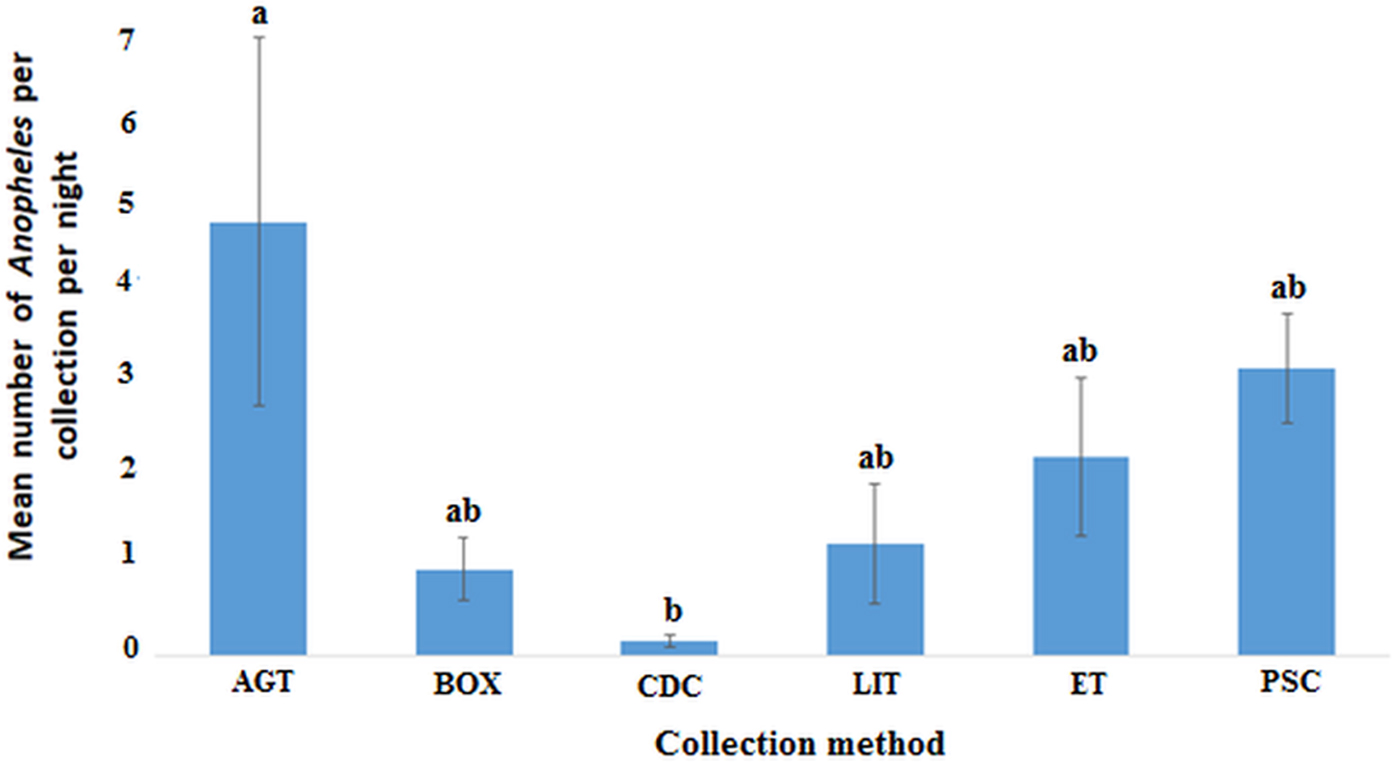
Fig. 4. Plots of the mean number of anopheline mosquitoes caught by each method per night in the Western region (P < 0.05). Bars with identical letters are not significantly different from each other. Error bars show standard error of the mean.
In the Northern region, the indoor resting collections had the highest mean number of anophelines per collection night (8.86, 95% CI 4.13–13.59) followed by AGT (7.33, 95% CI 0.83−13.831) with BGS catching the least (0.58, 95% CI 0.01–1.16). The observed differences in the mean number of anophelines was statistically significant between IRC and BGS but not AGT and BOX (F (3, 44) = 4.808; P < 0.01) (Fig. 5). However, the differences in mean between AGT and BGS, AGT and BOX or BGS and BOX were not statistically significant.
