1. Introduction
1.1. Background
Cigarette smoking is probably one of the most widespread and proven risk factors threatening public health that the world has ever faced, killing an estimated 6 million people every year(1,Reference Proctor2) . More than 5 million of those deaths are the result of direct smoking, while over 600 000 premature deaths result from the exposure of non-smokers to second-hand smoke(Reference Schroeder3). Smokers die, on average, more than a decade before non-smokers(Reference Jha, Ramasundarahettige and Landsman4). This premature mortality is because long-term exposure (lasting over three to four decades) to cigarette smoke (CS) is associated with an increased risk of developing serious CS-related diseases.
Evidence from epidemiologic and meta-analyses is sufficient to infer a causal relationship between CS and the development of lung cancer. CS accounts for 80 % and 50 % of the worldwide lung cancer outbreak in men and women, respectively(5,Reference Heuvers, Hegmans and Stricker6) . Although lung cancer incidence rates are decreasing in men in Europe and North America, they have increased in Asia and Africa(5) and in women, reflecting changes in smoking habits(Reference Lortet-Tieulent, Soerjomataram and Ferlay7). CS is the major recognised risk factor for oral cavity and pharyngeal cancer, with relative risks in the order of five to ten times higher for smokers than for non-smokers(8). A causal relationship has also been established between CS and chronic obstructive pulmonary disease (COPD), which causes a greater predisposition to the Mycobacterium tuberculosis disease(9). CS also exacerbates asthma, though data are only suggestive but not sufficient to infer a causal link between CS and asthma incidence(9,Reference Papaioannou, Koutsokera and Tanou10) .
Although CS-related diseases originate mainly in the organs that are directly exposed to CS, the toxic substances contained in CS can also reach other organs through the circulatory system and cause damage that eventually leads to the development of many other diseases. Indeed, the gas phase of CS crosses the lung alveolar wall and enters the bloodstream(Reference Yamaguchi, Nasu and Harada11), where it can interact with both water-soluble and lipid-soluble circulating plasma antioxidants, resulting in their depletion(Reference Alberg12). Plasma antioxidants, whose role and physiological activity have been exhaustively reviewed in a recent paper(Reference Stocker13), include low-molecular-mass vitamins A, C and E, uric acid, α- and γ-tocopherol, α- and β-carotene, β-cryptoxanthin, lutein and zeaxanthin, lycopene, and retinol(Reference Halliwell and Gutteridge14), low-molecular-mass reduced aminothiols (i.e. glutathione (GSH) and cysteine)(Reference Rossi, Giustarini and Milzani15), and high concentrations (∼600 μM, i.e. ∼43 mg/ml) of reduced albumin(Reference Colombo, Clerici and Giustarini16).
A causal relationship has been demonstrated between CS and colorectal polyps(Reference Figueiredo, Crockett and Snover17–Reference Davenport, Su and Zhao19), colorectal cancer and hepatocellular carcinoma(9). CS is also associated with a relatively modest increase in the risk of developing kidney cancer(Reference Chow, Dong and Devesa20). In women, CS also increases breast cancer risk(Reference Dossus, Boutron-Ruault and Kaaks21). The evidence at our disposal does not suggest the presence of any causal relationship between CS and the risk of developing prostate cancer, although there is a higher risk of death from prostate cancer in smokers than in non-smokers(9,Reference Islami, Moreirad and Boffetta22) . CS is also a proven risk factor for diabetes mellitus, rheumatoid arthritis and impairment of the immune system(9). Moreover, CS is responsible for an increased risk of cardiovascular diseases (CVD) and related atherosclerosis(23), acute coronary heart disease and stroke(Reference Mons, Müezzinler and Gellert24), and is the most important risk factor for abdominal aortic aneurysm(Reference Jahangir, Lipworth and Edwards25,Reference Tang, Yao and Roetker26) . Smoking is associated with enhanced oxidative stress, which favours the progression of CVD. Current smokers with CVD, who have survived a coronary heart disease event, such as acute myocardial infarction or ischaemia, would particularly benefit from quitting smoking, because this could prevent their next, potentially fatal CVD event(Reference Reiner27). Although the CVD risk as well as the possible death from CVD events is very high in such patients, a considerable number of them (up to about a quarter), astonishingly, still smoke and have no intention of quitting smoking(Reference Reiner27,Reference Prugger, Wellmann and Heidrich28) . In addition, exposure to passive smoking may compromise smoking cessation among patients with coronary heart disease(Reference Prugger, Wellmann and Heidrich29).
In general, the most important way to prevent CVDs is to constantly adopt a healthy lifestyle. A healthy diet, abstinence from smoking and avoidance of exposure to second-hand smoke are among the actions of the first prevention of CVD(Reference Arnett, Blumenthal and Albert30). The good news for smokers is that smoking cessation reduces CVD risk. A recent investigation analysed data from the Framingham Heart Study to determine the association between years since smoking cessation and subsequent CVD risk among former smokers versus persistent smokers and never smokers(Reference Duncan, Freiberg and Greevy31). The retrospective analysis included a population of 8770 individuals and found that, compared with current heavy smoking, smoking cessation among former heavy smokers was associated with lower CVD risk within 5 years of cessation, corroborating analogous findings demonstrated by others (e.g. ref. Reference Ahmed, Patel and Nyaku32 ). However, compared with never smokers, the CVD risk of former smokers remained significantly elevated beyond 5 years after smoking cessation(Reference Duncan, Freiberg and Greevy31).
Oxidative burden can play an important role in the pathological conditions resulting from long-term exposure to CS. It can be due to direct oxidative damage or indirect pathways, such as antioxidant depletion or inflammation. Indeed, low-grade systemic inflammation is evident in smokers as confirmed by elevated levels of inflammation biomarkers(Reference McElroy, Carmella and Heskin33,Reference Yanbaeva, Dentener and Creutzberg34) . Since CS is a primary source of oxidants, which induce oxidative stress/damage in smokers, animal models and in vitro cell models(Reference Colombo, Dalle-Donne and Orioli35–Reference Dalle-Donne, Garavaglia and Colombo40), the bolstering of body’s antioxidant defences through antioxidant supplementation or an antioxidant-rich diet could potentially mitigate some of the CS-induced damage.
Numerous studies have focused on the utility of antioxidant supplementation. However, whether antioxidant supplementation has any preventive and/or therapeutic effect in CVD has not yet been irrefutably established. Clinical human studies have supported the association between oxidative stress and cardiovascular events thanks also to the use of several oxidative stress biomarkers, which can also be used to assess the preventive/protective effect of antioxidant supplementation in patients with CVD risk, including smokers(Reference Cammisotto, Nocella and Bartimoccia41).
In this review, we focus on the antioxidant profile in plasma of smokers, the evaluation of which is an essential step in assessing the efficacy and safety of potential interventional strategies. Besides, we also discuss the effectiveness of antioxidant supplements and antioxidant-rich diets in smokers.
1.2. Composition of cigarettes and their regulation
Cigarette composition has changed over time. The main changes occurred in the 1950s: ‘tar’ and nicotine content declined, and other CS constituents changed correspondingly, primarily because of the introduction of filter tips, the selection of tobacco varieties, the utilisation of highly porous cigarette paper and the changing composition of the tobacco blend (with the incorporation of reconstituted and expanded tobaccos). According to the technical notes of the Joint Research Centre of the European Commission, prior to 1955, probably no brand of cigarettes had a tar content below 35 mg. Since then, the introduction of the new technologies for cigarette manufacturing have led to a decline in tar, nicotine and carbon monoxide content(Reference Geiss and Kotzias42). Concurrently, nitrate content increased. This enhanced the combustion of tobacco, decreasing the amount of polynuclear aromatic hydrocarbons, carbon monoxide and phenols, but simultaneously increasing the generation of nitrogen oxides that promote the formation of the carcinogenic N-nitrosamines, especially the tobacco-specific N-nitrosamines(Reference Hoffmann and Hoffmann43).
In the 1960s, many countries introduced the first laws to reduce nicotine and tar content and to inform consumers about the harmful compounds contained in cigarettes. In this regard, a recent and important European recommendation is the Directive 2001/37/EC, adopted by the European Parliament and the Council on 5 June 2001. This directive contained provisions for cigarette design to reduce tar content and to set limits for nicotine and carbon monoxide amount; it indicates warnings on cigarette packets and prohibits misleading descriptors(Reference McNeill, Joossens and Jarvis44). Nowadays, the revised Directive 2014/40/EU, approved by the European Parliament on 26 February, strengthened the rules on how tobacco products are manufactured, produced and presented in the European Union (EU) (e.g. each cigarette pack has to contain at least twenty sticks; messages on packs suggesting that a product is less harmful that another one are forbidden) and introduced specific rules for certain tobacco-related products, such as tobacco flavourings and electronic cigarettes (e-cigarettes)(45). About this last point, the Directive aims at ensuring an equal treatment across the EU for nicotine-containing e-cigarettes. Safety and quality requirements for e-cigarettes are necessary to monitor and learn more about these products, often seen as fashionable, less harmful and healthier than regular cigarettes, especially among young people.
