There is an increasing demand for fishmeal from the fish-farming industry at a time when current production levels are foreseen as unsustainable, and escalating costs have led to much research in the use of alternative proteins for aquafeeds. Tacon & Metian(Reference Tacon and Metian1) stated that fishmeal and fish oil use within aquafeeds will decrease in the long term and are becoming targeted as ingredients in higher value starter, finisher and broodstock feeds. Much attention has been focused on plant proteins as an alternative protein source in aquafeeds; however, their use is hindered by low palatability, poor essential amino acid profile, deficiencies in minerals as well as cumulative effects of anti-nutritional factors(Reference Gatlin, Barrows and Brown2, Reference Francis, Makkar and Becker3). It appears that dietary non-essential amino acid composition plays a decisive role in determining their effectiveness(Reference Gaye-Siessegger, Focken and Abel4). Many studies have investigated the essential amino acid requirements of different fish species(Reference Kim5–Reference Fournier, Gouillou-Coustans and Métailler10) and the effect of the dietary essential-to-non-essential amino acid ratio on growth performance(Reference Green, Hardy and Brannon11–Reference Peres and Oliva-Teles13); however, only a few studies have examined the importance of dietary non-essential amino acid composition in fish. In 1985, Hughes(Reference Hughes14) had already examined the influence of two non-essential amino acids in the diet on the growth performance of lake trout (Salvelinus namaycush) and rainbow trout (Oncorhynchus mykiss). He fed semi-purified diets containing either glutamic acid (Glu) or glycine (Gly) (replaced by isonitrogenous amount and in another diet by equal weight) as the only source of non-essential amino acids. Both species, fed the diet containing Glu, showed significantly better growth and feed conversion than those fed the diets with Gly. Hughes(Reference Hughes14) found that trout utilise Glu more efficiently than Gly when no other source of non-essential amino acids is present. However, he did not compare the effect of amino acid-based diets with a protein-based diet nor did he test a more complex composition of non-essential amino acids; therefore, he could not give any information about the influence of dietary non-essential amino acid composition on the growth performance of the trout. Schuhmacher et al. (Reference Schuhmacher, Münch and Gropp15) investigated the influence of two essential-to-non-essential amino acid ratios, and different sources of non-essential amino acids in the diet, on the body-mass gain and growth rate of rainbow trout. The synthetic amino acid diets contained Glu, Gly or glutamine (Gln) in isonitrogenous amounts (with a constant amount of proline (Pro)) as only sources of non-essential amino acid. Contrary to the aforementioned results of Hughes(Reference Hughes14), Gln was superior to Gly, and Gly was superior to Glu. Gaye-Siessegger et al. (Reference Gaye-Siessegger, Focken and Abel4) examined the importance of the dietary non-essential amino acid composition for the growth performance of Nile tilapia (Oreochromis niloticus). The purified diets did not contain any protein, only synthetic amino acids. The utilisation of synthetic amino acids by tilapia was poor; nevertheless, the non-essential amino acid composition of the diet had a significant effect on the lipid, protein and energy gain in tilapia. In a following experiment, rainbow trout were fed three purified diets differing in their composition of non-essential/conditional essential amino acids(Reference McCullagh, Gaye-Siessegger and Focken16). Diet 1 resembled exactly the amino acid composition of fishmeal, in diet 2, cysteine (Cys), Gly, Pro and tyrosine (Tyr) were replaced by their precursor amino acid, and in diet 3, alanine (Ala), asparagine and aspartate, Cys, Gly, Pro, serine (Ser) and Tyr were replaced by Glu. The diets were composed to be equal in lipid and energy content, and 30 % of the diets consisted of synthetic amino acids. The dietary non-essential amino acid composition had a significant effect on growth rates as well as on nitrogen and energy retention of trout. δ13C of individual amino acids in fish was determined by liquid chromatography–isotope ratio MS (LC-IRMS)(Reference McCullagh, Juchelka and Hedges17). The relatively new method of LC-IRMS allows the determination of isotopic signatures of individual amino acids from proteins without prior derivatisation. Results of that study led to the design of the present feeding trial with an improved experimental set-up, including the isonitrogenous replacement of non-essential amino acids, greater sample size as well as the addition of a 13C-enriched amino acid to be able to trace its absorption and use in metabolism.