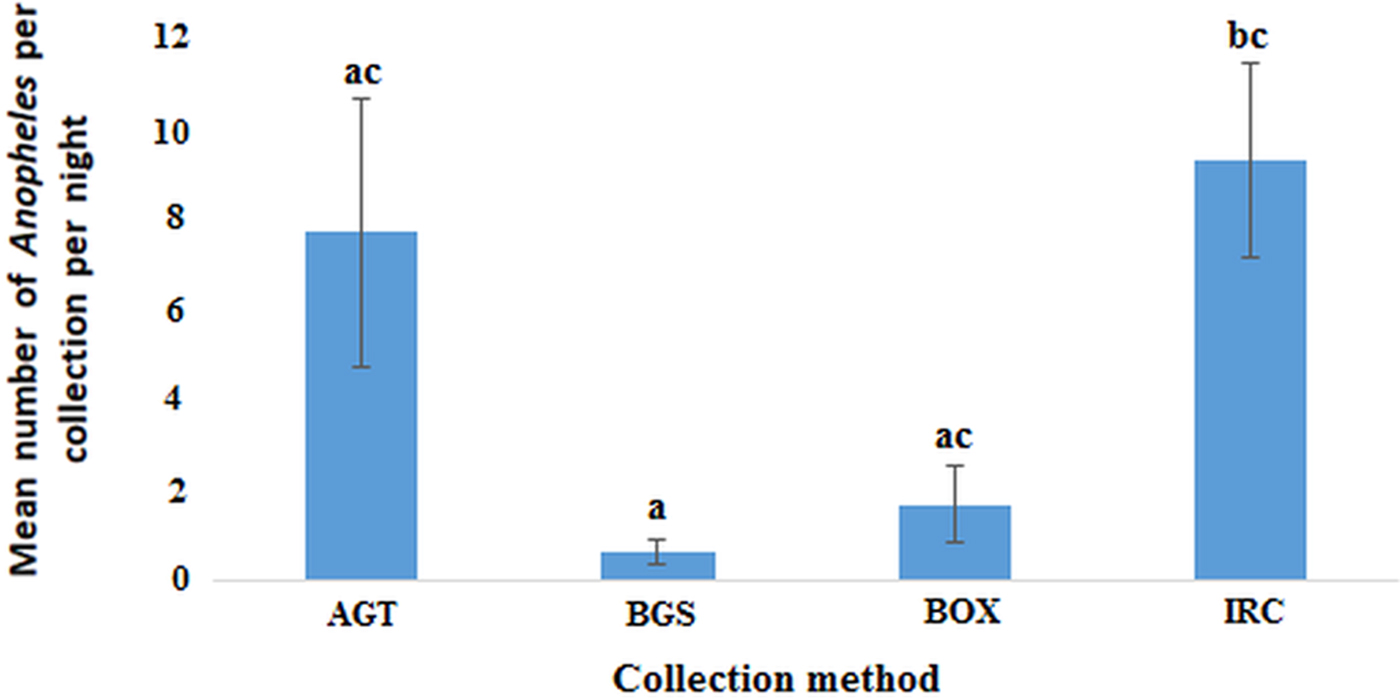
Fig. 5. Plots of the mean number of anophelines caught by each method per night in the Northern region (P < 0.05). Bars with identical letters are not significantly different from each other. Error bars show standard error of the mean.
Trapping efficiency for mosquitoes that have previously taken a blood meal
Estimating the number of mosquitoes that were likely to have previously taken a blood meal is important for xenomonitoring. These mosquitoes include those that were caught bloodfed, gravid or unfed and parous. The parity dissections were only conducted in the Western region study. The results show that AGT caught the highest mean number of An. gambiae s.l. previously exposed to at least one blood meal. PSC was second with CDC collecting the least number per collection night. As expected, the gravid traps caught a high proportion of gravid An. gambiae s.l. while the indoor collections caught a high proportion on bloodfed An. gambiae s.l. (Table 2).
