There is increasing interest in the potential health benefits of vitamin K beyond its role in coagulation. Several studies have reported functions for vitamin K beyond its classic role, including the improvement of bone health(Reference Cockayne, Adamson and Lanham-New1), and the reduction of vascular calcification and cardiovascular risk(Reference Beulens, Bots and Atsma2, Reference Geleijnse, Vermeer and Grobbee3). Moreover, several studies(Reference Beulens, Bots and Atsma2–Reference Spronk, Soute and Schurgers4) have suggested that menaquinones, also known as vitamin K2, could be more effective in these functions than phylloquinone, also known as vitamin K1. Nevertheless, menaquinones are generally not taken into consideration when developing dietary recommendations for vitamin K. Present recommendations for dietary vitamin K are defined for phylloquinone intake only, and are based on median intakes of phylloquinone in certain regions, such as North America(5–7). In some cases, the effects of phylloquinone on coagulation have also been accounted for. For healthy adults, adequate intakes of vitamin K range from 55 to 90 μg/d for adult women and 65–120 μg/d for adult men.
The International Life Sciences Institute (ILSI) Europe has selected experts on vitamin K from academia and industry to review the need for specific dietary reference values (DRV) for menaquinones. To achieve this objective, the expert group conducted a thorough review of existing literature on dietary menaquinones and their role in human health to evaluate: (a) whether unique recommendations for menaquinone intake are justified at this time and (b) what additional information is needed to have strong scientific underpinnings for establishing DRV for menaquinones. In defining nutrient requirements, the selection of criteria to establish nutrient adequacy is an important step. For most nutrients, a hierarchy of criteria for nutrient adequacy can be established, ranging from the prevention of clinical deficiency to the optimisation of body stores or status. The goal is to have a low probability of nutrient inadequacy while minimising the potential risk of excess(8). In light of this definition, we have reviewed the literature with a focus on the following criteria for setting DRV: chemical structure and function of menaquinones; dietary intake of menaquinones; absorption and metabolism of menaquinones; amount of menaquinones required to prevent deficiency, maintain optimal body stores and/or prevent chronic disease; factors influencing menaquinone requirements such as age, sex and safety. The evidence for individual menaquinones for each of these items is described and, if known, differences with phylloquinone are described. Based on this evidence, a conclusion on setting a DRV for menaquinones is drawn and recommendations for future research are made.
Chemical structure and function of menaquinones
Vitamin K is a generic term for a number of structurally related compounds that are characterised by their common functional methylated naphthoquinone ring system, and an aliphatic side chain composed of a number of isoprenoid residues. All differences between the various forms of vitamin K originate from the differences in the length and the saturation degree of the side chain(Reference Shearer and Newman9). Phylloquinone is a single compound with a side chain of four isoprenoid residues, three of which are saturated (Fig. 1). Menaquinones, commonly found in nature, have side chains of varying length between four and thirteen isoprene residues, most of which are unsaturated(Reference Collins and Jones10). However, some bacteria produce isoprenologues in which one or more of the prenyl units are saturated(Reference Shearer and Newman9). Menaquinones are generally denoted as MK-n, where n stands for the number of isoprene residues.
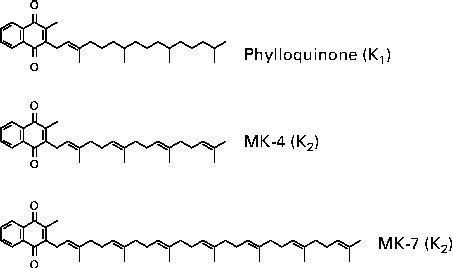
Fig. 1 Chemical structures of K vitamins. MK, menaquinone.
MK-4 is unique among the menaquinones in that it is not synthesised by bacteria. Instead, MK-4 is alkylated from menadione (vitamin K3), a synthetic form of vitamin K that is present in animal feeds, or is the product of tissue-specific conversion directly from dietary phylloquinone, with menadione as the postulated intermediate(Reference Booth and Suttie11, Reference Thijssen, Vervoort and Schurgers12). There is also speculation that longer-chain menaquinones, such as MK-7, can be converted to MK-4 as well(Reference Schurgers, Teunissen and Hamulyak13). The most abundant menaquinones in the human diet are the short-chain MK-4, which originates from animal products, and the long-chain MK-7, MK-8, MK-9 and MK-10.
