Exclusively breast-fed infants are susceptible to vitamin D deficiency rickets( Reference Shaw 1 ). Low infant vitamin D status predicts low bone mass at 9 years( Reference Javaid, Crozier and Harvey 2 ). Furthermore, vitamin D deficiency is associated with autoimmune disease( Reference Holick 3 ) and acute lower respiratory tract infections( Reference Dinlen, Zenciroglu and Beken 4 , Reference Ali and McDevitt 5 ) In addition, vitamin D deficiency in the 1st month of fetal life may affect brain cell growth and differentiation and may thereby contribute to psychiatric diseases, such as autism and schizophrenia, at a later age( Reference Eyles, Burne and McGrath 6 ).
The vitamin D status of exclusively breast-fed infants depends on sunlight exposure, vitamin D stores at birth and the breast milk vitamin D output( Reference Dawodu and Akinbi 7 ). Breast milk contains both parent vitamin D and 25-hydroxyvitamin D (25(OH)D). These are usually summed to the so-called antirachitic activity (ARA). Breast milk ARA in Western mothers ranges from 8 to 332 IU/l( Reference Dawodu and Tsang 8 – Reference við Streym, Hojskov and Moller 10 ). The current vitamin D adequate intake (AI) of the Institute of Medicine (IOM) for 0–6-month-old infants is 10 µg/d( 11 ), which translates to an ARA of 513 IU/l in mature breast milk at an average milk consumption of 780 ml/d( Reference Neville, Keller and Seacat 12 ). The AI of the IOM is based on limited data from Finland, South Africa and the USA, showing that 10 µg/d maintains infant serum 25(OH)D at 30–50 nmol/l in the first postnatal year and thereby supports normal bone accretion( 11 ). In view of the low breast milk vitamin D and the current advice to protect 0–6-month-old infants from direct sunlight exposure( 13 ), the American Academy of Pediatrics advices supplementation of all breast-fed infants with 10 µg vitamin D daily( Reference Wagner and Greer 14 ). Most guidelines in European countries are in line with this advice( Reference Braegger, Campoy and Colomb 15 ).
The determinants of milk ARA are maternal vitamin D intake from the diet and supplements, vitamin D stores and sunlight exposure. Vitamin D intakes from dietary sources are unlikely to influence milk vitamin D to a great extent. In the Netherlands, the median dietary vitamin D intake of 19–30-year-old Dutch women is 2·6 μg/d. This intake is only slightly higher than the Dutch AI of 2·5 µg/d( 16 ), and is remote from the daily requirement of 10 µg/d( 17 ). Mushrooms exposed to UV-B harbour highest amounts of vitamin D2, whereas fish like halibut, carp, mackerel, eel and salmon contain highest amounts of vitamin D3 ( 18 ). Supplementation of pregnant and/or lactating women by up to 50 µg/d increases milk with only 23–35 IU/l( Reference Ala-Houhala, Koskinen and Parviainen 9 , Reference Wall, Stewart and Camargo 19 – Reference Saadi, Dawodu and Afandi 21 ). Little is known on the relative contribution of maternal vitamin D stores on milk ARA( Reference Dawodu and Tsang 8 ). Ala-Houhala et al.( Reference Ala-Houhala, Koskinen and Parviainen 9 ) demonstrated seasonal differences in milk ARA in lactating Finnish women (mean ARA 124 IU/l in summer; 14 IU/l in winter) that are driven by the more abundant UV-B radiation in summer.
From an evolutionary point of view, it is puzzling why milk ARA is so low that infants need to be supplemented with vitamin D. To our knowledge, there are no data available on milk ARA of women with lifetime abundant sunlight exposure causing a high vitamin D status. We were interested to see whether milk vitamin D of mothers with lifetime abundant sunlight exposure reaches the current AI for 0–6-month-old infants. In the present study, we investigated the relationship of milk ARA with latitude, maternal vitamin D intake from fish and maternal vitamin D status. For this, we measured milk vitamin D concentrations, milk long-chain n-3 PUFA and maternal plasma 25(OH)D of lactating mothers living in countries at different latitudes and with diverse cultural backgrounds.
Methods
This cross-sectional study is part of the ‘ZOOG’ (‘Zonder Ontsteking Oud en Gezond’ translated: ‘Without Inflammation Old and Healthy’) project. The aim is to study relationships between maternal nutrient status and milk composition in various geographical regions with different cultural backgrounds with the ultimate aim to optimise human milk and formula compositions.