The aim of the present study was to test whether the dietary non-essential/conditionally essential amino acid (herein referred to as non-essential amino acid) composition has an effect on growth performance, protein utilisation and δ13C of individual amino acids in fish tissue and whether non-essential amino acids, not provided in the diet, were derived from Glu, the dietary amino acid provided in excess, or other sources. 13C-enriched Glu was used to trace its utilisation in amino acid metabolism.
Materials and methods
Feed
Diets were made from wheat starch, potato dextrin, wheat germ oil, fish oil, vitamin and mineral premixes, cellulose, carboxymethylcellulose, titanium dioxide (TiO2) and different compositions of synthetic amino acids (Table 1). Diet 1 was formulated to have the same amino acid composition as fishmeal which was determined previously. In diet 2, Cys, Gly, Pro and Tyr were isonitrogenously replaced by their precursor amino acids, and in diet 3, Ala, asparagine and aspartate, Cys, Gly, Pro, Ser and Tyr were isonitrogenously replaced by Glu. Diets 4, 5 and 6 resembled diets 1, 2 and 3 except for Glu, which was enriched in 13C (Glu contained 0·1 % 13C-enriched Glu (99 %), labelling position 1). Vitamin and mineral mixtures were made as given in Meyer-Burgdorff et al. (Reference Meyer-Burgdorff, Müller and Becker18). In a control diet, synthetic amino acids were replaced by defatted fishmeal. All diets were composed to be equal in nitrogen, lipid and energy content. Betaine was included in the purified diets as a feed attractant. Ingredients of diets were mixed well, moistened and made into 2 mm pellets, which were then dried.
Table 1 Ingredients (g/100 g) of the experimental diets

AA, amino acids; CMC, carboxymethylcellulose.
* Enriched Glu (1-13C) was obtained from Chemotrade (Leipzig, Germany); remaining ingredients were obtained from Sigma-Aldrich (Taufkirchen, Germany).
Experimental procedure
Fish were obtained from the Fischzucht Störk (Wagenhausen, Bad Saulgau, Germany, farm free of viral haemorrhagic septicaemia and infectious haematopoietic necrosis in accordance with Council Directive 2006/88/EC) and were all from the same batch. A total of forty-two fish with an average body mass of 4·7 (sd 0·57) g were randomly distributed in aquaria supplied with a constant flow-through of water. The feed used during the rearing of the fish before the experiment was a commercial trout diet. Fish were assigned randomly to one of seven groups and were fed individually (one fish/tank) one of the purified diets or the fishmeal-based diet at a level of 3·5 % body mass equivalents/d. The feed was offered by automatic feeders five times/d. The experiment lasted 10 weeks.
Once every week, the fish were weighed to adjust the feeding ration, and the aquaria were cleaned. The water temperature was maintained at 15 ± 0·5°C, and the dissolved oxygen content was >10 mg/l. An artificial lighting system was provided, with a photoperiod regimen of 12 h light–12 h dark.
The experiment was performed in accordance with applicable German and European laws. The experimental facilities are approved by the respective authorities and operated under the supervision of the Animal Welfare Commissioner at Universität Hohenheim.
Analytical methods
The samples were prepared as described by Gaye-Siessegger et al. (Reference Gaye-Siessegger, Focken and Abel4). Sample preparation and analysis for LC-IRMS were based on methods described by McCullagh(Reference McCullagh19). Briefly, fish samples were acid hydrolysed and reconstituted in Milli-Q water at approximately 1 mg/ml.
Amino acid δ13C analysis of fish samples was carried out using an LC-IRMS system consisting of a Thermo Surveyor HPLC system interfaced with a Delta V Advantage through a Thermo Finnigan LC-IsoLink (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Of the sample solution, 10 μl was injected onto the column (Primesep A, 4·6 × 250 mm, 5 μm, 100 A; SIELC Technologies, Prospect Heights, IL, USA), via partial loop injection.
HPLC separation was based on a modified version of the chromatographic method described by McCullagh et al. (Reference McCullagh, Gaye-Siessegger and Focken16). The original method utilised a linear gradient, which did not adequately separate Gly and threonine (Thr). In order to obtain reliable δ13C values for these two amino acids, modification to the gradient elution conditions was made, and the column dimensions were changed.
