We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings.
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to .
To save content items to your Kindle, first ensure no-reply@cambridge.org is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle.
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
A porous material that has been contaminated with a hazardous chemical agent is typically decontaminated by applying a cleanser solution to the surface and allowing the cleanser to react into the porous material, neutralising the agent. The agent and cleanser are often immiscible fluids and so, if the porous material is initially saturated with agent, a reaction front develops with the decontamination reaction occurring at this interface between the fluids. We investigate the effect of different initial agent configurations within the pore space on the decontamination process. Specifically, we compare the decontamination of a material initially saturated by the agent with the situation when, initially, the agent only coats the walls of the pores (referred to as the ‘agent-on-walls’ case). In previous work (Luckins et al., European Journal of Applied Mathematics, 31(5):782–805, 2020), we derived homogenised models for both of these decontamination scenarios, and in this paper we explore the solutions of these two models. We find that, for an identical initial volume of agent, the decontamination time is generally much faster for the agent-on-walls case compared with the initially saturated case, since the surface area on which the reaction can occur is greater. However for sufficiently deep spills of contaminant, or sufficiently slow reaction rates, decontamination in the agent-on-walls scenario can be slower. We also show that, in the limit of a dilute cleanser with a deep initial agent spill, the agent-on-walls model exhibits behaviour akin to a Stefan problem of the same form as that arising in the initially saturated model. The decontamination time is shown to decrease with both the applied cleanser concentration and the rate of the chemical reaction. However, increasing the cleanser concentration is also shown to result in lower decontamination efficiency, with an increase in the amount of cleanser chemical that is wasted.
Evaporation within porous media is both a multiscale and interface-driven process, since the phase change at the evaporating interfaces within the pores generates a vapour flow and depends on the transport of vapour through the porous medium. While homogenised models of flow and chemical transport in porous media allow multiscale processes to be modelled efficiently, it is not clear how the multiscale effects impact the interface conditions required for these homogenised models. In this paper, we derive a homogenised model, including effective interface conditions, for the motion of an evaporation front through a porous medium, using a combined homogenisation and boundary layer analysis. This analysis extends previous work for a purely diffusive problem to include both gas flow and the advective–diffusive transport of material. We investigate the effect that different microscale models describing the chemistry of the evaporation have on the homogenised interface conditions. In particular, we identify a new effective parameter, 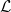 $\mathcal{L}$, the average microscale interface length, which modifies the effective evaporation rate in the homogenised model. Like the effective diffusivity and permeability of a porous medium,
$\mathcal{L}$, the average microscale interface length, which modifies the effective evaporation rate in the homogenised model. Like the effective diffusivity and permeability of a porous medium, 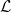 $\mathcal{L}$ may be found by solving a periodic cell problem on the microscale. We also show that the different microscale models of the interface chemistry result in fundamentally different fine-scale behaviour at, and near, the interface.
$\mathcal{L}$ may be found by solving a periodic cell problem on the microscale. We also show that the different microscale models of the interface chemistry result in fundamentally different fine-scale behaviour at, and near, the interface.

Email your librarian or administrator to recommend adding this to your organisation's collection.