Subjects, sample collection, storage and analysis
Lactating mothers living in the Netherlands (latitude: 53°N), Curaçao (12°N), Vietnam (varying from 10–21°N), Malaysia (3°N) and Tanzania (2°S) were invited to participate in the study. Inclusion criteria were that the women were apparently healthy and well nourished and gave birth to an apparently healthy term infant. Another inclusion criterion was that there were no pregnancy complications. ‘Health and well nourishment’ were self-proclaimed and by visual observation. The inhabitants of The Netherlands and Curaçao consumed typically Western diets, consisting of high carbohydrate and meat intakes, and low vegetable, fruit and fish intakes. One of the participants in Curaçao and all Dutch mothers took supplements that provided 10 µg vitamin D/d, among other vitamins and minerals, during pregnancy and lactation. Most women from the Netherlands and Curaçao had a high socio-economic status. The Halong Bay province is located next to the Gulf of Tonkin, where seafood is widely consumed. Phu Tho is a rural province in northern Vietnam. It is among the poorest areas, where tea cultivation is one of the main agricultural activities. The diet is predominantly composed of rice. Tien Giang is a province in southern Vietnam located in the Mekong Delta. Agricultural and freshwater aquatic products are widely available. Ho Chi Minh City and Hanoi are the largest cities in the south and north of Vietnam, respectively. Most Vietnamese women had a low to average socio-economic status. Especially the women in Phu Tho and Tien Giang had a low socio-economic status. The Malaysian mothers consumed typical Malaysian diets, composed of rice, some fish and meats and vegetables. Most of them completed post-secondary school or college. In Tanzania, we included women from two different tribes: the Maasai and the people in Ukerewe. The Maasai have a semi-nomadic lifestyle. Most women had a low socio-economic status. The current diet of the Maasai consists mainly of beans, ugali (maize porridge), rice and chapatti (maize wheat pancakes) complemented with curdled milk and meat( Reference Oiye, Olesimel and Oniango 22 , Reference Luxwolda, Kuipers and Koops 23 ). The people in Ukerewe (island in Lake Victoria) have very high intakes of freshwater fish (>7 times/week). Besides fish, their diets consist of some typically African staple foods such as ugali, muhogo (cassava root), beans and plantain (baked banana)( Reference Luxwolda, Kuipers and Koops 23 ).
Earlier studies by Luxwolda et al. ( Reference Luxwolda, Kuipers and Kema 24 ) in Tanzania, Africa, showed that lactating women in Sengerema (n 28–30), Same (n 23–30) and Maasai (n 6) had mean plasma 25(OH)D levels between 80·1 (sd 27·5) and 99·3 (sd 20·9) nmol/l. Ho-Pham et al. ( Reference Ho-Pham, Nguyen and Lai 25 ) and Nguyen et al. ( Reference Nguyen, von Schoultz and Nguyen 26 ) showed that Vietnamese women living in the South had a higher mean serum 25(OH)D level of 75·3 (sd 14·8) nmol/l compared with 58·0 (sd 18·5) nmol/l for women living in the Northern parts of Vietnam. Using the data of Luxwolda, Ho-Pham and Nguyen, we performed a power analysis and estimated that six to thirty women should be sufficient to provide insight whether women living in regions with adequate vitamin D status have adequate amounts of vitamin D in their milk. Differences in plasma 25(OH)D between two groups of eighteen subjects with mean plasma 25(OH)D concentrations of 99 and 80 nmol/l, respectively, and between two groups of eleven subjects with mean serum 25(OH)D concentrations of 75 and 58 nmol/l, respectively, can be detected with 80 % power at P<0·05.
Samples were collected in the Netherlands, Curaçao, Halong Bay, Phu Tho, Tien Giang, Ho Chi Minh City and Hanoi (all in Vietnam), Kuala Lumpur in Malaysia and in Tanzania from Maasai mothers living in Ruvu and mothers in Ukerewe (Lake Victoria)( Reference Luxwolda, Kuipers and Koops 23 ). The studies in Curaçao, Vietnam, Malaysia and Tanzania were approved by the Ethics Committee of the St. Elisabeth Hospital in Willemstad, Curaçao; the Ethics Committee of The Family Food and Nutrition Institute in Hanoi, Vietnam; the International Medical University (IMU) Joint Committee of the Research and Ethics Committee in Kuala Lumpur, Malaysia; and the National Institute for Medical Research in Dar-es-Salaam, Tanzania (NIMR/HQ/R.8a/Vol.IX/8000), respectively. Women in the Netherlands merely provided us with a milk sample, which waves the need of official medical ethical clearance for its non-invasive nature. All women gave informed and/or written consent. The study was in agreement with the Helsinki declaration of 1975 as revised in 2013.