1.3. Oxidative stress, inflammation and oxidative stress: the vicious cycle of cigarette smoke
CS contains nicotine, polycyclic aromatic hydrocarbons, quinones, benzo(α)pyrene, hydrogen cyanide, carbon monoxide and dioxide, pyridine alkaloids, ammonia, phenols, N-nitrosamine, reactive oxygen species (ROS) and reactive nitrogen species (RNS), and saturated (acetaldehyde, formaldehyde) and α,β-unsaturated aldehydes (acrolein and crotonaldehyde), which are particularly harmful because of their high reactivity and toxicity(Reference Lu, Cai and Kong46,Reference Rodgman and Perfetti47) . Aldehydes are long-lived compared with most ROS/RNS and oxidising intermediates, with half-lives ranging from a few hours to days, and therefore, they can spread over long distances, causing airway inflammation that leads to the development of respiratory diseases(Reference Moretto, Facchinetti and Southworth48–Reference Yeager, Kushman and Chemerynski50). Moreover, aldehydes may get into systemic circulation and can cause damage to the vasculature and to different organ systems(Reference Grootveld, Percival and Leenders51).
There is clear evidence that long-term exposure to CS can cause oxidative stress to the entire organism, as reflected by increased levels of oxidative stress biomarkers in the plasma and urine of smokers, including plasma F2-isoprostanes(Reference Seet, Lee and Loke52), malondialdehyde(Reference Lykkesfeldt, Viscovich and Poulsen53–Reference Chelchowska, Ambroszkiewicz and Gajewska55), carbonylated proteins(Reference Pignatelli, Li and Boffetta56–Reference Kocyigit, Selek and Celik58) and urinary eicosanoids(Reference McElroy, Carmella and Heskin33).
CS also stimulates an inflammatory response characterised by the activation of macrophages and the recruitment/activation of neutrophils, eosinophils, monocytes and lymphocytes in the respiratory tract(Reference Rahman59,Reference Crotty Alexander, Shin and Hwang60) . An important, though not unique, mechanism by which CS produces an inflammatory response is the activation of the nuclear factor (NF)-κB pathway(Reference Goncalves, Coletta and Silverio61,Reference Rom, Avezov and Aizenbud62) via the generation of ROS/RNS and aldehydes, such as acrolein and crotonaldehyde(Reference Rom, Avezov and Aizenbud62). This results in NF-κB translocation into the cell nucleus, where it induces transcription of many genes involved in immune regulation. The release of chemo-attractants is not just limited to inflammatory cells directly exposed to CS. Structural lung cells such as pulmonary fibroblasts, alongside their important role in maintaining the extracellular matrix and in repairing tissue after injury, may also play a role in inflammatory responses by releasing interleukin-8 (IL-8) and other chemokines(Reference Numanami, Koyama and Nelson63,Reference Li, Ning and Matthay64) . CS also activates endothelial cells, resulting in the release of inflammatory mediators and chemo-attractive cytokines such as IL-6 and IL-8(Reference Crotty Alexander, Shin and Hwang60). Furthermore, CS induces the release of IL-8 from human airway smooth muscle cells, and the effect is enhanced by tumour necrosis factor (TNF)-α(Reference Oltmanns, Chung and Walters65). CS also contributes to additional oxidative stress by the release of ROS/RNS from macrophages, neutrophil and eosinophil granulocytes, which migrate from the blood to lung parenchyma(Reference Rahman59) (Fig. 1). In addition to an elevated oxidant load and inflammation in the respiratory tract and lungs, smokers experience increased systemic oxidative stress and inflammation(Reference Yanbaeva, Dentener and Creutzberg34,Reference Rueff-Barroso, Trajano and Alves66,Reference Barreiro67) .
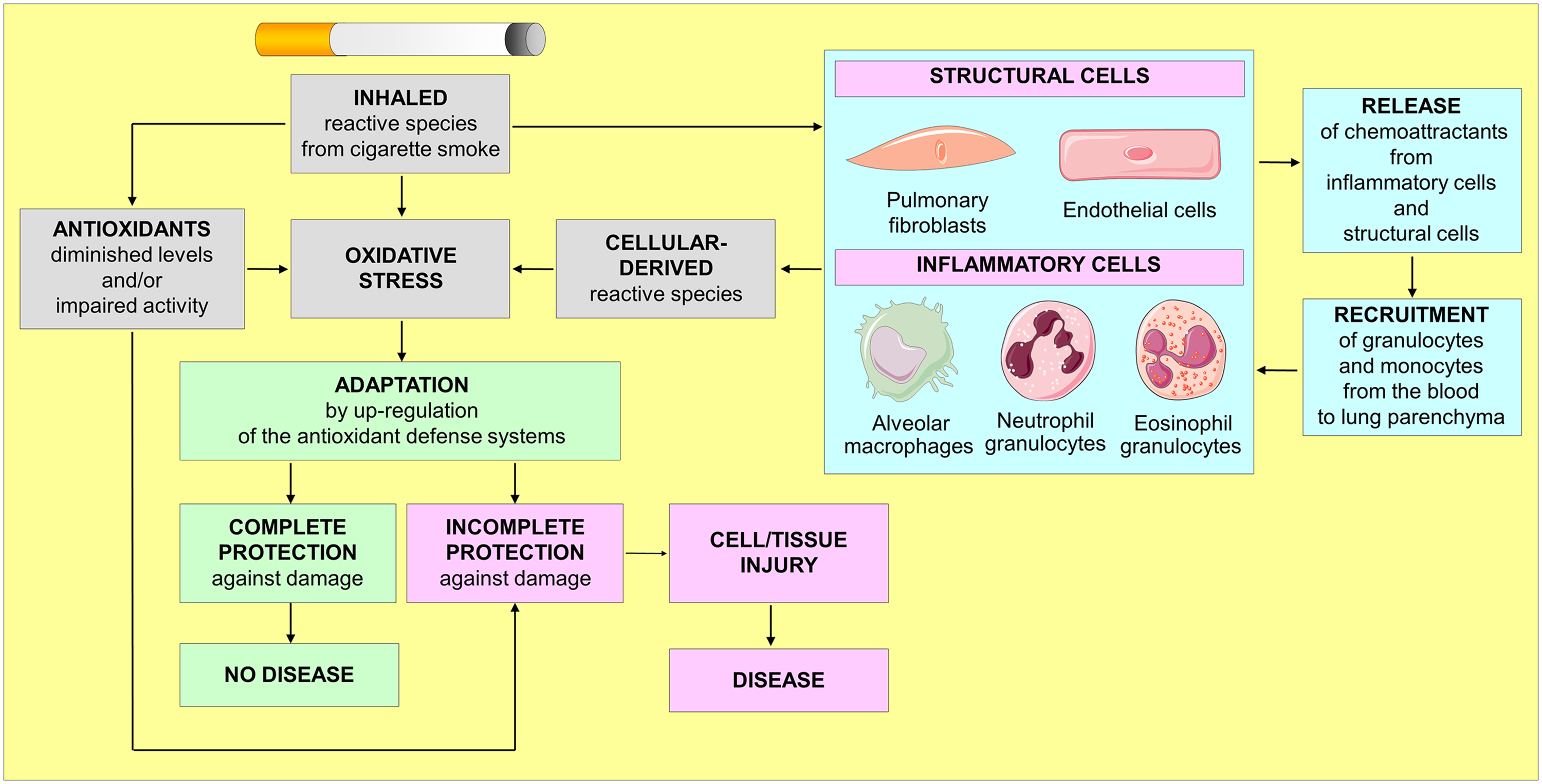
Fig. 1. The vicious cycle of oxidative stress in smokers.
The inhaled CS reactive species represent only a portion of the total oxidative stress eventually experienced by smokers, as the CS also contributes to the formation of further endogenous reactive species formation from inflammatory cells. When activated, inflammatory cells together with structural cells (e.g. pulmonary fibroblasts and endothelial cells) initiate an inflammatory cascade that triggers the release of inflammatory mediators which sustain the inflammatory process and lead to tissue damage as well as a range of systemic effects. After years of chronic smoking, this vicious cycle leads to a permanent oxidative injury of cells and tissue, thereby promoting diseases.