A 2·1 × 350 mm (2·1 × 250 mm+2·1 × 100 mm columns inline) Primesep A mixed-mode column was used for the separations with a step gradient. Mobile phase A consists of 100 % Milli-Q water at 25°C and mobile phase B (0·2 % of 12 m-H2SO4). Details of the LC-IsoLink set-up were as described by McCullagh(Reference McCullagh19).
Calculations and statistical evaluation
Calculations were performed on the data for each individual fish. Specific growth rate was calculated as (ln (final body mass (g)/initial body mass (g)) × 100)/number of days over the experimental period. Protein gain was determined following the principles of the comparative slaughter technique(Reference Lawes and Gilbert20). Protein content of trout from a previous experiment (comparable body mass, same commercial trout diet) was used to estimate the initial protein content of trout. The effects of dietary amino acid composition and 13C-enriched Glu on specific growth rate, protein gain as well as on δ13C of individual amino acids in fish were tested with a two-way ANOVA using the software STATISTICA 6.0 (StatSoft GmbH, Hamburg, Germany), followed by Tukey's honestly significant difference test. Differences were regarded as statistically significant at a P level of 0·05.
Results
The proximate composition and energy content of the diets are presented in Table 2. Although the diets were composed to be equal in N, lipid and energy content, small differences occurred. The elevated ash content of the fishmeal-based diet resulted from the high mineral content of fishmeal. No mortality occurred. Fish fed the fishmeal-based diet showed significantly higher final body mass (18·2 (sd 5·50) g) than fish fed the amino acid diets (P < 0·05) (Table 3). Growth rates of fish fed diets 1 and 4, resembling the amino acid composition of fishmeal, did not differ significantly from fish fed diets 2, 3, 5 and 6 (P = 0·059). Dietary amino acid composition significantly influenced the protein gain (P = 0·025), with the highest gain in trout fed the diets with the full amino acid spectrum (Fig. 1).
Table 2 Proximate composition and gross energy of the purified diets and the fishmeal-based diet
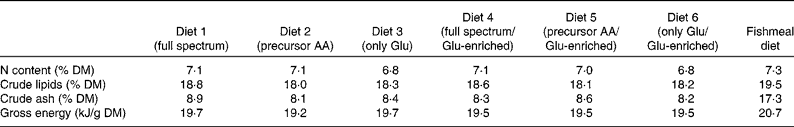
AA, amino acids.
Table 3 Body mass and growth of trout fed the purified diets
(Means values and standard deviations, n 6)
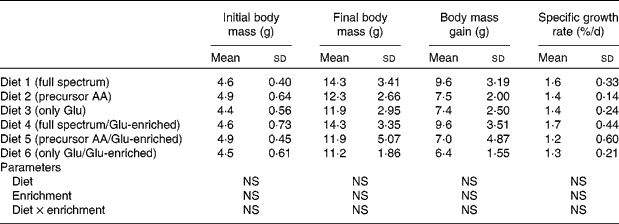
AA, amino acids.