The mothers in the Netherlands, Curaçao and Vietnam were instructed to save a 25 ml of milk sample that was taken from a completely emptied breast around noon. A quantity of 25 ml milk samples in Malaysia were collected in the morning as far as possible. Tanzanian mothers collected a midstream sample at an undefined time during the day. Non-fasting venous EDTA-anticoagulated blood samples were taken from the women in Curaçao, Vietnam and in Tanzania–Ukerewe on the day of breast milk collection. Plasma was isolated by centrifugation at 2500 g for 10 min. We also had the opportunity to investigate six lactating mother–infants pairs living in Tanzania–Ukerewe (ethics clearance: National Institute for Medical Research in Dar-es-Salaam, Tanzania (NIMR/HQ/R.8a/Vol.IX/8000)). For this we collected milk and blood of the mothers and infants at 4–22 postpartum (PP) weeks. All samples were collected in tubes and immediately frozen and stored at −20°C (the Netherlands, Curaçao, Malaysia and Tanzania) and −80°C (Vietnam). All samples were transported to the University Medical Center Groningen (the Netherlands) on dry ice. They were stored at −20°C until analysis.
Before analysis, the breast milk samples were thawed at 37°C. A 500-µl aliquot was used for analysis of the vitamin D profile. Saponification and liquid–liquid extraction were performed essentially as described by Corso et al.( Reference Corso, Rossi and De 27 ). Analysis took place by liquid-chromatography tandem MS (LC-MS/MS) after derivatisation with 4-[2-(3,4-dihydro-6,7-dimethoxy-4-methyl-3-oxo-2-quinoxalinyl)ethyl]-3H-1,2,4-triazole-3,5(4H)-dione (DMEQ-TAD), as described by Kamao et al.( Reference Kamao, Tsugawa and Suhara 28 ). The inter-assay and intra-assay CV at 1·7–34·8 nmol/l were <15 and 10, respectively, for all four analytes. The quantification limit was 0·1 nmol/l for vitamin D3 and D2 and 0·2 nmol/l for 25(OH)D3 and 25(OH)D2. Vitamin D3 and vitamin D2 were summed to vitamin D, whereas 25(OH)D3 and its D2 analogue were combined to 25(OH)D. For milk ARA calculation, 1 IU/L was equal to 25 pg/ml vitamin D and 5 pg/ml 25(OH)D.
Plasma 25(OH)D3 and 25(OH)D2 (together referred to as 25(OH)D) were measured with isotope dilution-online solid-phase extraction LC-MS/MS, as described by Luxwolda et al.( Reference Luxwolda, Kuipers and Kema 24 ). The milk fatty acid composition was measured by GC with flame ionisation detection( Reference Volmer, Meiborg and Muskiet 29 ). Milk EPA and DHA acids were expressed in g/100 g fatty acids (g%). The inter-assay and intra-assay variations for EPA (0·06 g%) and DHA (0·45 g%) were <15 %( Reference van der Steege, Muskiet and Martini 30 ). We used the milk EPA+DHA content as a proxy for fish intake.
Data analysis and statistics
Cut-off values for vitamin D of 25, 50 and 80 nmol/l( 11 , Reference Henry, Bouillon and Norman 31 , Reference Zittermann 32 ) were applied to report vitamin D deficiency (<25 nmol/l), vitamin D insufficiency (25–49·9 nmol/l), hypovitaminosis D (50–79·9 nmol/l), vitamin D sufficiency (80–249·9 nmol/l) and vitamin D toxicity (≥250 nmol/l).
The IBM PASW Statistics 22 software was used. As not all data were Gaussian distributed, we reported medians and ranges. Results below the limit of quantification were used, because this did not alter our conclusions. Relationships were analysed by use of bivariate correlation analysis and using the Spearman’s ρ test for calculating r. A linear regression model was made, using backwards regression, to assess determinants of milk ARA. The milk ARA was log-transformed as these data were not normally distributed. As covariates, we used maternal plasma 25(OH)D, latitude, milk DHA+EPA, maternal age, lactation duration and BMI. A P value <0·05 was considered significant.
Results
Study population, milk antirachitic activity, plasma 25-hydroxyvitamin D and milk EPA+DHA
Table 1 shows the characteristics of the investigated mothers and their infants, together with the milk ARA, maternal plasma 25(OH)D (available for Curaçao (n 9), Vietnam (n 100) and Tanzania-Ukerewe (n 20)), and the milk EPA+DHA contents. None of the 181 lactating mothers, that is nine in the Netherlands, ten in Curaçao, 101 in Vietnam, twenty in Malaysia and forty-one in Tanzania, reached the vitamin D IOM AI in their milk. Milk ARA was mainly composed of vitamin D3 and 25(OH)D3. Their D2 analogues were generally below the detection limit. The median milk ARA of the total population was 45 IU/l (range: 1–247 IU/l).