Under physiological conditions, the human body counterbalances the excessive production of ROS/RNS with the antioxidant defence systems, which neutralise ROS/RNS and maintain the balance between oxidants and antioxidants(Reference Halliwell and Gutteridge14). Antioxidant defence systems consist of enzymatic and non-enzymatic systems (endogenous antioxidants) and a variety of low-molecular-weight molecules, most of which derive from dietary sources (exogenous antioxidants) (Fig. 2). Diet-derived antioxidants dissipate during their reaction with reactive species and can be replenished through the diet. Furthermore, many antioxidant enzymes, such as catalase, glutathione peroxidase, heme oxygenase and superoxide dismutase, require micronutrient cofactors such as selenium, iron, copper, zinc and manganese for optimal catalytic activity.

Fig. 2. The endogenous/exogenous antioxidant defence systems.
An antioxidant is any substance (endogenous or exogenous, natural or synthetic) which, when present at low concentration compared with that of an oxidisable substrate, significantly prevents its oxidation, or delays it. The antioxidant defence system consists of both enzymatic and non-enzymatic (endogenous antioxidants) systems, along with a variety of low-molecular-weight antioxidants, most of which are derived from dietary sources (exogenous antioxidants). In the human body, endogenous antioxidant defence systems work in synergy with exogenous antioxidants.
In smokers, inhaled CS overwhelms all these antioxidant defence systems and causes a subsequent decrease in antioxidant levels, thus promoting additional oxidative stress. This vicious cycle leads to an enduring and systemic pro-oxidative state within the body that causes oxidative modifications of nucleic acids, lipids and proteins, eventually promoting cell/tissue injury and diseases(Reference Crotty Alexander, Shin and Hwang60).
2. Antioxidants in smokers
2.1. Antioxidant profile in blood plasma of smokers
Although the inhaled CS mainly reaches the respiratory tract, where it causes an adaptative local antioxidant response in the lung (i.e. boost of GSH levels)(Reference Gould, Min and Gauthier68,Reference Gould, Min and Gauthier69) , which is lower in older smokers than in younger (18–30 years) smokers(Reference Gould, Min and Huang70), the toxic substances contained in CS can also reach other organs through the circulatory system, thus causing the lowering of the concentration of almost all low-molecular-mass antioxidants in the blood plasma.
Some studies have shown that smokers have lower plasma concentrations of almost all low-molecular-mass antioxidants than non-smokers (Table 1). This condition results from at least two factors, one that depends on the diet and one caused directly by CS. Epidemiological studies showed that smokers consume less fruit and vegetable than non-smokers(Reference Dallongeville, Marecaux and Fruchart71–Reference Wei, Kim and Boudreau74). A recent population-based cohort analysis on a population of 1000 smokers (age ≥ 25 years) confirmed that smokers consume less fruit and vegetable rich in antioxidants than non-smokers(Reference Haibach, Homish and Giovino75). Smokers can thus be particularly lacking in exogenous antioxidants. For example, the intake of vitamin C in smokers ranges from +0·3 % to −38 % compared with non-smokers(Reference Northrop-Clewes and Thurnham76); therefore, the plasma vitamin C concentration is usually lower in smokers than in non-smokers (Table 1). Besides, smokers consume approximately 10·8–39·4 % less β-carotene than non-smokers, and the comparison between heavy smokers (≥ 20 cigarettes/d, or ≥ 10 years of smoking) and non-smokers showed lower plasma β-carotene levels in smokers (between −17·0 % and −50·7 %)(Reference Northrop-Clewes and Thurnham76). In addition to dietary differences, some studies, in which dietary intakes of antioxidants have been corrected, show that at least vitamin C and possibly β-carotene are depleted by CS itself(Reference Alberg12,Reference Lykkesfeldt, Christen and Wallock77–Reference Lykkesfeldt, Halliwell and Poulsen79) . Smokers also have lower plasma levels of vitamins A and E, GSH and Cys than non-smokers (Table 1).
Table 1. Plasma profile of antioxidants in smokers

HPLC, high-performance (pressure) liquid chromatography; SBD-F, ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate.
(a) Standard deviation
(b) Standard error
(c) Range
(1) This SD is inferred.
(2) Values were adjusted for age, race/ethnicity, education level, family annual income, leisure physical activity, vitamin/mineral supplement intake and body mass index.
(3) Tocopherol levels were corrected for the sum of total cholesterol and triacylglycerol concentrations.
(4) Values were adjusted for age, sex, body mass index, alcohol intake and dietary intake of the nutrient in question.
(5) Values were adjusted for age and sex.
(*) % difference = concentrations in non-smokers−smokers/non-smokers × 100 %.
(**) Values estimated from graph.
Sex affects levels of β-carotene, lycopene and selenium in smokers. Compared with non-smokers, male smokers have lower circulating levels of lycopene and selenium (Table 1). Conversely, no statistically significant differences were found in the plasma levels of lycopene and selenium in female smokers(Reference Wei, Kim and Boudreau74). A study involving thirty-seven males and forty-two females showed that current female smokers had higher serum β-carotene levels (0·24 ± 0·23 μg/ml) than current male smokers (0·10 ± 0·10 μg/ml)(Reference Hakim, Harris and Garland80).
Extensive research has been conducted to evaluate the influence of cigarette smoking on plasma vitamin E status, biokinetics and metabolism. The antioxidant effect of vitamin E is ascribable to its ability to scavenge peroxyl radicals and limit the propagation of lipid peroxidation. In a recent study conducted to determine some plasma lipophilic and haematological components in middle-aged Romanians, who considered themselves ‘healthy’ − 58 non-smokers, 58 conventional cigarette smokers − a significant increase (p < 0·0001) in the level of vitamin E was observed in smokers compared with non-smokers, but only for smokers who consume more than ten cigarettes per day(Reference Badea, Gaman and Delia81). However, the prevalence of the most recent research articles on the correlation between cigarette smoking and plasma vitamin E levels show an opposite result, namely a reduction in plasma vitamin E concentration after exposure to both active and passive CS(Reference Karademirci, Kutlu and Kilinc82–Reference Shah, Khand and Uddin86).
Vitamin E consists of eight different lipophilic compounds: four tocopherols (α, β, γ and δ) and four tocotrienols (α, β, γ and δ). Of these, α- and γ-tocopherols are the most important biologically(Reference Jiang87). Divergent results were obtained concerning the effects of CS on plasma α- tocopherol levels (Table 1). Some authors found statistically significant differences in the plasma levels of α-tocopherol between smokers and non-smokers(Reference Chelchowska, Ambroszkiewicz and Gajewska55,Reference Sobczak, Golka and Szoltysek-Boldys88–Reference Kayan, Naziroğlu and Celik91) , but not others(Reference Gabriel, Liu and Crott92,Reference Leonard, Bruno and Paterson93) . The same discrepancy was observed in several studies concerning plasma levels of γ-tocopherol which, in smokers, are reduced(Reference Zerbinati, Galli and Regolanti94), do not differ(Reference Jeanes, Hall and Proteggente95) or even increase(Reference Dietrich, Block and Norkus78) compared with those seen in smokers. Further confusing the matter was the observation that, even though plasma or erythrocyte α- and γ-tocopherol concentrations do not significantly differ, smokers have reduced levels of α-tocopherol in lymphocytes and platelets compared with non-smokers(Reference Jeanes, Hall and Proteggente95). These conflicting results may be due to the reference values of γ-tocopherol in plasma, which are influenced by the dietary patterns of the population under examination and by other aspects that have not been explored so far, which may include genetic and metabolic factors. Furthermore, the poor sensitivity and differential response of γ-tocopherol detection methods may limit the accuracy of its determination in plasma.
The regulatory mechanisms that influence vitamin E levels in smokers(Reference Lodge96) as well as in non-smokers(Reference Borel, Preveraud and Desmarchelier97) are largely unknown. It has been hypothesised that the reduced dietary nutrient intake observed in smokers may be a contributing factor to the observed reduction in plasma vitamin E levels(Reference Raatz, Jahns and Johnson98). However, plasma vitamin E turnover is different between smokers and non-smokers. Faster disappearance rates of α-tocopherol in the plasma of smokers compared with non-smokers were demonstrated in several biokinetic studies(Reference Munro, Burton and Kelly99,Reference Bruno, Ramakrishnan and Montine100) and were attributed to the increase in plasma clearance of α-tocopherol rather than decreased absorption(Reference Bruno, Ramakrishnan and Montine100–Reference Bruno, Leonard and Atkinson102). In addition, smokers and non-smokers show differences in the extent of vitamin E metabolism via a cytochrome P450-mediated pathway that leads to the formation of carboxy-ethyl-hydroxy-chroman excreted in the urine(Reference Jeanes, Hall and Proteggente95,Reference Bruno, Ramakrishnan and Montine100,Reference Bruno, Leonard and Li103) .