Table 2. Estimated mean number of A. gambiae s.l. from the Western region which have previously taken a blood meal

AGT, Anopheles gravid trap; BOX, Box gravid trap; CDC, CDC-gravid trap; LIT, light trap; ET, Exit trap and PSC, Pyrethrum spray catches.
a Estimates per collection night in the case of AGT, BOX, CDC, LIT, ET and per room for PSC.
Infection prevalence in mosquitoes
Pools of heads and thoraces were split from abdomens and screened separately for W. bancrofti and Plasmodium spp DNA. The results are presented in Table 3. In the Western region, none of the head/thorax pools were positive for either. However, W. bancrofti and Plasmodium spp. DNA were detected in pools of mosquito abdomens. A 0.80% and 2.15% prevalence of W. bancrofti and Plasmodium spp. was observed in the An. gambiae collected from the Western region. These included mosquitoes (i.e. 3 pools of 15 mosquitoes) that had been captured using PSC (the only method that had mosquitoes that were positive for W. bancrofti). The Plasmodium positives pools were obtained from 3, 4 and 1 pool of 14, 11 and 2 mosquitoes that were caught in AGT, BOX and ET, respectively. The Plasmodium spp. positives were recovered from the pools that contained unfed, bloodfed and gravid Anopheles mosquitoes, indicating that, immature stages of the parasite were present in the midgut. Unsurprisingly, the W. bancrofti DNA positive pools were all from those that were bloodfed. Screening of pools of Mansonia show a prevalence of 3.57% (95% CI 0.11–17.08%) Plasmodium spp. and 3.86% (95% CI 0.12–18.45) of W. bancrofti DNA. In the Northern region, we observed a higher overall prevalence of W. bancrofti at 3.01% (95% CI 1.60–5.05) and Plasmodium spp. at 3.27% (1.78–5.40). The W. bancrofti positive pools were from 2, 3 and 7 pools of 5, 9 and 29 for AGT, BOX and IRC, respectively. While that of Plasmodium spp. were from 1 to 11 pools of 5 and 52 mosquitoes that were captured using BOX and IRC, respectively. In addition, a small number of head/thorax pools were positive for W. bancrofti (0.3%, 95% CI 0.01–1.5) and Plasmodium spp. (0.9%, 95% CI 0.2–2.5), indicating the developing stage of filarial worms were present in the thorax, and the infective stage sporozoites were present in the salivary glands. The positive mosquitoes were collected from IRC and AGT and these mosquitoes were An. gambiae and Culex spp.
Table 3. Infection prevalence of W. bancrofti and Plasmodium spp. in mosquito pools

a The table above shows the estimated prevalence of W. bancrofti and Plasmodium spp. DNA in the mosquitoes collected using PoolScreen 2.0.
Discussion
Monitoring parasite infection in vector populations can be used to assess transmission as well as infection in the human population (Boakye et al., Reference Boakye, Wilson, Appawu and Gyapong2004; Ughasi et al., Reference Ughasi, Bekard, Coulibaly, Adabie-Gomez, Gyapong, Appawu, Wilson and Boakye2012; de Souza et al., Reference de Souza, Sesay, Moore, Ansumana, Narh, Kollie, Rebollo, Koudou, Korama, Bolay, Bocckarie and Boakye2014). However, there is a challenge identifying efficient vector collection methods for anopheline mosquitoes, the primary vectors in sub-Saharan Africa for both LF and malaria. Using gravid traps increases the chance of catching infected mosquitoes as gravid mosquitoes would have taken at least one blood meal (Irish et al., Reference Irish, Moore, Derua, Bruce and Cameron2013). CDC gravid traps, purposely designed for Culex mosquitoes, are used routinely for the surveillance of diseases such as dengue fever and lymphatic filariasis transmitted by Culex mosquitoes (L'Ambert et al., Reference L'Ambert, Ferré, Schaffner and Fontenille2012; Hapairai et al., Reference Hapairai, Plichart, Naseri, Silva, Tesimale, Pemita, Bossin, Burkot, Ritchie, Graves, Melrose and Joseph2015). However, an equivalent for anophelines is not widely used for monitoring, and this has been a challenge for surveying these populations. This study reports on the first evaluation of an Anopheles gravid trap for monitoring LF and malaria in vectors in Ghana.