Table 1 Mothers and infants (Medians and ranges; numbers and percentages)
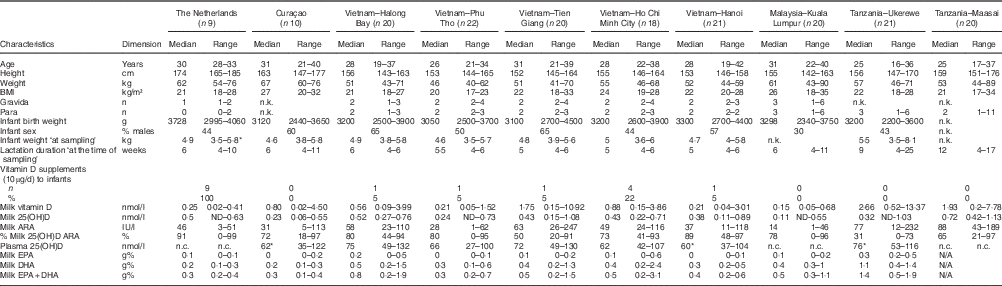
n.k., not known; ARA, antirachitic activity; 25(OH)D, 25-hydroxyvitamin D; n.c., not collected; N/A, not analysed.
* Not complete: 1 missing.
Fig. 1 shows, for each of the subpopulations, the milk ARA as box plots (medians, ranges, 25th–75th percentiles and 5th–95th percentiles). The women in The Netherlands, Malaysia, Vietnam–Hanoi and Vietnam–Phu Tho exhibited the lowest milk ARA, whereas those in Tanzania–Ukerewe, Tanzania–Maasai and Vietnam–Tien Giang showed the highest. It appeared that low latitude is no guarantee for adequate milk ARA. We found median plasma 25(OH)D concentrations of 71 (range 27–132) nmol/l in the mothers from Curaçao, Vietnam and Tanzania–Ukerewe.
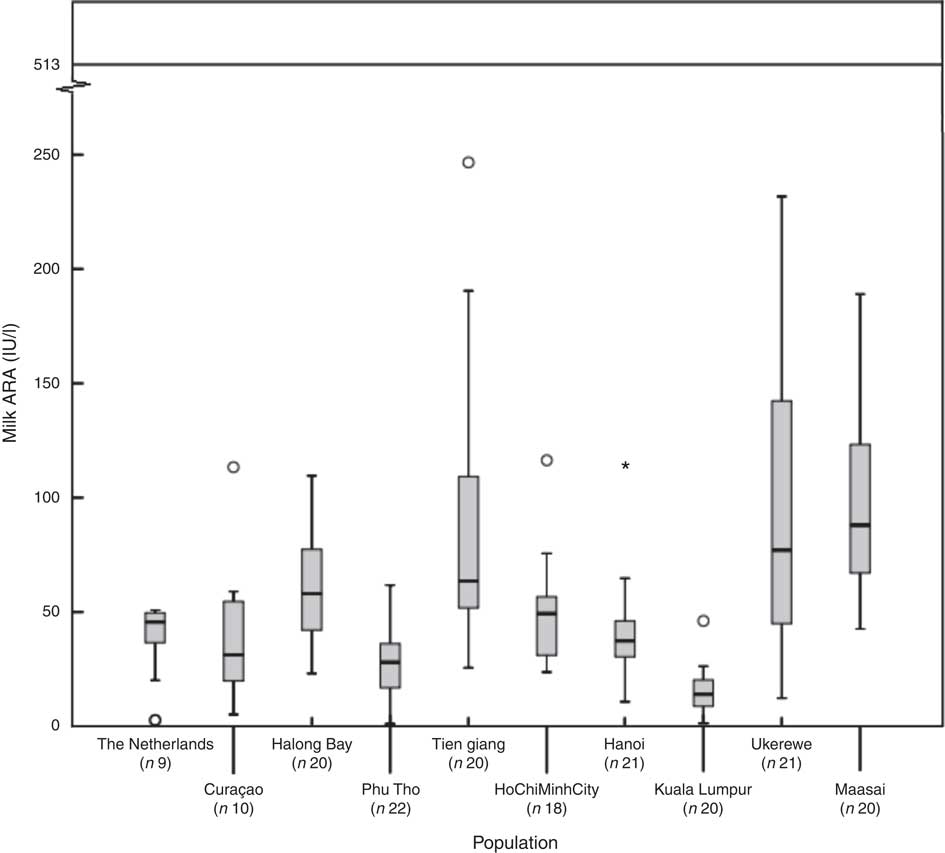
Fig. 1 Milk antirachitic activity (ARA) of the investigated populations. Adequate intake of 10 μg vitamin D/d (milk ARA of 513 IU/l) for 0–6-month-old infants, as set by the Institute of Medicine. *Outlier.
We found that none of these women had vitamin D deficiency (25(OH)D <25 nmol/l), 19·4 % had vitamin D insufficiency (25–49·9 nmol/l), 47·3 % had hypovitaminosis D (50–79·9 nmol/l) and 33·3 % had sufficient vitamin D status (80–249·9 nmol/l). The milk EPA+DHA content ranged from 0·1 to 3·1 g% with a median of 0·4 g%. Lowest medians were found in the Netherlands, Curaçao and Vietnam–Phu Tho and highest in Tanzania–Ukerewe (located at Lake Victoria).