A study based on data obtained from the Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality Study (baseline examination from 1988 to 1994; mortality follow-up through 2006) was conducted on a total of 1492 adults, of which 668 were current smokers, 403 former smokers and 421 never smokers with obstructive lung function(Reference Ford, Li and Cunningham104). In general, antioxidant concentrations were the lowest in current smokers and the highest in participants who have never smoked(Reference Ford, Li and Cunningham104). Even though higher amounts of variously oxidised proteins were measured in the smokers’ blood and/or plasma, no study detected carbonylated albumin in plasma(Reference Pignatelli, Li and Boffetta56,Reference Marangon, Devaraj and Jialal105) . It is worth noting that albumin is the main carbonylated protein in the bronchoalveolar lavage fluid of long-term elder smokers, even in the absence of pulmonary diseases(Reference Nagai, Betsuyaku and Kondo106,Reference Suzuki, Betsuyaku and Ito107) , and in parenchymal lung tissue of smokers(Reference Hackett, Scarci and Zheng108). We showed a drastic decrease in the albumin Cys34 sulfhydryl group and a marked carbonylation when the purified albumin was exposed to whole-phase CS extract(Reference Colombo, Aldini and Orioli109). Mass spectrometry analysis detected acrolein and crotonaldehyde Michael adducts at Cys34, Lys525, Lys351 and His39 and a Schiff base with acrolein at Lys541 and Lys545(Reference Colombo, Aldini and Orioli109). Erythrocytes contain ∼3 mM GSH, whereas plasma GSH concentration is in the low micromolar range(Reference Rossi, Giustarini and Milzani15). We demonstrated that erythrocytes protected both albumin and other plasma proteins from CS-induced carbonylation and thiol oxidation(Reference Colombo, Rossi and Gagliano110). An efficient intracellular antioxidant machinery, coupled with their high blood concentration, makes erythrocytes an effective ‘‘sink’’ of oxidants(Reference Minetti and Malorni111,Reference Cimen112) . The high GSH concentration in human erythrocytes could at least partly explain why oxidised albumin was found within the bronchoalveolar lavage fluid and parenchymal lung tissue of smokers, but not within blood plasma.
2.2. Antioxidant supplementation in smokers
When the efficiency of the plasma antioxidant defence barrier is lowered, oxidants are no longer adequately countered, and tissue injury may therefore be triggered. As current evidence suggests lower plasma concentrations of almost all low-molecular-mass antioxidants (Table 1) and increased oxidative stress markers in smokers, it has been speculated that specific antioxidant supplements (i.e. antioxidant vitamins: β-carotene, vitamin C and vitamin E) could be particularly beneficial to smokers.
In the general population, the preventive potential of some antioxidant supplements, obtained either by extraction from natural food or by chemical synthesis, has been studied in numerous epidemiologic studies and meta-analyses. Even if high doses (more than the recommended dietary daily allowance, RDA(113,114) ) of individual antioxidant supplements are receiving enthusiastic feedback, driven mainly by the pharmaceutical industries that produce and sell them, supporting experimental scientific evidence is moderately positive in some cases and, in other cases, rather negative(Reference Giustarini, Dalle-Donne and Tsikas115–Reference Bjelakovic, Nikolova and Gluud122). Just to give an example, considering the cancer incidence in clinical trials, β-carotene supplementation significantly increased the onset of aggressive prostate cancer(Reference Neuhouser, Barnett and Kristal123). Recent reviews report complete overviews of the positive or negative effects of antioxidant administration in the general population(Reference Salehi, Martorell and Arbiser124) or regarding the development of asthma and cancer in smokers(Reference Alsharairi125). Many epidemiological studies and meta-analyses investigated the effectiveness of antioxidant supplements to decrease the risk of CS-related diseases in cigarette smokers (Table 2). Overall, in smokers, the situation is even worse than what is known for the non-smoker population, as the evidence supporting the beneficial effects of antioxidant supplements is still ambiguous, if not entirely negative. Results from the vitamins and lifestyle (VITAL) study, for example, suggest that long-term use of single β-carotene, retinol and lutein supplements should not be recommended for lung cancer prevention, particularly among smokers(Reference Satia, Littman and Slatore126). A Cochrane review also found that subjects at risk for lung cancer (i.e. smokers) taking β-carotene supplement had a statistically significant increased risk of lung cancer incidence, lung cancer mortality and all-cause mortality(Reference Cortés-Jofré, Rueda and Corsini-Muñoz127). However, this finding has not been ascertained among those not at risk of lung cancer(Reference Cortés-Jofré, Rueda and Corsini-Muñoz127). In smokers, the meta-analysis of randomised controlled trials suggests that β-carotene supplementation was associated with an increased risk not only of lung cancer but also of gastric cancer(Reference Middha, Weinstein and Männistö128). Strong evidence from randomised controlled trials conducted in heavy smokers and asbestos-exposed workers, i.e. the β-carotene and retinol efficacy trial (CARET)(Reference Omenn, Goodman and Thornquist129) and the α-tocopherol and β-carotene cancer prevention (ATBC) study(130), showed that high-dose β-carotene supplements may increase the risk of lung cancer and of death from lung cancer, CVDs, and any cause. This harmful effect was not observed among healthy male physicians in the Physicians’ Health Study in the USA, which found lack of effect of long-term supplementation with β-carotene on the incidence of malignant neoplasms and CVD(Reference Hennekens, Buring and Manson131).
Table 2. Studies evaluating the effect of antioxidant supplements in smokers.

It is worth noting that the negative effect of β-carotene on lung cancer incidence and mortality among subjects at high risk of lung cancer at baseline (i.e. smokers) was persistent even when combined with vitamin A or E(Reference Fortmann, Burda and Senger121,Reference Fortmann, Whitlock and Burda132) . These studies confirm that prevention of cancer through β-carotene supplementation should not be recommended in smokers.
Within this context, according to the 2018 report of the World Cancer Research Fund/American Institute for Cancer Research (https://www.wcrf.org/dietandcancer), β-carotene supplements and, in general, dietary supplements are not recommended for cancer prevention, especially in smokers, while it is advisable to take natural antioxidants and vitamins through the diet. Therefore, caution should be taken, especially in smokers, when considering dietary supplementation with β-carotene.
Concerning the negative effect of β-carotene, it has been proposed that high intake of carotenoids may even enhance smoke-induced oxidative stress in smokers(Reference Palozza, Serini and Trombino133). A possible explanation is that CS modifies the chemical composition of these antioxidants, turning them into pro-oxidants(Reference Hurst, Contreras and Siems134). Indeed, β-carotene can easily form oxidation products with pro-oxidant effects, especially at high concentrations in the oxidative environment of the smoker’s lung characterised by increased cell oxidative stress and decreased antioxidant defence(Reference Palozza, Simone and Mele135). Moreover, excessive intake of antioxidant supplements could lead to ‘antioxidative stress’, that is, the increased antioxidant potential of the cell, where antioxidants might attenuate or block the cellular adaptive stress responses that are induced by low levels of ROS acting as regulators of intracellular signalling pathways(Reference Poljsak and Milisav136). This suggests that dysregulated ROS homeostasis may contribute to many human diseases(Reference Finkel137). High-dose antioxidant supplements may alter the redox balance with deleterious effects on cellular functions, which results in a consequent alteration of the organ functionality.
Furthermore, male smokers supplemented with β-carotene developed metabolomic profiles consistent with the induction of cytochrome P450 enzymes, the primary metabolisers of xenobiotics(Reference Mondul, Sampson and Moore138). These findings may shed light on the increased mortality associated with β-carotene supplementation in the ATBC study, and suggest the need to explore potential interactions between drugs and food supplements(Reference Mondul, Sampson and Moore138).
Some encouraging results have also been achieved on the administration of antioxidant supplements to smokers. A secondary analysis of the α-tocopherol, β-carotene cancer prevention study in Finland on male smokers (n = 7469, age 50–69 years, who started smoking ≥5 cigarettes/d at ≥21 years) suggests that intervention with 50 mg/d of vitamin E for 5–8 years decreases the incidence of pneumonia(Reference Hemilä139), which is a major risk factor for the development of the acute respiratory distress syndrome(Reference Tasaka, Amaya and Hashimoto140).