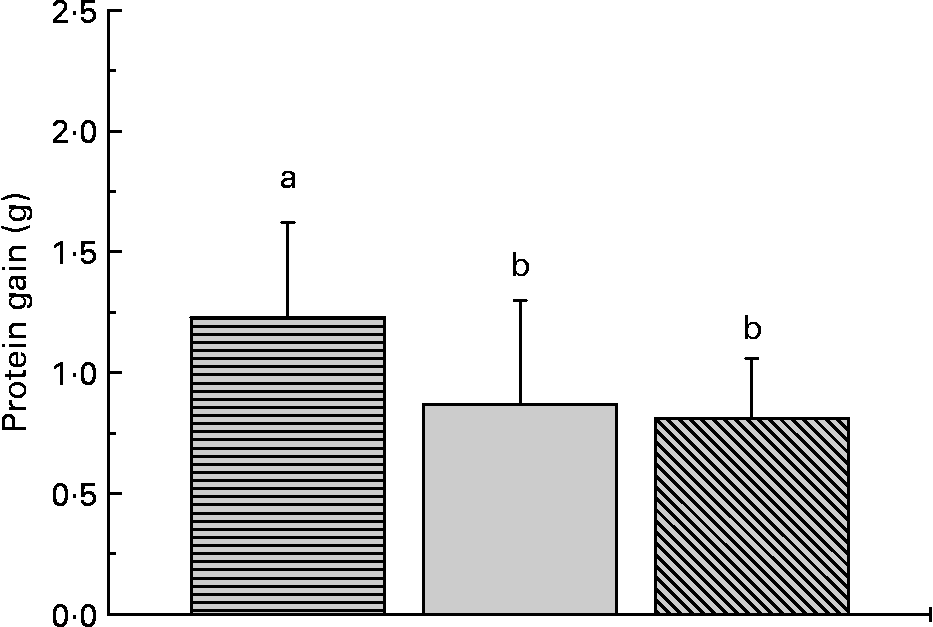
Fig. 1 Dietary amino acid composition significantly influenced protein gain in trout. Values are means, with standard deviations represented by vertical bars (n 12). ![]() , Full spectrum;
, Full spectrum; ![]() , precursor amino acids;
, precursor amino acids; ![]() , only glutamic acid. a,b Mean values with unlike letters were significantly different (P < 0·05).
, only glutamic acid. a,b Mean values with unlike letters were significantly different (P < 0·05).
δ13C of amino acids in the fishmeal diet and in fish fed this diet did not differ for Ala, lysine (Lys), methionine (Met), phenylalanine (Phe), Thr and Tyr (derived via pyruvate, oxaloacetate or phosphoenolpyruvate) (Fig. 2). While arginine (Arg), histidine (His), Pro and Ser (α-ketoglutarate-, 3-phosphoglycerate- and ribose-5-phosphate-derived amino acids) in fish were only slightly enriched in 13C compared with dietary amino acids; Gly was strongly enriched.
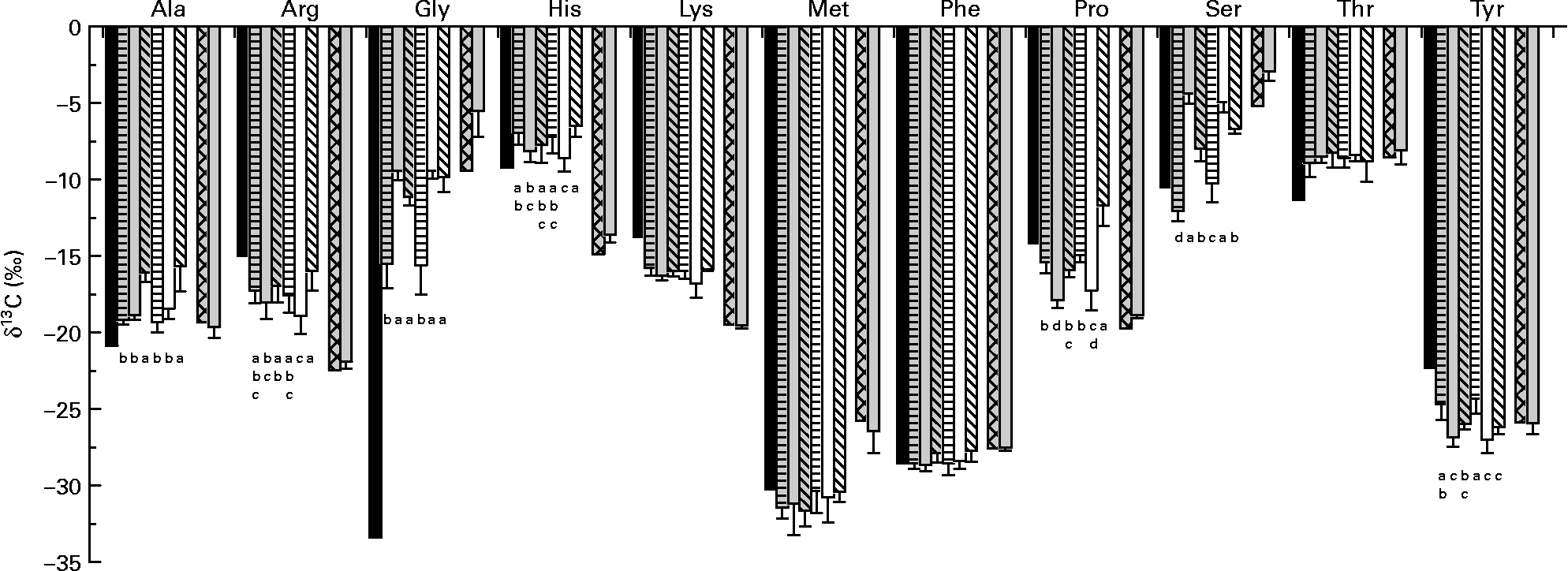
Fig. 2 δ13C of individual amino acids in diets (average of diets) and fish tissue. Values are means, with standard deviations represented by vertical bars (n 6). Dietary amino acid composition significantly affected δ13C of alanine (Ala), arginine (Arg), glycine (Gly), histidine (His), phenylalanine (Phe) and tyrosine (Tyr) of trout fed the purified diets. ■, Amino acids in the purified diets; ![]() , fish on diet 1 full spectrum;
, fish on diet 1 full spectrum; ![]() , fish on diet 2 precursor amino acids;
, fish on diet 2 precursor amino acids; ![]() , fish on diet 3 only glutamic acid (Glu);
, fish on diet 3 only glutamic acid (Glu); ![]() , fish on diet 4 full spectrum (Glu-enriched); □, fish on diet 5 precursor amino acids (Glu-enriched);