Determinants of milk antirachitic activity
Fig. 2 shows the relationships between maternal plasma 25(OH)D and milk ARA (Fig. 2(a); r 0·48, P<0·01), maternal plasma 25(OH)D and milk vitamin D (Fig. 2(b); r 0·43, P<0·01) and maternal plasma 25(OH)D and milk 25(OH)D (Fig. 2(c); r 0·38, P<0·01), for mothers in Curaçao, Vietnam and Tanzania–Ukerewe. Fig. 3 shows the relationships between milk EPA+DHA and milk ARA (Fig. 3(a); r 0·41, P<0·01), milk EPA+DHA and milk vitamin D (Fig. 3(b); r 0·51, P<0·01) and milk EPA+DHA and milk 25(OH)D (Fig. 3(c); r 0·15, P=0·06), for mothers in the Netherlands, Curaçao, Vietnam, Malaysia and Tanzania–Ukerewe.
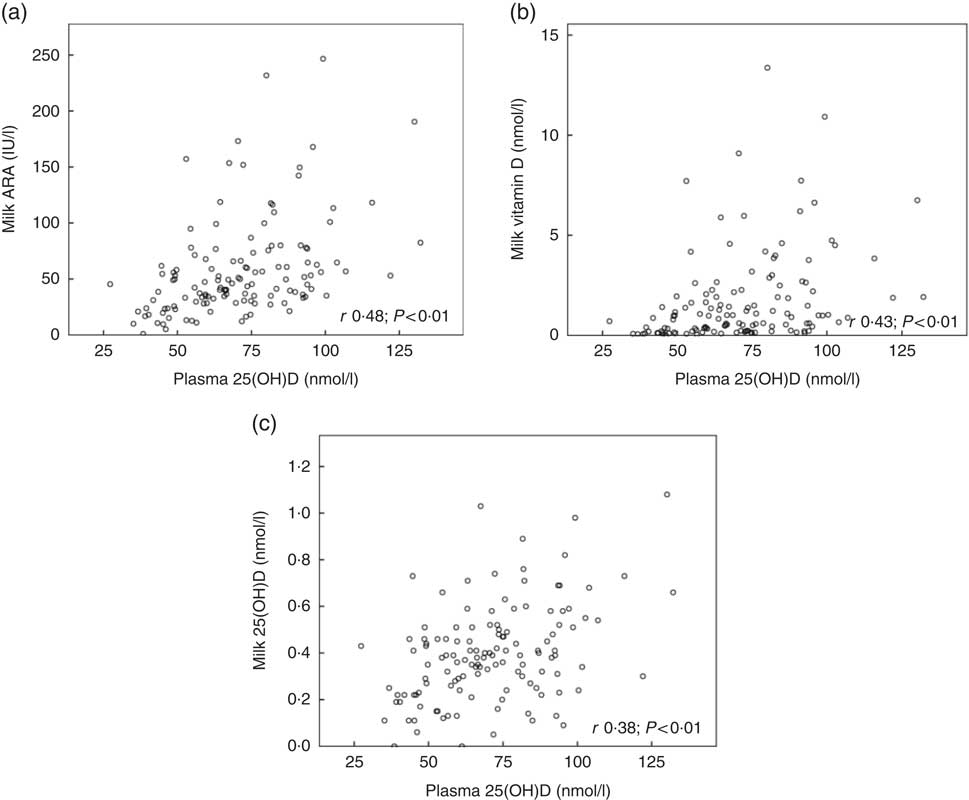
Fig. 2 Relationships between maternal plasma 25-hydroxyvitamin D (25(OH)D) and milk antirachitic activity (ARA) (a), maternal plasma 25(OH)D and milk vitamin D (b) and maternal plasma 25(OH)D and milk 25(OH)D (c) for 129 healthy lactating mothers in Curaçao, Vietnam and Tanzania-Ukerewe.