Short-term (7 d) γ-tocopherol-rich supplementation in combination with smoking cessation improved vascular endothelial function beyond that from smoking cessation alone in young smokers, probably by decreasing proinflammatory mediators, TNF-α and myeloperoxidase(Reference Mah, Pei and Guo141). In healthy smokers who received nicotine replacement therapy, oral administration of a γ-tocopherol-rich mixture of tocopherols improved vascular endothelial function and decreased oxidative stress, which was assessed by urinary 8-iso-15(S)-prostaglandin F2α and was inversely correlated to endothelial function(Reference Mah, Pei and Guo142). Long-term (36 months) supplementation with vitamin E lowered oxidative stress by 21 % in smokers, as measured by urinary 8-iso-prostaglandin F2α, whereas no effect was observed for combined vitamin E and selenium or selenium alone intervention(Reference Guertin, Grant and Arnold143). As CS-induced oxidative stress is thought to lower levels of plasma omega-3 fatty acids, a study evaluated the effects of omega-3 fatty acid supplementation on oxidative stress in heavy-smoker males, showing that high dose of omega-3 fatty acid supplements for 3 months decreases oxidative stress index (i.e. total oxidant status/total antioxidant capacity)(Reference Sadeghi-Ardekani, Haghighi and Zarrin144).
Taken together, all these studies on smokers highlight that, if you take an antioxidant supplement, the best choice is a balanced multivitamin supplement containing no more than 100 % of the recommended dietary allowance (RDA) of most micronutrients(113,114) . In this regard, in a randomised, double-blind, placebo-controlled trial of 14 641 male physicians (ex-smokers n = 5856; current smokers n = 527; age 64·3 ± 9·2 years), daily multivitamin supplementation modestly but significantly reduced the risk of total cancer(Reference Gaziano, Sesso and Christen117). Excessive (more than expected according to the RDA) intake of antioxidants can have deleterious effects.
2.3. Antioxidant-rich diet in smokers
The human diet represents a source of many different compounds endowed with antioxidant activity, mainly vitamins, polyphenols and flavonoids (Fig. 2). While the intake of fruits and vegetables worldwide remains low(Reference Hall, Moore and Harper145), the beneficial effect on health of a diet rich in fruit and vegetable is well known in the general population(Reference Boeing, Bechthold and Bub146–Reference Wang, Ouyang and Liu149). Just to give an example, the European Prospective Investigation Into Cancer and Nutrition (EPIC), a multi-centre prospective study carried out in ten European countries (Denmark, France, Germany, Greece, Italy, The Netherlands, Norway, Spain, Sweden and the United Kingdom), including 519 978 participants (366 521 women and 153 457 men, age 35–70 years)(Reference Leenders, Sluijs and Ros147) found that a diet high in fruit and vegetable was inversely associated with all-cause mortality (but it was driven mainly by CVD mortality), presumably due to the protection offered by diet-derived antioxidants against the development of diseases related to oxidative stress. The findings from the EPIC study also showed that the risks of upper gastrointestinal tract cancers, colorectal cancer and liver cancer were inversely associated with intakes of fruit and/or vegetables and/or fibre(Reference Bradbury, Appleby and Key150).
Overall, all these studies highlighted that, in the general population, there is evidence of beneficial effects of a higher intake of fruits and vegetables. Nevertheless, further findings from the EPIC cohort, obtained combining data from the previous EPIC study and analysing total fruit and vegetable consumption as well as individual subtypes, do not support a clear inverse association between fruit and vegetable consumption and colon or rectal cancer beyond a follow-up of more than 10 years(Reference Leenders, Siersema and Overvad151). These findings agree with the conclusion by the Continuous Update Project of the World Cancer Research Fund and American Institute for Cancer Research, highlighting limited evidence of an inverse association between fruit and vegetable consumption and colon and/or rectal cancer(152).
The low plasma concentrations of almost all low-molecular-mass antioxidants commonly found in smokers (Table 1) suggest that a diet rich in antioxidants may be particularly beneficial for maintaining good health in this population. High dietary antioxidant intake from fruits and vegetables are thought to be protective against oxidative stress, thus providing a potential mechanism through which they can prevent CS-related diseases.
Over the past two decades, many epidemiologic studies and meta-analyses have investigated the effectiveness of an antioxidant-rich diet to decrease the risk of smoking-related diseases in smokers (Table 3). For example, in the EPIC study, among 1830 incident cases of lung cancer (mean follow-up of 8·7 years), the risk of squamous cell carcinomas in current smokers was reduced by 15 % with an increase of 100 g/d of combined fruit and vegetables (HR 0·85; 95 % CI 0·76, 0·94), while no clear effects were seen for the other histological subtypes of lung cancer(Reference Büchner, Bueno-de-Mesquita and Linseisen153). Moreover, the inverse association between fruit and vegetable consumption and mortality seemed stronger for participants with a body mass index > 30 or with high alcohol consumption (>30 g/d in women and >60 g/d in men), and it was suggested even for smokers(Reference Leenders, Sluijs and Ros147).
Table 3. Studies evaluating the effect of an antioxidant-rich diet (i.e. fruit- and vegetable-rich diet) in smokers
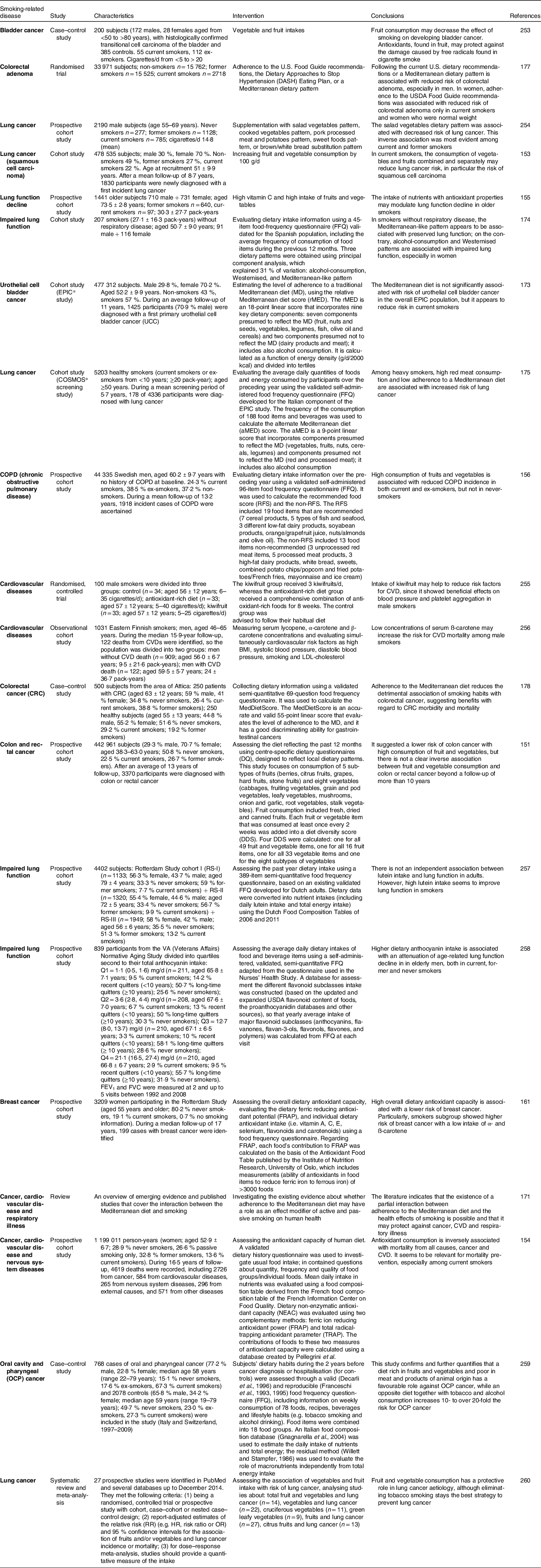
A recent large prospective study (the E3N prospective cohort study initiated in 1990) of middle-aged French women has included 4619 deaths among 1 199 011 person-years of follow-up (no smokers 20 978, passive smokers 17 844, ex-smokers 23 814, current smokers 9876). It highlights the importance of fruit and vegetable consumption for the prevention of all-cause and CVD mortality (but not for cancer mortality), especially among current smokers(Reference Bastide, Dartois and Dyevre154). Antioxidant-rich diets are also associated with a slower rate of lung function decline in older current smokers(Reference Bentley, Kritchevsky and Harris155).