In the Western region, about 40% of the total An. gambiae s.l. collected were An. melas. Anopheles melas is usually associated with mangroves, which were present in the study communities. Tuno et al. (Reference Tuno, Kjaerandsen, Badu and Kruppa2010) also reported on the abundance of An. melas in the western coast of Ghana. In the Northern Region, the Anopheles sampled included An. coustani, An. funestus, An. rufipes and An. gambiae s.l. which are also implicated in malaria transmission (Tabue et al., Reference Tabue, Awono-Ambene, Etang, Atangana, Antonio-Nkondjio, Toto, Patchoke, Leke, Fondjo, Mnzava, Knox, Tougordi, Donnelly and Bigoga2017). These Anopheles species have been reported to exhibit ‘limitation’ which favours transmission even when mf prevalence is low (McGreevy et al., Reference McGreevy, Bryan, Oothuman and Kolstrup1978; Bryan, Reference Bryan, McMahon and Barnes1990; Amuzu et al., Reference Amuzu, Wilson and Boakye2010).
For this study, trapping efficiency was evaluated by comparing the mean number of Anopheles per trap night. Amongst the gravid traps used, the AGT performed better than the Box gravid trap (BOX) and CDC-gravid trap (CDC), even though all three were baited with water from larval habitats. AGT had the highest proportion of An. gambiae s.l exposed to a blood meal as well as the highest mean number of An. gambiae s.l compared with the other collection methods in the Western region. Its efficiency at trapping exposed mosquitoes was approximately 1.6 and 2.2 times better than PSC and ET, respectively. Amongst the outdoor collection methods, it was 5.1, 27 and 9.4 times better than BOX, CDC and LIT. The improved performance of AGT could be due to a bigger fan, which provided a suction effect over the entire water surface. Whereas, the suction effect for the BOX and CDC were only strong at the opening to the collection chambers but the effect was less towards the periphery. AGT resulted in a higher catch than Exit trap (ET), CDC-LIT and pyrethrum spray collection (PSC), when standardized per location per night. Similar observations were made in the testing of AGT in Kenya (Lindh et al., Reference Lindh, Dugassa, Lindsay and Fillinger2016). The Kenyan study evaluated the trapping efficiency of the OviART gravid trap designed to collect gravid Anopheles. The OviART gravid trap, which is similar to the AGT used in this study, collected 2.3 times the number of An. gambiae s.l. compared with BOX. In our study, the AGT also showed good trapping efficiency for An. melas, even though this species prefers to breed in high salinity water compared with other anophelines. AGT also caught the highest mean number of Anopheles that had previously taken a blood meal. Similarly, Lindh et al. (Reference Lindh, Dugassa, Lindsay and Fillinger2016) observed a higher proportion (90%) of gravid mosquitoes in the OviART trap. The main difference in the AGT and OviART trap is the oviposition tray. The AGT had a 6 L rectangular basin unlike the OviART which had 8 L circular basin in which water was kept serving as an attractant for gravid mosquito. The rectangular basin of the AGT provides a larger surface area for oviposition, compared with the circular basin of the OviART. However, it is not clear whether the aforementioned variations, affects the performance of these traps.
Some of the limitations to the use of the AGT are due to bulkiness and the use of a 12 V car battery which limits portability. There is no protective shield on the traps and the basins get flooded during rains wetting the collection chambers and trapped mosquitoes. Rains can also damage the fan and the battery. As such, improved designs aimed at protecting the components of the trap while also improving portability will make it more useful in the field.