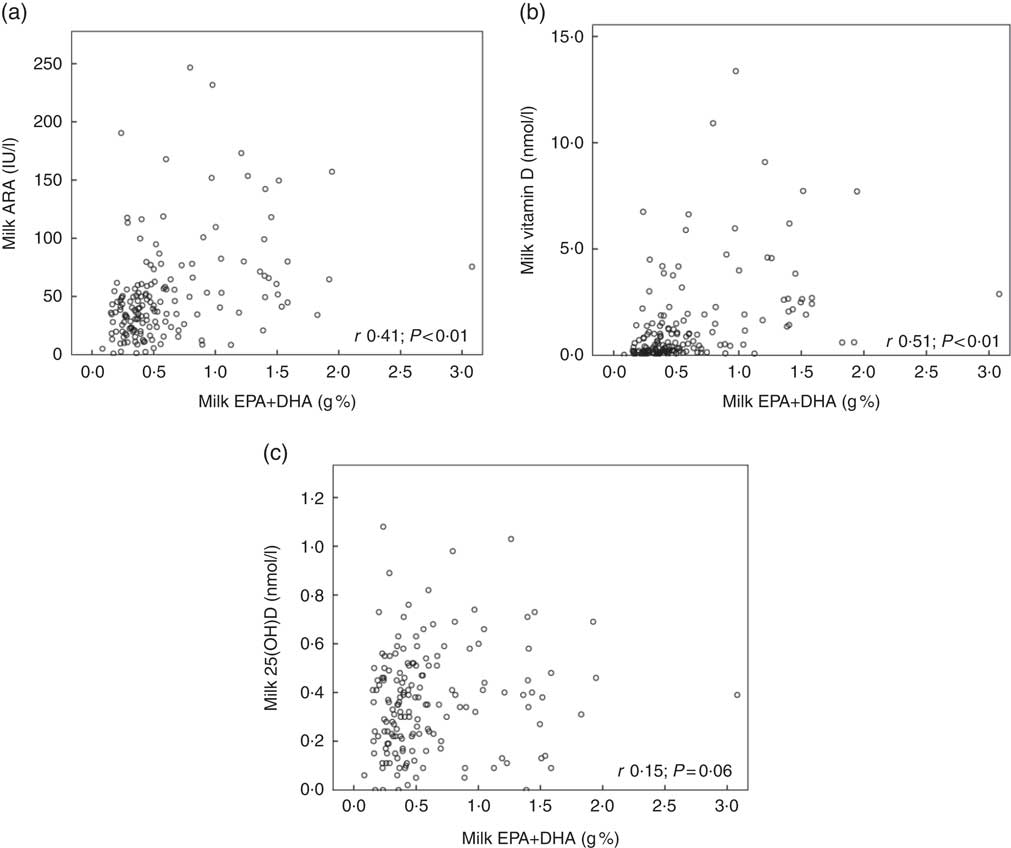
Fig. 3 Relationships between milk EPA+DHA and milk antirachitic activity (ARA) (a), milk EPA+DHA and milk vitamin D (b) and milk EPA+DHA milk and 25-hydroxyvitamin D (25(OH)D) (c) for 161 healthy lactating mothers in the Netherlands, Curaçao, Vietnam, Malaysia and Tanzania-Ukerewe. Milk DHA+EPA were used as a proxy of fish intake.
Using backwards regression in a linear regression model with integrated data from Curaçao, Vietnam and Tanzania–Ukerewe, we found that milk ARA was related to maternal plasma 25(OH)D (β 0·40; P<0·01), latitude (β −0·21; P<0·01) and milk EPA+DHA (β 0·20; P<0·05). The model had an adjusted R 2 of 0·34. In the linear regression model with backwards regression, we found that maternal age, lactation duration and BMI were unrelated with milk ARA.
Six lactating mother–infant pairs living in Tanzania–Ukerewe
The online Supplementary Fig. S1 shows the individual mother–child relationship for plasma 25(OH)D at 4–22 PP weeks. Maternal plasma 25(OH)D ranged from 53 to 93 nmol/l; their exclusively breast-fed infants exhibited 52–85 nmol/l, whereas the milk ARA ranged from 34 to 173 IU/l.
Discussion
In this study, we determined the milk ARA of mothers living in five different countries (the Netherlands, Curaçao, Vietnam, Malaysia and Tanzania), located at latitudes ranging from 2°S to 53°N. The median milk ARA concentration of the entire study population was 45 IU/l (range: 1–247 IU/l; n 181; Table 1). None of the milk samples reached the IOM AI of 513 IU/l (Fig. 1). The median maternal 25(OH)D of the women in Curaçao, Vietnam and Tanzania–Ukerewe was 71 (range: 27–132) nmol/l. Maternal plasma 25(OH)D, latitude and milk EPA+DHA were determinants of milk ARA (Fig. 2 and 3), whereas maternal age, lactation duration and BMI were unrelated.