, fish on diet 4 full spectrum (Glu-enriched); □, fish on diet 5 precursor amino acids (Glu-enriched); ![]() , fish on diet 6 only Glu (Glu-enriched);
, fish on diet 6 only Glu (Glu-enriched); ![]() , amino acid fishmeal diet;
, amino acid fishmeal diet; ![]() , fish on a fishmeal diet. Lys, lysine; Met, methionine; Pro, proline; Ser, serine; Thr, threonine. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
, fish on a fishmeal diet. Lys, lysine; Met, methionine; Pro, proline; Ser, serine; Thr, threonine. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
For fish fed the purified diets, Arg, Lys, Pro (except for fish fed diet 6) and Tyr in fish were 13C-depleted compared with dietary amino acids, whereas Ala, Gly, His and Thr were enriched. δ13C of Met exhibited strong individual deviations; δ13C of Phe showed only small differences between diet and fish, and for Ser, the picture was not uniform. Dietary amino acid composition significantly affected δ13C of Ala, Arg, Gly, His, Phe and Tyr (Table 4). For Glu, Pro and Ser, there was a significant interaction between dietary amino acid composition and enrichment.
Table 4 Effect of dietary amino acid composition and 13C-enriched Glu on protein gain and δ13C of individual amino acids in trout fed the purified diets (n 6)

n.i., Non-interpretable due to significant interaction.
*P < 0·05, **P < 0·01, ***P < 0·001.
δ13C of fish tissue Glu differed significantly between individuals on different diets (Fig. 3). Glu in fish fed the diets with enriched Glu (diets 4–6) was only slightly enriched compared with fish without enriched amino acid in the diet.