Former or current tobacco smoking is the predominant risk factor for development of COPD, a well-known risk factor for lung cancer. Exposure to CS is a mutual aetiology underlying both diseases, accounting for almost 90 % of cases. Inflammatory responses in smokers and patients with COPD involve the activation of redox-sensitive transcription factors, such as NF-κB, which in turn amplify inflammation and oxidative stress. Two recent large population-based prospective studies in Swedish men, including 44 335 men, aged 45–79 years(Reference Kaluza, Larsson and Orsini156), and women, including 34 739 women, aged 48–83 years(Reference Kaluza, Harris and Linden157), with no history of COPD at baseline, confirmed the inverse and independent association between high long-term consumption of fruits (in both men and women) and vegetables (in men only) and incidence of COPD (35 % lower risk in men, 37 % lower risk in women). The preventive effect of dietary antioxidants was more evident among current and ex-smokers, probably because of increased oxidative stress due to smoking compared with never smoking, and the persistent oxidative burden even after smoking cessation(Reference Kaluza, Larsson and Orsini156,Reference Kaluza, Harris and Linden157) .
A pooled analysis with more than 3000 case subjects suggests that a diet high in carotenoid-rich fruits and vegetables (e.g. carrots for α-carotene, sweet potatoes and green leafy vegetables for β-carotene, tomatoes for lycopene, citrus fruits for β-cryptoxanthin, and green leafy vegetables for lutein and zeaxanthin(158)) offers many health benefits, including a possible reduced risk of breast cancer in the general population(Reference Eliassen, Hendrickson and Brinton159). This result agrees with a recent case–control study on Chinese women (561 cases and 561 controls), which showed an inverse association between the consumption of dietary carotenoids and breast cancer risk(Reference Wang, Li and Pan160). This protective effect has also been observed in smokers. The Rotterdam study reported indeed that a low intake of dietary α-carotene and β-carotene was associated with a higher risk of breast cancer among smokers(Reference Pantavos, Ruiter and Feskens161). These results agree with findings from the Swedish Mammography Screening Cohort, which found a decreased risk of breast cancer in smokers with high intake of dietary α-carotene and β-carotene(Reference Larsson, Bergkvist and Wolk162).
The Japan public health centre based prospective study investigated the association between dietary intake of antioxidant vitamins and the incidence of total and ischemic stroke(Reference Uesugi, Ishihara and Iso163). The study involved (between 1995 and 1997) 82 044 Japanese men and women aged 45–74 years, and, during 983 857 person-years of follow-up until the end of 2009, it documented 3541 incident total strokes and 2138 ischemic strokes. Dietary intakes of α-carotene, β-carotene, α-tocopherol and vitamin C were not inversely associated with the incidence of total stroke and ischemic stroke adjusted for cardiovascular risk factors. When stratified by current smoking status, dietary vitamin C intake was inversely associated with the incidence of total stroke and ischemic stroke among non-smokers but not smokers(Reference Uesugi, Ishihara and Iso163).
A diet rich in antioxidants reputed for its beneficial effects on human health is the traditional Mediterranean diet (the dietary pattern usually consumed among the populations bordering the Mediterranean Sea), which has been inscribed on the heritage list of the United Nations Educational, Scientific and Cultural Organization(164). The Mediterranean diet is based on high consumption of fruits and vegetables, cereals, olive oil, potatoes, poultry, beans, nuts, lean fish and dairy products, low intake of red meat, and moderate consumption of red wine(Reference Giacosa, Barale and Bavaresco165). Protective mechanisms of the Mediterranean diet include antioxidant activity, anti-inflammatory activity, anti-mutagenic and anti-proliferative properties, involvement in cell signalling, cell cycle regulation, and angiogenesis(Reference Dai, Jones and Goldberg166,Reference Tyrovolas and Panagiotakos167) . Even if the exact mechanisms by which an increased adherence to the traditional Mediterranean diet exerts its positive health effects have not yet been clarified, the most important adaptations induced by adherence to the Mediterranean diet have been summarised in a recent article(Reference Tosti, Bertozzi and Fontana168).
A meta-analysis that investigated more than four million subjects suggested that the Mediterranean diet is associated with significant reduction in overall mortality and CVD and cancer risks in the general population(Reference Ros, Martínez-González and Estruch148,Reference Sofi, Macchi and Abbate169) . Just to give an example, a recent study on the elderly adults with high cardiovascular risk (n = 7216, age 55–80 years, 6-year follow-up) showed that participants who consumed in total nine or more servings/d of fruits plus vegetables had a hazard ratio 0·60 of CVD in comparison with those consuming <5 servings/d(Reference Buil-Cosiales, Toledo and Salas-Salvadó170).
The Mediterranean diet could have a modestly but significantly protective role against active and passive CS effects(Reference Vardavas, Flouris and Tsatsakis171). Cigarette smoking is associated with urothelial cell damage, leading to chronic inflammatory bladder disease and, therefore, increasing urothelial cell bladder cancer risk(Reference Kispert, Marentette and Campian172). The EPIC evaluated the association between adherence to the Mediterranean diet and risk of urothelial cell bladder cancer, the most common form of bladder cancer. It suggested that adherence to the Mediterranean diet may decrease the risk of urothelial cell bladder cancer in current smokers, especially among heavy long-term smokers, although the interaction was not significant(Reference Buckland, Ros and Roswall173).
As expected, the effects of smoking on the functionality of the respiratory system are direct and could be serious and lasting. Therefore, the possible benefits of the Mediterranean diet were evaluated also in respiratory organs. In a recent cross-sectional study, which analysed baseline data from randomised representative smokers without respiratory diseases (n = 207, aged 35–70 years), the Mediterranean diet appears to be associated with preserved lung function(Reference Sorli-Aguilar, Martin-Lujan and Flores-Mateo174). Moreover, a study based on 5203 participants, aged ≥50 years, who were current smokers or had quit smoking for <10 years and had smoked at least 20 pack-years, showed that adherence to the Mediterranean diet was significantly associated with reduced lung cancer risk(Reference Gnagnarella, Maisonneuve and Bellomi175).
Given the association between smoking and colorectal cancer development(9), studies have been conducted on the possible protective effects of the Mediterranean diet in smokers. A meta-analysis revealed that ever smokers had 18 % higher risk of colorectal cancer as compared with never smokers, and this association was dose-dependent regarding pack-years(Reference Botteri, Iodice and Bagnardi176). Epidemiological data indicated that the Mediterranean diet has protective effect against the development of colorectal adenomas, more markedly among current smokers(Reference Dixon, Subar and Peters177). A case–control study conducted on colorectal cancer patients (n = 250; age 63 ± 12 years; 26·4 % current smokers) versus non-diseased subjects (n = 250; age 55 ± 13 years; 29·2 % current smokers) indicated that adherence to the Mediterranean diet may mitigate the adverse effects of smoking on colorectal cancer development, suggesting indirect benefits of adherence to this antioxidant-rich diet over colorectal cancer morbidity and mortality(Reference Kontou, Psaltopoulou and Soupos178).
The beneficial effects of fruits, vegetables and some other foods (e.g. dry legumes and cereals) and beverages (e.g. green tea) have been attributed, at least in part, to their high content of polyphenols (of which the largest group is flavonoids), which are recognised compounds able to reduce oxidative stress and inflammation(Reference Tsuda179). Polyphenols are present in fruits and vegetables in concentrations up to several 100 mg/100 g, thereby constituting the major class of diet-derived antioxidants(Reference Meulenberg180).
The effects of a diet rich in polyphenol-filled foods have been analysed and summarised in a recent systematic review(Reference Del Bo’, Bernardi and Marino181). Here we will focus our attention on studies specifically conducted on smokers. A randomised controlled clinical trial in male smokers suggested that dietary polyphenols modulate the expression of genes related to cellular stress defence in smokers’ blood cells(Reference Bøhn, Myhrstad and Thoresen182). A further randomised controlled trial suggested a protective role of blueberries (a single 300 g serving of fresh-frozen blueberries) on reactive hyperaemia and systolic blood pressure in young smokers (n = 16, age 23·6 ± 0·7 years, smoking 15 ± 1 cigarettes/d) after acute exposure to the smoke of one cigarette(Reference Del Bo’, Porrini and Fracassetti183).
Polyphenols are also the main bioactive constituents of green tea, which in recent years has earned great attention in relation to its health benefits against a variety of human diseases(Reference Butt, Ahmad and Sultan184). A review summarising the results of the intervention studies on the consumption of green tea and related antioxidant effects, published until June 2010, concluded that there is limited evidence that regular consumption of green tea in amounts of at least 0·6–1·5 l/d may increase plasma antioxidants, with beneficial effects of green tea consumption being more likely in smokers(Reference Ellinger, Müller and Stehle185). By contrast, evidence from epidemiological studies suggests an inverse association between green tea intake and lung cancer risk among never smokers but not among smokers(Reference Yuan186). It is also worth considering that, although the antioxidant activity of polyphenols is well recognised in the prevention of a variety of human diseases (e.g. CVD and certain types of cancer) in the general population(Reference Pandey and Rizvi187), polyphenols can even display pro-oxidant activities at high doses or in the presence of transition metals, such as iron and copper(Reference De Marchi, Biasutto and Garbisa188,Reference Bouayed and Bohn189) , as in gastric juice and intestinal content(Reference Halliwell, Zhao and Whiteman190). Moreover, if consumed very frequently (>1 l/d) as a hot drink, green tea has been associated with increased incidence of oesophageal cancer in some countries, such as India(Reference Ganesh, Talole and Dikshit191).