The finding of maternal plasma 25(OH)D, latitude (a proxy for UV-B exposure) and milk EPA+DHA (proxy for fish intake) as determinants of milk ARA is in agreement with the influences of maternal sunlight exposure, maternal vitamin D intake and possibly maternal stores( Reference Dawodu and Tsang 8 , Reference Wall, Stewart and Camargo 19 ). The milk ARA outputs were not even close to the AI of the IOM for 0–6-month-old infants. The low milk ARA, especially in mothers with sufficient vitamin D status, or those with lifetime exposure to all-year-round abundant sunshine, are puzzling from an evolutionary point view. This finding raises the question of milk vitamin D adequacy for infants who in the past were born at latitudes with insufficient vitamin D UV-B synthesising capacity, notably in winter. The loss of skin pigmentation following the Out-of-Africa diaspora, occurring independently in Europeans and Asians, is widely acknowledged to reflect adaptation to the lower UV-B exposure that comes along with higher latitudes( Reference Chaplin and Jablonski 33 ). That sunlight exposure seems unable to raise milk ARA to the IOM AI was previously suggested by the study of Greer et al.( Reference Greer, Hollis and Cripps 34 ). They demonstrated a maximal milk vitamin D3 increase of only 60 IU/l, following 2 d total body UV-B exposure of healthy lactating white women to a 1·5 minimal erythemal dose. This level of exposure approximated 30 min sunshine at mid-day on a clear summer day at temperate latitudes. The inability of lactating mothers to reach the IOM AI of 10 μg vitamin D/d by natural means became also illustrated by the very high supplemental dose needed to reach this level. Daily supplementation of 50 μg (2000 IU) from 27 gestational weeks resulted in a milk ARA of only 64 IU/l at 2 weeks PP and 56 IU/l at 2 months PP( Reference Wall, Stewart and Camargo 19 ). Wagner et al.( Reference Wagner, Hulsey and Fanning 35 ) showed that supplementing lactating mothers with 160 µg (6400 IU) vitamin D/d from 1 month PP for 6 months caused a gradual increase of the milk vitamin D concentration to reach an ARA of 873 IU/l at the study end. This study was followed by a multi-year, two-site RCT demonstrating this approach to be both safe and effective for providing adequate vitamin D to the nursing infant( Reference Hollis, Wagner and Howard 36 ). Even though there are no apparent safety risks with an intake of 160 µg vitamin D/d, this dosage is well above the current upper limit of 100 µg/d( 37 ) and corresponds with the daily consumption of about 1·7 kg average pelagic marine fish( Reference Sioen, De Henauw and Van Camp 38 ).
The inability of milk to reach the IOM vitamin D AI by natural means suggests that either the needed oral intake for 0–6-month-old infants is much lower than the currently recognised 10 µg AI or that breast-fed infants should rely on their vitamin D stores. The high plasma 25(OH)D concentrations in the six lactating mother–child pairs in Tanzania–Ukerewe suggest that a lifetime high maternal vitamin D status of the mother is capable of guaranteeing an adequate vitamin D status in her exclusively breast-fed child, despite her relatively low milk ARA. In view of the local culture and habits, other potential infant vitamin D sources, such as sun exposure, non-human milk dietary sources or supplements, are unlikely. Little is known on the sizes of vitamin D stores in humans and the factors determining their mobilisation. However, fasting of previously vitamin-D-loaded rats caused a slower 25(OH)D decline, compared with ad libitum-fed counterparts( Reference Brouwer, van Beek and Ferwerda 39 ). Didriksen et al. ( Reference Didriksen, Burild and Jakobsen 40 ) recently showed that vitamin D3 increased in abdominal subcutaneous fat tissue after supplementation with vitamin D3. It is well known that the body fat mass of non-pregnant subjects exhibits an inverse relation with plasma 25(OH)D( Reference Snijder, van Dam and Visser 41 ).
Our previous study showed a higher 25(OH)D in pregnant women in Tanzania from the first trimester until delivery, when compared with non-pregnant counterparts( Reference Luxwolda, Kuipers and Kema 24 ). After delivery, plasma 25(OH)D dropped steeply to reach non-pregnant values. Such changes were not observed in women living in Western countries( Reference Dent and Gupta 42 – Reference Salle, Delvin and Lapillonne 44 ). We suggested that the observed course of 25(OH)D in traditionally living pregnant women in Tanzanian may reflect mobilisation of vitamin D from adipose tissue stores( Reference Luxwolda, Kuipers and Kema 24 ) and/or 25(OH)D from muscle( Reference Abboud, Rybchyn and Rizk 45 ). Among the various organs, adipose tissue has been estimated to contain about 75 % of total body vitamin D, whereas 25(OH)D is more evenly distributed with high contents in adipose tissue, serum and muscle( Reference Heaney, Horst and Cullen 46 ). The driver of vitamin D mobilisation from fat might be the state of ‘accelerated starvation and facilitated anabolism’ that is operational throughout pregnancy to ensure sufficient fluxes of glucose, fatty acids and other nutrients for the benefit of the fetus( Reference Hadden and McLaughlin 47 ). Trapping of vitamin D in the maternal circulation may occur by oestrogen-induced augmentation of circulating vitamin