All forms of vitamin K have one well-known function. They all serve as a cofactor for the post-translational enzyme γ-glutamate carboxylase, which is established by the common naphthoquinone ring structure(Reference Rishavy and Berkner14). This enzyme converts certain protein-bound glutamate residues into γ-carboxyglutamate, generally known as Gla. Currently, seventeen members of the Gla protein family are known, including seven proteins involved in blood coagulation (all synthesised in the liver), osteocalcin (OC; bone), matrix Gla protein (MGP; mainly cartilage and vessel wall), growth arrest-specific protein 6, Gla-rich proteins, two proline-rich Gla proteins, two transmembrane Gla proteins, periostin and periostin-like factor(Reference McCann and Ames15). With the exception of the clotting factors OC (bone formation) and MGP (inhibitor of soft tissue calcification), the physiological importance of these proteins is not yet fully understood(Reference McCann and Ames15). At this time, conformation-specific assays are available for two extra-hepatic Gla proteins (OC and MGP).
Dietary intake of menaquinones
Menaquinones generally are of microbial origin. Important dietary sources are cheese, curd and natto (a traditional Japanese food composed of fermented soya beans)(Reference Schurgers and Vermeer16), while dietary phylloquinone is mainly found in green vegetables, notably spinach, broccoli, kale and Brussels sprouts(Reference Bolton-Smith, Price and Fenton17, Reference Shearer and Bolton-Smith18). Estimated intake of phylloquinone and menaquinones in The Netherlands and Germany has suggested that between 10 and 25 % of total vitamin K intake are provided by menaquinones(Reference Schurgers and Vermeer16, Reference Nimptsch, Rohrmann and Linseisen19). Information on the dietary intake of menaquinones is, however, limited. This is mainly due to the lack of complete food composition tables that list menaquinone concentrations in common foods. Currently, most food composition data for menaquinones are restricted to single foods such as cheese or yogurt(Reference Schurgers and Vermeer16, Reference Kamao, Suhara and Tsugawa20). However, a regularly updated and expanding food composition table of foods on the Dutch market is produced at VitaK's laboratories in The Netherlands.
Based on the content of menaquinones of certain foods, regional differences exist both for the form and the amount of menaquinones consumed. For example, in Japan, MK-7 intake has mostly been documented in the diet due to the consumption of natto, while long-chain menaquinones, MK-7 to MK-10, are predominantly consumed by high dairy intake populations, such as the Dutch population. In addition, cheese is the most important source of menaquinones in the European food supply, and therefore menaquinone concentrations in the cheeses of Dutch, German, Swiss, British and French origins were tested(Reference Schurgers and Vermeer16). Since different lactic acid bacteria are used in European cheeses, a large variability in menaquinone content among the cheeses was found(Reference Fox and McSweeney21). However, it remains to be seen whether these data are applicable to non-European countries.
The few studies that have provided estimates for menaquinone intakes have been mainly performed among Japanese or European populations for which menaquinone-rich foods are present in the diet. A study by Kamao et al. (Reference Kamao, Suhara and Tsugawa20) measured the menaquinone content of foods and the estimated intake of MK-4 and MK-7 of 125 young Japanese women using a 3 d weighed food record. The MK-7 intake of this population was originally estimated at 57·4 (sd 83·7) μg/d and accounted for approximately 25 % of the total intake of vitamin K. However, this estimate is mainly driven by natto consumption, as it accounted for 99 % of the MK-7 intake and almost half of the population studied consumed natto. After stratifying by natto consumption, intake of MK-7 was estimated at 154·1 (sd 87·8) μg/d among natto consumers, but data on non-natto consumers were not provided.
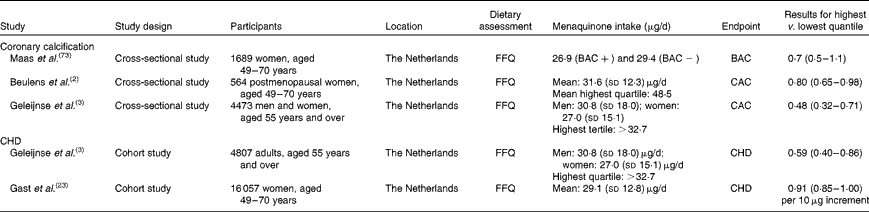
Several Dutch studies investigating the associations of menaquinone intake with disease incidence obtained estimates for menaquinone intake(Reference Beulens, Bots and Atsma2, Reference Geleijnse, Vermeer and Grobbee3, Reference Beulens, Van der and Grobbee22, Reference Gast, de Roos and Sluijs23) (Table 1) using FFQ. These intake estimates were based on direct measurements of menaquinones in foods in combination with published data(Reference Schurgers and Vermeer16, Reference Elder, Haytowitz and Howe24). The self-reported mean intake of menaquinones was approximately 31 μg/d(Reference Geleijnse, Vermeer and Grobbee3, Reference Beulens, Van der and Grobbee22). In the EPIC-Netherlands cohort, cheese contributed 53 % of menaquinone intake, while milk products and meat contributed 19 and 17 %, respectively. The most prominent long-chain menaquinone reported in the diet was MK-9. In the Rotterdam Study, MK-5 to MK-10 contributed 23·1 (sd 16·3) μg/d for men and 20·7 ± 13·8 μg/d for women. These data on food contents of menaquinones have also been applied to a German cohort of approximately 12 000 men(Reference Nimptsch, Rohrmann and Linseisen19). Similar estimates of 34·7 μg/d (interquartile range 25·7–45·7) were reported for all menaquinones and 18·0 μg/d (11·7–27·0) for MK-5 to MK-9, with cheese being the most important food source of menaquinones(Reference Nimptsch, Rohrmann and Linseisen19).