The high numbers and the different species caught in the AGT show that, not only is it efficient for sampling LF and malaria vectors, it can also be employed in sampling other mosquito vectors including Culex which is implicated in LF and arbovirus transmission elsewhere (Mak, Reference Mak2007; Lutomiah et al., Reference Lutomiah, Koka, Mutisya, Yalwala, Muthoni, Makio, Limbaso, Musila, Clark, Turell and Kioko2011; Jones et al., Reference Jones, Machin, Mohammed, Majambere, Ali, Khatib, McHa, Banson and Kelly-Hope2012). Information such as vector abundance and diversity within a locality, can help inform local health authorities on vector distribution and implication in the transmission which can be the basis for deploying control measures (Ciota and Kramer, Reference Ciota and Kramer2013; Oduola et al., Reference Oduola, Olojede, Oyewole, Otubanjo and Awolola2013). Further, parasite positivity in mosquitoes collected with these traps indicates the presence of infection in the community, providing information that may require intensified efforts to manage and control vector-borne diseases (Kouassi et al., Reference Kouassi, de Souza, Goepogui, Narh, King, Mamadou, Diakité, Dadzie, Boakye, Utzinger, Bockarie and Koudou2015).
None of the head/thorax pools of mosquitoes were positive for W. bancrofti and Plasmodium spp. in the Western region, suggesting that these mosquitoes were not carrying any infective stages. However, a small number of pools were positive from the Northern region. The mosquitoes that were positive for W. bancrofti DNA were An. coluzzii and An. melas, the primary vectors of W. bancrofti in these areas (de Souza et al., Reference de Souza, Kelly-Hope, Lawson, Wilson and Boakye2010). The number of samples analysed was few, however, they illustrate the utility of detecting parasite DNA in mosquitoes, even when infection prevalence is very low in the community. Detection of parasite DNA confirms the presence of infection in humans and indicate ongoing transmission to mosquitoes. Furthermore, the positive pools obtained from the traps, supports the evidence that these methods are useful for sampling epidemiologically relevant mosquitoes (ie. mosquitoes that were exposed to at least a blood meal). Hence, they can be employed in monitoring vector populations which can provide valuable information to support the decision to stop MDA.
However, comparison of the differences in infection prevalence in mosquitoes between collection methods of Anopheles species and locations were not performed. This is a limitation as these information are relevant and can inform vector monitoring campaigns.
In conclusion, AGT was a very efficient collection method compared with the other traps in both study regions, but particularly in the Western region where few mosquitoes were found resting indoors. We found that, on average, AGT collected over 2 times as much as blood exposed Anopheles compared with the indoor methods and 5–27 times compared with the other gravid traps. The collection of indoor resting mosquitoes, either by PSC or mechanical aspiration is efficient in areas with high numbers of indoor resting anophelines such as in the Northern region since many rooms can be screened for indoor resting mosquitoes by one team of collectors. While the AGT showed efficiency in trapping mosquitoes, there are limitations to the number of traps that can be set and rotated by a team because of the heavy car battery, which needs to be recharged every few days.
Acknowledgements
We appreciate the chiefs, elders and opinion leaders of Agyan, Akonu, Asemko, Dugli and Sekyerekura for their co-operation during sampling. A special thanks to field assistants who helped with the mosquito collections. We appreciate the support and contributions of Angela Hughes, Harun Njoroge, Joseph Harold Nyarko Osei, Kwaku Osei Akuoko and Sellase Pi-Bansa. We are also grateful to Dr Dugassa for providing guidance on the requirements for the AGT.
Financial support
This research received financial support from the WellcomeTrust (200076/Z/15/Z).
Conflict of interest
None.
Ethical standards
Ethical approval for the study was obtained from the IRB of the Noguchi Memorial Institute for Medical Research (approval number: 100/15-16) and the Liverpool School of Tropical Medicine's Ethical Committee (approval number: M11-17). Consent was obtained from members of households before undertaking indoor collections. Written informed consent was obtained before testing individuals for W. bancrofti parasites.