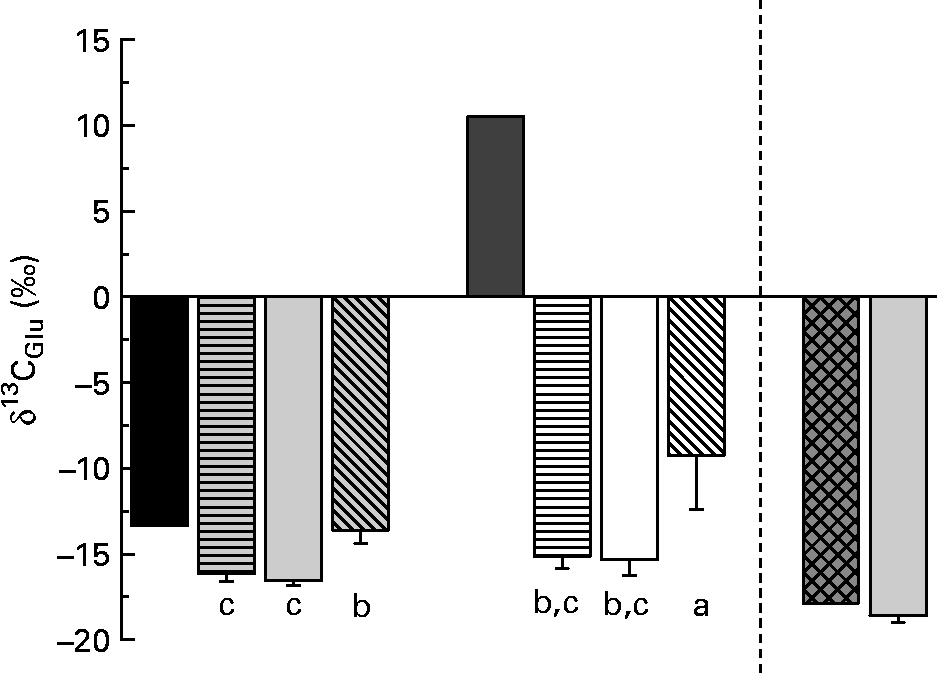
Fig. 3 δ13C of glutamic acid (Glu) in the purified diets and the fishmeal-based diet as well as in fish tissue. Values are means, with standard deviations represented by vertical bars (n 6). Glu in fish fed the diets with enriched Glu was only slightly enriched compared with fish without enriched amino acid in the diet. ■, Diets 1–3; ![]() , fish on diet 1 full spectrum;
, fish on diet 1 full spectrum; ![]() , fish on diet 2 precursor amino acids;
, fish on diet 2 precursor amino acids; ![]() , fish on diet 3 only Glu;
, fish on diet 3 only Glu; ![]() , diets 4–6;
, diets 4–6; ![]() , fish on diet 4 full spectrum (Glu-enriched); □, fish on diet 5 precursor amino acids (Glu-enriched);
, fish on diet 4 full spectrum (Glu-enriched); □, fish on diet 5 precursor amino acids (Glu-enriched); ![]() , fish on diet 6 only Glu (Glu-enriched);
, fish on diet 6 only Glu (Glu-enriched); ![]() , fish meal diet;
, fish meal diet; ![]() , fish on a fishmeal diet. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
, fish on a fishmeal diet. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
Discussion
Trout immediately ingested the purified diets offered. Nevertheless, trout fed the fishmeal-based diet grew significantly better than trout fed the synthetic amino acid diets exhibiting the same amino acid composition (diets 1 and 4). The slightly higher energy content of the fishmeal-based diet compared with the purified diets could not account for differences in growth. However, compared with previous studies, it has been observed that salmonids seem to utilise free amino acids in their diets more efficiently than warm water species such as tilapia(Reference Gaye-Siessegger, Focken and Abel4, Reference Ng, Hung and Herold21). Ng et al. (Reference Ng, Hung and Herold21) suggested that the absorbed amino acids in the plasma are catabolised and excreted faster in warm water species. Walton et al. (Reference Walton, Adron and Coloso22) found that the growth of rainbow trout given semi-defined diets containing large amounts of synthetic amino acids was markedly inferior to the growth of fish given diets with the same amino acid composition in proteinaceous form. However, Kim et al. (Reference Kim, Kayes and Amundson6, Reference Kim, Kayes and Amundson7) observed high growth rates in rainbow trout given diets which contained up to 75 % synthetic amino acids. In the present study, experimental diets were composed of synthetic amino acids as the only N source. Growth rates of trout fed these diets with a diverse pattern of non-essential amino acids differed considerably (but not statistically significant). Fish fed diets with the amino acid composition of fishmeal had a significantly higher protein gain than fish fed diets with a reduced amino acid spectrum, demonstrating the relevance of the dietary non-essential amino acid composition.