Epidemiological studies, short-term randomised controlled trials, and preclinical studies also attributed the benefits associated with the consumption of fruit and vegetables to the presence of flavonoids, a class of polyphenolic compounds abundantly present in foods and beverages of vegetable origin (e.g. red/blue coloured fruits, citrus fruits, apples, broccoli, peppers, black and, especially, green tea)(Reference Carocho and Ferreira192). Results from both epidemiological studies and short-term randomised trials showed beneficial effects of dietary flavonoids on CVD in the general population(Reference van Dam, Naidoo and Landberg193). For example, 93 600 women (aged 25–42 years) from the Nurses’ Health Study (NHS) II, healthy at baseline (1989), were followed for 18 years to examine the relationship between anthocyanins and other flavonoids (total flavonoid intake 58–643 mg/d; anthocyanin intakes 2–35 mg/d) and risk of myocardial infarction(Reference Cassidy, Mukamal and Liu194). The combined intake of anthocyanin-rich food (i.e. blueberries and strawberries) seemed to be associated with a decreased risk of myocardial infarction in young women, comparing those consuming more than three servings/week to those with lower intake. However, in stratified analyses, the inverse association between anthocyanins and myocardial infarction was stronger among women who never smoked compared with those who currently smoked(Reference Cassidy, Mukamal and Liu194). A study conducted recently on 56 048 participants of the Danish Diet, Cancer, and Health cohort has shown that an achievable (of approximately 500 mg/d) dietary intake of total and individual flavonoid subclasses is associated with a lower risk of all-cause, related to CVD, and cancer-related mortality especially in current smokers and individuals with high alcohol consumption, with the highest flavonoids intakes being more beneficial(Reference Bondonno, Dalgaard and Kyrø195).
3. Discussion and perspectives
Since there is clear evidence that long-term exposure to CS can result in systemic oxidants–antioxidants imbalance to the entire organism, as reflected by low levels of plasma antioxidants in smokers (Table 1), it has been speculated that specific antioxidant supplements or an antioxidant-rich diet could be particularly beneficial for smokers. The potential beneficial effects of antioxidants to reinforce the body’s antioxidant defence systems in smokers are overemphasised by a plethora of studies in animal models and/or in vitro cellular models of exposure to CS. These are important tools in the assessment of CS-induced oxidative stress; however, the species-specific functional differences limit the predictive value of animal experiments in the translation of these observations to humans, and the variations of cell functionality deriving from the emergent properties of complex cellular systems (tissues and organs) cannot be inferred from the knowledge of the single system components(Reference Dalle-Donne, Colombo and Gornati38,Reference Halliwell196) . For example, rats and mice appear to be more sensitive to antioxidants than humans; therefore, antioxidant supplementation is more likely to reduce oxidative damage in rodents than in humans(Reference Halliwell and Gutteridge14,Reference Halliwell197) .
Many randomised intervention trials and meta-analysis failed to detect any beneficial effect of antioxidant supplements in smokers, and sometimes the use of antioxidants has turned out to be even counterproductive (Table 2). In this regard, evidence from large randomised trials and meta-analyses suggests that a long-term use and/or high dose of individual β-carotene, retinol and lutein supplements should be avoided, especially in smokers(Reference Bardia, Tleyjeh and Cerhan198,Reference Myung, Kim and Ju199,Reference Satia, Littman and Slatore126–Reference Middha, Weinstein and Männistö128) (Table 2).
The benefit of a diet rich in fruit and vegetables has been attributed to their content of many phytochemical microelements with various antioxidant activity and chemical properties. But why is the ingestion of specific types of food more beneficial than the intake of the isolated antioxidants? It can be assumed that the additive and synergistic effects of the micronutrients in fruits and vegetables could be responsible for their beneficial antioxidant activities(Reference Wolfe, Kang and He200,Reference Song, Derito and Liu201) . Moreover, the antioxidants present in vegetables, which have hormetic effects as many phytochemicals, are supplied in a balanced way, not only in terms of composition but also of the necessary dose, thus maintaining the fragile redox homeostasis of the organism(Reference Martel, Ojcius and Ko202). This may partially explain why no antioxidant supplement can replace the combination of micronutrients in fruits and vegetables to achieve the observed health benefits. Therefore, the increase in fruit and vegetable consumption could be a logical strategy to increase the dietary antioxidant intake and to decrease oxidative stress, potentially leading to reduced risk of oxidative stress-related diseases. In this regard, dietary guidelines for Americans recommend that it would be beneficial to eat at least nine servings (4·5 cups) of fruits and vegetables a day, specifically four servings (two cups) of fruits and five servings (2·5 cups) of vegetables, in a 2000 kcal diet(203).
In smokers, an antioxidant-rich diet potentially may provide a preventive strategy to lower the risk of CS-related diseases. The health benefits of antioxidants were observed predominantly when they were consumed within their natural food matrices, while preventive effects derived from an optimal antioxidant-rich diet may not be reproduced with high doses (>RDA) of the single antioxidant supplements. However, large, randomised intervention trials and meta-analysis demonstrated that the preventive strategy based on a diet rich in fruits and vegetables in smokers showed only a modest protective effect to reduce the risk of CS-related diseases (Table 3). Hence, it is insufficient to effectively counteract the CS-related oxidative stress and diseases.
So, as CS is a modifiable risk factor, quitting smoking is the best prevention and the most effective way to reduce the detrimental health effects of CS, although it may not be sufficient to repair all the oxidative damages caused by long-term exposure to CS(Reference Jha, Ramasundarahettige and Landsman4,Reference Mons, Müezzinler and Gellert24,Reference Louhelainen, Rytilä and Haahtela204,Reference Jha and Peto205) . A randomised controlled cessation trial examined the effects of smoking cessation in a population of 434 current smokers in 12 months of follow-up. Quitters (n = 55) had significantly increased levels of blood plasma GSH compared with subjects who continued to smoke (p < 0·01)(Reference Mons, Muscat and Modesto206).
A recent systematic review and meta-analysis evaluated the association between smoking reduction and some health risks in observational studies. Smokers were categorised as follows: heavy smokers smoked ≥15–20 cigarettes/d, moderate smokers smoked 10–19 cigarettes/d and light smokers smoked <10 cigarettes/d(Reference Chang, Anic and Rostron207). Compared with current heavy smokers, decreased lung cancer risk was ascertained for people who reduced smoking by more than 50 %, from heavy to moderate or to light. The meta-analysis also showed a lower risk of CVD for smokers who reduced their smoking habit from heavy to light, but not smokers who reduced by more than 50 % and reduced from heavy to moderate smoking. Therefore, substantial smoking reduction can decrease lung cancer risks, although the risk of lung cancer remains high, whereas the relationships between smoking reduction and CVDs as well as all-cause mortality remain doubtful. Thus, complete smoking cessation is by far the most effective strategy for cancer and CVD prevention in smokers.
E-cigarettes are among the electronic nicotine delivery systems (ENDS), a heterogeneous class of products in which an electrically powered coil is used to heat a liquid matrix, or e-liquid, that contains nicotine, solvents (e.g. propylene glycol, vegetable glycerine) and, usually, flavouring(Reference Breland, Soule and Lopez208).
The concentration of nicotine in e-liquid varies substantially: it can reach 36 mg/ml or more(Reference Breland, Soule and Lopez208). Some ENDS can deliver to venous blood the same dose of nicotine, at the same rate as a conventional cigarette, while others cannot(Reference Farsalinos, Spyrou and Stefopoulos209,Reference Dawkins and Corcoran210) . This heterogeneity in ENDS nicotine delivery is in contrast with the regulated nicotine replacement products used to help to stop smoking, such as chewing gum and nicotine patch, which deliver nicotine more reliably, but to lower plasma concentrations and at a slower rate(Reference Henningfield211,Reference Choi, Dresler and Norton212) . E-cigarettes too are claimed as a smoking cessation aid, but even if some research suggests that e-cigarettes could help smokers to quit or reduce smoking(Reference Polosa, Caponnetto and Morjaria213–Reference Caponnetto, Campagna and Cibella215), additional studies are necessary to determine whether this is true long term(Reference Rom, Pecorelli and Valacchi216).