Table 1 Menaquinone intake, arterial calcification and risk of CHD

BAC, breast arterial calcification; CAC, coronary artery calcification.
Finally, the European Food Safety Authority reported data on UK intake of menaquinones based on the UK National Dietary and Nutrition Survey(Reference Bresson, Flynn and Heinonen25). This study used weighed dietary records, albeit based on the menaquinone content from a limited number of food items(Reference Bresson, Flynn and Heinonen25). The overall estimated intake of menaquinones ranged from 36 μg/d (female adults) to 54 μg/d (male teenagers). The mean estimated intake of menaquinones among male adults was 43 μg/d, which is similar to the intakes reported for other European countries.
It should be noted that estimates from the Dutch and German populations were obtained from FFQ that are designed to estimate the relative dietary intake of large populations, but not to estimate the absolute dietary intake. These limitations should be kept in mind when interpreting these data. In order to obtain more precise estimates of menaquinone intakes, studies using (weighed) food records and concentrations of individual menaquinones obtained from representative foods from different food supplies are required. Nonetheless, current studies have shown estimated intakes of menaquinones ranging between 30 and 50 μg/d, which account for up to 25 % of intake of total vitamin K.
Absorption and metabolism of menaquinones
Phylloquinone is primarily obtained from green, leafy vegetables in which it is tightly bound to the membranes of plant chloroplasts, and thus less bioavailable compared with phylloquinone obtained from plant oils and/or dietary supplements(Reference Booth and Suttie11). Menaquinones, which are primarily derived from animal-based sources, are consumed in food matrices containing more fat that may improve absorption and lead to higher bioavailability than phylloquinone(Reference Gijsbers, Jie and Vermeer26). However, this has yet to be systematically tested for all menaquinones.
Following intestinal absorption, all vitamin K forms are incorporated into TAG-rich lipoproteins and transported primarily to the liver, but also to other target tissues. Circulating TAG-bound forms of vitamin K peak at around 4–10 h after intake and the majority of phylloquinone and MK-4 are removed from the circulation by 24 h postprandially(Reference Schurgers, Teunissen and Hamulyak13, Reference Schurgers and Vermeer16, Reference Sato, Schurgers and Uenishi27). Currently, human data on the absorption of menaquinones from food sources are limited to MK-7. These MK-7 data show similar peaks at 4 h after intake, but MK-7 does not appear to be completely removed from the circulation after 72–96 h(Reference Schurgers, Teunissen and Hamulyak13, Reference Schurgers and Vermeer16). The different pharmacokinetics among various vitamin K forms also result in very different plasma half-life times. Whereas phylloquinone has a relatively short half-life time(Reference Novotny, Kurilich and Britz28), MK-7 has a reported half-life time of several days(Reference Schurgers, Teunissen and Hamulyak13, Reference Sato, Schurgers and Uenishi27). Available data indicate higher absorption and bioavailability of MK-7 than phylloquinone, which may facilitate its uptake by various target tissues.
Another difference between the short-chain forms of vitamin K (phylloquinone and MK-4) and the long-chain forms relates to tissue distribution. For example, one study showed that MK-9 is preferentially incorporated in LDL, which facilitates its transport to non-hepatic target tissues(Reference Schurgers and Vermeer29). It is not known whether other long-chain menaquinones have similar transport differences. MK-4 is unique among the menaquinones in its tissue distribution, which relates to its non-bacterial origin. As recently shown in a rodent model using stable isotopes(Reference AL Rajaba, Booth and Peterson30), phylloquinone consumed in the form of leafy greens is converted to MK-4 in some, but not all, tissues. The labelled MK-4 was most abundant in the brain, kidney, fat and reproductive organs. In contrast to phylloquinone as the sole dietary source of vitamin K, there was no conversion of phylloquinone to MK-4 in the liver nor were there detectable amounts of labelled MK-4 in serum. These data confirm earlier rodent studies that have reported differences in tissue distribution between phylloquinone and MK-4(Reference Ronden, Thijssen and Vermeer31, Reference Thijssen and Drittij-Reijnders32).