It has previously been shown that growth and protein utilisation significantly affect δ13C of bulk fish material(Reference Focken23–Reference Trueman, McGill and Guyard25). In the present experiment, dietary non-essential amino acid composition significantly affected δ13C of some individual amino acids in fish tissue. McCullagh et al. (Reference McCullagh, Gaye-Siessegger and Focken16) fed rainbow trout with a commercial trout feed and three purified diets differing in their composition of non-essential/conditional essential amino acids. The dietary amino acid composition, had a significant effect on growth rate as well as on nitrogen and energy utilisation. But only δ13C of Tyr was significantly influenced by dietary amino acid composition, presumably due to low sample size. In agreement with McCullagh et al. (Reference McCullagh, Gaye-Siessegger and Focken16), δ13C of Tyr in the present experiment differed significantly between treatments. Tyr in fish fed diets with a reduced amino acid spectrum was significantly depleted in 13C compared with Tyr in fish fed diets 1 and 4. Tyr can be synthesised in the body from the essential amino acid Phe(Reference Stryer26). Diets 2, 3, 5 and 6 did not contain Tyr; therefore, fish had to use dietary Phe, which was strongly depleted in 13C (δ13CPhe = − 28·6 ‰), to synthesise Tyr.
Ala and Gly in fish tissue were 13C-enriched compared with dietary amino acids. Ala in fish fed diets 3 and 6, which contained no Ala, was once more enriched. Ala is biosynthetically derived from pyruvate(Reference Stryer26). The pyruvate dehydrogenase complex, which splits pyruvate into acetyl coenzyme A and CO2, discriminates strongly against the heavier isotope(Reference DeNiro and Epstein27). The acetyl coenzyme A molecules are 13C-depleted relative to the remaining pyruvate. The acetyl group of acetyl coenzyme A is the main source of C for lipid biosynthesis(Reference Stryer26). Remaining ‘enriched’ pyruvate is used for the synthesis of Ala. In agreement with McCullagh et al. (Reference McCullagh, Gaye-Siessegger and Focken16), Gly in fish tissue was strongly enriched in 13C compared with the dietary amino acid. Most proteins contain only small quantities of Gly, but collagen has about 35 % Gly (plus 11 % Ala, 21 % Pro and hydroxyproline)(Reference Nelson and Cox28). In addition, Gly is involved in many anabolic reactions other than protein synthesis including the synthesis of purine nucleotides, haem, glutathione and creatine(Reference Murray, Granner and Mayes29). It may be that enzymes involved in these reactions discriminate strongly against molecules with 13C, the remaining Gly becoming 13C-enriched. Gly in fish fed diets with a reduced amino acid spectrum was significantly enriched compared with Gly in fish fed diets with the full spectrum of amino acids (diets 1 and 4). Gly is synthesised in the body from the amino acid Ser(Reference Stryer26). Dietary Ser was more enriched in 13C than dietary Gly.
Ser in fish fed diet 4 was significantly enriched in 13C compared with fish fed diet 1. In fish fed diets with a reduced spectrum, Ser was 13C-enriched with the highest δ13C found for fish fed diets 2 and 5. These two diets contained the highest amounts of Ser. Fish fed these diets built up Gly from dietary Ser. The main pathway to Gly is a reversible reaction catalysed by the enzyme serine hydroxymethyltransferase(Reference Stryer26).
While Pro in fish fed diets 1, 3 and 4 was slightly depleted in 13C compared with dietary Pro, it was strongly depleted in fish fed diets 2 and 5 (lacking Cys, Gly, Pro and Tyr). Pro is normally biosynthetically derived from Glu(Reference Stryer26). McCullagh et al. (Reference McCullagh, Gaye-Siessegger and Focken16) suggested that enzymes involved in the biosynthesis of Pro discriminate strongly against 13C. Fish fed diets without Pro had to synthesise this amino acid from dietary Glu ( − 13·4 %) and showed 13C-depleted values in comparison with Glu. Fish fed diet 6 (containing a high amount of enriched Glu) showed significantly enriched Pro compared with fish fed diet 3.
For fish, proteinogenic amino acids are commonly divided into ten essential and ten non-essential amino acids. Reeds(Reference Reeds30) stated that the classification of amino acids into essential and non-essential has become increasingly imprecise. While Arg is classified as an essential amino acid for birds, carnivores and young mammals, it is a semi-essential amino acid for adult mammals(Reference Tapiero, Mathé and Couvreur31). Glu is also involved in the synthesis of citrulline, Arg and glutathione(Reference Stryer26, Reference Reeds, Burrin and Stoll32). In catfish, dietary Glu is used for the synthesis of Arg especially when dietary Arg is deficient in the diet(Reference Buentello and Gatlin33). It is not possible to rule out that fish fed the high-Glu diets synthesised Arg, and thus the isotopic composition of the amino acid in fish tissue was impaired.