ENDS toxicant emissions depend on many factors, including device construction, device power, liquid constituents and user behaviour. Toxicants in ENDS are still present in the liquid, or they are produced when the liquid is heated. The main toxicants present in the liquid are propylene glycol and vegetable glycerine, which represent the 80–97 % of the content of most e-liquids(Reference Han, Chen and Zhang217). Both are toxic, especially at high doses(Reference Neale, Mesler and Young218), but little is known about their effects after long-term chronic exposition. E-liquid also contains flavourings, compounds that are usually added to food, whose effects on health after being heated and aerosolised are unknown(219). Some contaminants findable in e-liquids are diethylene glycol, ethylene glycol and ethanol(Reference Hutzler, Paschke and Kruschinski220). Most e-cigarette aerosols contain many metals, probably originating from some atomiser components, such as cadmium, nickel and lead(Reference Williams, Bozhilov and Ghai221). Other e-cigarettes toxicants are formaldehyde, acetaldehyde, acrolein, acetone, furans and benzene. Some studies reported 9–450 times lower toxicant concentrations in e-cigarettes than conventional cigarettes (e.g. ref. Reference Goniewicz, Knysak and Gawron222 ). However, other studies demonstrated that the toxicant level in e-cigarettes can be higher than in conventional cigarettes(Reference Rom, Pecorelli and Valacchi216). This discrepancy could depend on the fact that e-cigarette components vary widely, since they are almost unregulated and produced by numerous companies, making it difficult to evaluate their safety(Reference Harrell, Simmons and Correa223).
Regarding health risks, e-cigarettes are reported to cause adverse effects after short-term use, comparable to some tobacco-smoking effects (e.g. increase in impedance, peripheral airway flow resistance and oxidative stress)(Reference Vardavas, Anagnostopoulos and Kougias224), while little is known about the health impact of long-term use(Reference Schraufnagel, Blasi and Drummond225). Reviews on e-cigarettes’ impact on health concluded that ENDS are not harmless but are generally less dangerous than regular cigarettes(Reference Dinakar and O’Connor226,Reference Glasser, Collins and Pearson227) . Cancer risk is in most cases lower, even if in some cases, for some products, it can be comparable to that of tobacco smoke(Reference Stephens228). Regarding cardiovascular risk, mainly dependent on the presence of nicotine, some studies comparing e-cigarettes and conventional cigarettes found fewer CVD biomarkers for ENDS, while others observed no differences(Reference Shields, Berman and Brasky229). Finally, the toxicity in the lung remains unknown, even if it has been speculated that e-cigarettes, inducing inflammation, can increase the risk for lung cancer and COPD(Reference Levy, Borland and Villanti230).
Given these premises, we do not have the basic knowledge necessary to speculate if an antioxidant-rich diet or antioxidant supplements could be beneficial to an e-cigarette smoker likewise a conventional cigarette smoker.
CS remains a strong risk factor for premature mortality in older subjects. Current smokers and former smokers had an increased mortality of 2 and 1·3 times, respectively, compared with non-smokers. In any case, quitting smoking is beneficial even at advanced age(Reference Müezzinler, Mons and Gellert231). Efforts to support smoking cessation at all ages should be therefore a public health priority. Nevertheless, most smokers are unable or unwilling to quit smoking, and for this reason, the problem of ensuring an improvement in their health quality remains of interest.
Future studies to better define interventional dietary strategies in smokers should focus on three points. Firstly, exhaustive macro- and micronutrient databases are needed to evaluate the absorption, bioavailability and metabolism of dietary antioxidant in smokers. It is worth noting that only a part of the diet’s antioxidants, that is, the bioavailable portion, is absorbed, reaches the systemic circulation without undergoing any chemical modification, and can carry out its healthy effects. For diet-derived antioxidants and phytochemicals to be absorbed, they must be released from the food matrix and presented to the brush border of the small intestine in such a state that they can enter the enterocytes by passive diffusion or active transport systems. Metabolic modifications (e.g. dehydroascorbate to ascorbate), or transformations (e.g. β-carotene to retinol), or packaging in the enterocyte (e.g. β-carotene, retinol, vitamin E), occur before secretion in a biocompatible form, through the enterocyte basal membrane, into blood capillaries (water-soluble micronutrients) or the chyliferous vessels (liposoluble micronutrients). Understanding this concept of bioavailability is essential for the production of food and supplements, for nutritional assessment and for determining diet–health relationships, both in the general population and in smokers who, among various diseases, also have intestinal dysfunctions(Reference Fricker, Goggins and Mateer232) that could alter phytochemical absorption. For these last reasons, more exhaustive studies are needed to determine whether a diet rich in fruit and vegetable with high levels of bioavailable antioxidants could protect against oxidative damage and the subsequent development of CS-related diseases.
Secondly, it seems appropriate to carry out further studies on the correlation between the diet and the profile of plasma antioxidants, on oxidative damage to lipids, proteins and DNA, and on biomarkers of oxidative damage. The potential benefits of a high fruit and vegetable intake on the plasma antioxidant profile, on oxidative damage to lipids, proteins and DNA, and on biomarkers of oxidative stress (e.g. GSH/glutathione disulphide ratio) were evaluated in a randomised, free-living, open placebo-controlled cross-over trial of 3 weeks, with a 2-week washout period between treatments. This study included twenty-two male smokers aged 18–50 years, with a relatively low vegetable and fruit consumption(Reference van den Berg, van Vliet and Broekmans233). The high intake of fruit and vegetable increased plasma levels of vitamin C, α-carotene, β-carotene, β-cryptoxanthin and zeaxanthin. However, no effects were demonstrated on any biomarker of oxidative damage to lipids, proteins and DNA or biomarkers of oxidative stress(Reference van den Berg, van Vliet and Broekmans233). It could be that the increased levels of antioxidants were not sufficiently high to show beneficial effects to the selected biomarkers, or, alternatively, the method of selection of male smokers with a relatively low fruit and vegetable intake might have been inadequate to select subjects with really increased oxidative stress. Also, the population studied is small and limited to male smokers, so results should be replicated in larger studies to corroborate these findings.
More recently, it has been demonstrated that the induction of cell-mediated cytoprotective pathways, including antioxidant enzymes, protein chaperones, growth factors and mitochondrial proteins, is responsible, with an hormetic process, for the beneficial effects of a diet rich in fruits and vegetables(Reference Martel, Ojcius and Ko202,Reference Mattson234) . A study in which the effects of a plant-based diet were measured in smokers’ blood cells using microarray genome technology demonstrated the up-regulation of target genes for transcription factors involved in stress responses through antioxidant and non-antioxidant activity, offering some potential mechanistic explanations for the beneficial health effects of diets high in fruit and vegetable in smokers(Reference Bøhn, Myhrstad and Thoresen182).
Thirdly, it is very important to consider that the hypothesis suggesting beneficial effects of antioxidants for the prevention or treatment of CS-related diseases might be simplistic because antioxidants can also have negative effects if they alter the delicate redox balance of the organisms(Reference Halliwell235).
4. Conclusions
Global projections of mortality data estimate that total tobacco-attributable deaths will increase to over 8 million in 2030(Reference Mathers and Loncar236). Because of the widespread diffusion of the CS habit and its consequent severe impact on human health, interventions to reduce CS-related diseases should have a high priority worldwide. Despite the small or modest reduction of CS-related disease risk by a diet rich in fruit and vegetable observed in smokers, the most efficient way to prevent smoking-related oxidative stress and CS-related diseases remains smoking cessation. Therefore, smoking cessation should be the main objective of public health for the prevention of CS-related diseases and to limit the economic impact for community. As stated by Beaglehole and colleagues: ‘A tobacco-free world by 2040, where less than 5 % of the world’s adult population use tobacco, is socially desirable, technically feasible, and could become politically practical. This will prevent hundreds of millions of unnecessary deaths during the remainder of this century and safeguard future generations from the ravages of tobacco use activity’(Reference Beaglehole, Bonita and Yach237).
Waiting for a ‘tobacco-free world’, smokers have to consider that smoking cessation, consuming a diet rich in fruit and vegetable, avoiding obesity, hyperglycaemia and hypercholesterolemia(Reference Halliwell238), and exercising regularly (a mild pro-oxidant challenge that triggers a beneficial adaptation(Reference Jackson239)) could minimise levels of oxidative stress/damage. This lifestyle is therefore essential for maintaining a reasonably good health for as long as possible.
Acknowledgments
Figures were prepared using and combining medical clip arts and illustrations available within the Servier’s Medical Art section, by courtesy of Servier International.
Disclosure statement
The authors have no conflicts of interest to declare.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.