D binding protein (DBP). Plasma DBP has short half-life of 2·5–3 d and falls immediately after delivery( Reference Cooke and Haddad 48 ). Vitamin D may easily traverse the placenta because of its relatively low affinity for DBP, whereas the more tightly bound 25(OH)D may traverse mostly via the cubilin-megalin system( Reference Hollis and Wagner 49 ). The TAG in the rapidly growing, mostly subcutaneous, fetal adipose compartment, are about 80 % de novo synthesised from polar precursors, notably glucose and lactate. This rapidly growing, vitamin-D-naive, fat compartment, harbouring 50 % TAG at the pregnancy end( Reference Kuipers, Luxwolda and Offringa 50 ), might exert high attraction for the (hydrophobic) vitamin D from maternal origin. The concerted action of oestrogens, DBP and the lower insulin sensitivity in pregnancy may jointly facilitate an almost one-way transplacental transfer of hydrophobic nutrients, and thereby contribute to the equilibration of vitamin D between maternal and fetal stores. Following this line of reasoning, it would be the maternal adipose tissue vitamin D content, that is in particular the maternal lifetime sun exposure and vitamin D intake, that determines the magnitude of the infant’s vitamin D store at birth. Taken together, we propose that the vitamin D status of exclusively breast-fed infants might notably be dependent on maternal stores and the ability to mobilise these stores during pregnancy and lactation. Like the infant status of Fe( Reference Ziegler, Nelson and Jeter 51 ) and vitamin B12 (E Stoutjesdijk, unpublished results), the vitamin D status of exclusively breast-fed infants might be strongly dependent on the size of their stores acquired during fetal life. Finally, although postnatal adequate infant vitamin D status can be reached by supplements or human milk( Reference Wagner, Hulsey and Fanning 35 ), it might be better to ensure adequate vitamin D status during both fetal and early postnatal life. This notion shifts the attention of adequate vitamin D status from pregnancy and lactation to preconception.
Limitations of this study are the inter-individual differences in the duration of lactation (4–25 weeks), the different milk collection methods, the incomplete collection of blood samples and differences in season. We had no information on the plasma 25(OH)D levels of the women living in the Netherlands, Malaysia and Maasai. In our linear regression model, we did not identify lactation duration as a confounder. Moreover, the IOM AI is defined for the entire period from birth to 6 postnatal months. Ala-Houhala et al.( Reference Ala-Houhala, Koskinen and Parviainen 9 ) and við Streym et al.( Reference við Streym, Hojskov and Moller 10 ) found differences in milk vitamin D and 25(OH)D between fore- and hind milks. These differences in median milk ARA did not exceed 50 IU/l, suggesting that sampling imperfections did not affect our conclusions. Regarding differences in season, as most of our studied population live close to the equator, other factors such as short and longer rainy periods, air pollution, clothing and sun-avoiding behaviour could be greater sources of inter-individual variation. Other limitations are that we did not collect dietary data. An analytical limitation is that many of the milk vitamin D and 25(OH)D concentrations were close to the detection limit of our method. This is, however, a problem with all published ARA data until now and does not take away the message that milk ARA does not reach the 10 µg vitamin D AI of the IOM. Finally, the number of studied mothers and mother–infant pairs was low.
In conclusion, milk ARA of mothers, including mothers with lifetime abundant sunlight exposure, is not even close to the 10 µg vitamin D AI for 0–6-month-old infants. Maternal plasma 25(OH)D, latitude and milk EPA+DHA are related with milk ARA. The increase of plasma 25(OH)D in traditionally living pregnant women, the increase of DBP during pregnancy and the intra-uterine growth of a sizeable vitamin-D-naive fetal adipose compartment suggest that, during pregnancy, maternal vitamin D may become transferred across the placenta to subsequently equilibrate with fetal stores. Storage of 25(OH)D in muscle and its mobilisation by physical activity( Reference Abboud, Rybchyn and Rizk 45 ) is another, yet poorly understood, option. Our data may point at the importance of adequate fetal vitamin D stores and shifts the attention from adequate vitamin D status during pregnancy and lactation to adequacy starting before conception. Information on maternal and fetal adipose tissue vitamin D contents, 25(OH)D in muscle and the factors governing their mobilisation are needed.
Acknowledgements
The authors thank the UMCG Laboratory for Special Chemistry for analyses, Martine Luxwolda, Remko Kuipers, Jan van der Molen and master students Willem Abma, Valesca Wangsawirana and Wietske Hemminga for their participation in this study.
This work was supported by Ministry of Economic Affairs, the Provinces of Groningen and Drenthe.
Designed research: all authors; conducted research: E. S., N. V. N. and G. L. K.; statistical analysis: E. S.; wrote the paper: E. S., D. A. J. D.-B. and F. A. J. M.; primary responsibility for final content: F. A. J. M. All Authors have read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451700277X