Dietary Glu enters the Krebs cycle via deamination (aminotransaminase or glutamate dehydrogenase) that directly produces α-ketoglutarate(Reference Stryer26). During the step from α-ketoglutarate to succinyl-CoA, the C-1 atom is dissociated. In the present experiment, the C-1 atom was 13C-enriched. Therefore, only small effects of enriched Glu in diets on δ13C of individual amino acids were expected. But also Glu in fish fed the 13C-enriched diets was only slightly enriched compared with fish fed the other diets. This suggests a low contribution of dietary Glu to fish tissue Glu. In infant pigs, Reeds et al. (Reference Reeds, Burrin and Jahoor34) found that a large part of Glu was already used in the intestine as the energy source (95 %) and did not enter the amino acid pool. They concluded that Glu and Gln in the body derived almost entirely from de novo synthesis. For diet 6 (with enriched Glu), this is less consistent with the observed enrichment of Pro in fish tissue. If energy metabolism in the gut removes Glu, our evidence suggests that it is not fully removed when Glu is the only non-essential amino acid in the diet. However, the low enrichment compared with the diet nevertheless suggests extensive cycling and excretion of the Glu carbon skeleton, considering the continuous uptake of 13C-enriched Glu throughout the fish's lifetime. Excretion may be via a combination of the tricarboxylic acid cycle and Ala cycles (via the excretion of nitrogen) as well as via gut energy metabolism. Further research is needed to determine the importance of Glu as an energy source in fish.
Only a few studies have been conducted on carbon isotope analysis of individual amino acids in animals, e.g. Lorrain et al. (Reference Lorrain, Graham and Ménard35) determined δ13C and δ15N of individual amino acids in the blood of four penguin species that forage in different oceanic regions. The aim of their study was among others to test whether the δ13C of individual amino acids could resolve species foraging locations. They found that δ13C of all amino acids decreased with latitude and were correlated with bulk δ13C. But they were not able to determine additional ecological information from these results and asked for more controlled experiments. For Phe, they found only small differences in δ13C between blood and bulk values, and suggested that it may be the most appropriate amino acid for tracking changes in the baseline δ13C. In accordance with these observations, the present experiment also found only tiny differences between diet and body tissue Phe δ13C values. In similar agreement, Howland et al. (Reference Howland, Corr and Young36) found only little fractionation for Phe between diet and bone collagen in pigs raised on controlled diets in order to investigate the use of dietary macronutrients for the synthesis of bulk bone biochemical components.
Conclusion
Compound-specific δ13C analysis in the present study suggests that the carbon isotopic composition for non-essential amino acids remains remarkably similar from diet to fish tissue despite removal of several dietary non-essential amino acids and presumably their biosynthesis from alternative carbon sources. The reasons for this similarity are not clear, but it may be that non-essential amino acids used for tissue development are largely biosynthesised irrespective of the dietary non-essential amino acid composition and that the precursor amino acids are not the only significant carbon contributors. This has been shown in other animals for some amino acids including Glu and Gln. It is not clear to what degree the percentage contribution of carbon substrates for non-essential amino acid biosynthesis is influenced by diet, nutrition or differences in amino acids or dietary regimen. Further experiments to help determine the relative importance of dietary carbon substrates are planned (using isotopically enriched dietary amino acids) in order to help understand which dietary macronutrients support biosynthesis when non-essential amino acid requirements are met and when they are restricted in the diet.
Acknowledgements
The present study was partly supported by the John Fell Fund, University of Oxford. The authors wish to thank B. Fischer and H. Baumgärtner for their support in the laboratory at Hohenheim University, C. Serve-Rieckmann for his help in carrying out the feeding trial and Professor R. E. M. Hedges and the Research Laboratory for Archaeology for use of the LC-IRMS laboratory. J. G.-S. designed the study and wrote the manuscript, J. S. O. M. determined δ13C of amino acids by LC-IRMS and wrote the manuscript, U. F. designed the study, conducted the feeding trial and contributed to the manuscript. All authors read and approved the findings of the study. All authors have no conflicts of interest.