A caveat to these conclusions is that the data for phylloquinone are much more comprehensive than those for menaquinones. Many investigators have studied phylloquinone pharmacokinetics using different study designs, including stable isotopes(Reference Fu, Peterson and Hdeib33–Reference Jones, Bluck and Wang35). In contrast, menaquinone pharmacokinetic data are limited: (1) in the forms studied (mainly limited to MK-7); (2) from a lack of replication by independent laboratories; (3) by an absence of using stable isotope technology. More research is clearly required to quantify the differences in absorption and bioavailability among the various forms of vitamin K in order to set nutrient requirements.
Microbiotic production of menaquinones
Most aerobic Gram-positive bacteria and the majority of anaerobic bacteria produced by the gut use menaquinones in their electron transport pathways. The length of the side chain, as indicated by different menaquinones, is controlled by specific bacteria(Reference Collins and Jones10). The reasons for this are not entirely clear, but the length and degree of saturation of the menaquinone side chain are often dependent on the growth temperature of a given species(Reference Nowicka and Kruk36). Based on qualitative bacteriological analyses, several bacteria have been identified to produce specific menaquinones. Menaquinones produced by the gut flora have been tabulated by previous studies(Reference Fernandez and Collins37–Reference Mathers, Fernandez and Hill39). For example, Bacteroides fragilis produces MK-10 to MK-12, while Eubacterium lentum produces MK-6. Likewise, bacteria used as starter cultures for the production of foods such as cheese may also produce specific menaquinones. For example, the lactic acid bacteria Lactococcus lactis ssp. lactis and L. lactis ssp. cremoris produce mainly MK-8 and MK-9(Reference Morishita, Tamura and Makino40), while propionibacteria produce mainly MK-9(Reference Hojo, Watanabe and Mori41). The implications for the relative bioavailability of dietary menaquinones produced by bacteria in the food supply need to be considered relative to the bioavailability of menaquinones produced by bacteria in the human intestine.
It was once stated that up to 50 % of the human requirement for vitamin K was fulfilled by the intestinal production of menaquinones(Reference Conly and Stein42, Reference Suttie43). The 50 % estimate was based on semi-quantitative measurements of the vitamin K content of the human liver, in which one-half of the vitamin K content was phylloquinone and the other half was a mixture of long-chain menaquinones(Reference Duello and Matschiner44). However, subsequent studies indicated that phylloquinone accounted for less than 10 % of the vitamin K content in the human liver, with a greater preponderance of MK-10, MK-11 and MK-12 than previously assumed(Reference Suttie43, Reference Usui, Tanimura and Nishimura45). Based on these hepatic menaquinone concentrations, one would predict that circulating long-chain menaquinones would be in much higher concentrations than phylloquinone should these menaquinones have a major contribution to the human requirement for vitamin K. This, however, does not appear to be the case, and the route of absorption of bacterially produced menaquinones is still unclear. The absorption of all vitamin K forms takes place in the small intestine via a process requiring bile salts(Reference Olson46). However, bile salts are absent in the colon where the majority of menaquinones are produced, suggesting a low absorption of these vitamin K forms(Reference Conly and Stein47). This was confirmed by Ichihashi et al. (Reference Ichihashi, Takagishi and Uchida48), who showed that the absorption of intestinally produced menaquinones in rats is low and that the absorption rates decrease markedly with the length of the side chain. A study in infants also indicated that intestinally produced menaquinones are not well absorbed(Reference Fujita, Kakuya and Ito49). This study compared faecal and serum concentrations of phylloquinone and menaquinones of formula-fed infants with breast-fed infants. Formula-fed infants had higher serum and faecal phylloquinone concentrations as well as a higher MK-5 to MK-9 faecal concentration compared with breast-fed infants. Serum menaquinones were undetected in most formula-fed infants, suggestive of poor absorption(Reference Fujita, Kakuya and Ito49).
Another consideration is that most bacterially produced menaquinones are within the bacterial membranes, hence not readily bioavailable. It has been postulated that these bacterially synthesised menaquinones may be important in maintaining normal coagulation among severely ill patients with prolonged vitamin K deficiency(Reference Ramotar, Conly and Chubb50); however, current data are inconclusive regarding the relative contribution of menaquinones to fulfilling the dietary requirements for vitamin K.
The amount of menaquinones required to prevent deficiency and maintain optimal body stores
To understand the impact of menaquinones on health, it is necessary to demonstrate the link between the intakes of menaquinones and the nutritional status of vitamin K. Several biochemical markers of vitamin K status are available and all have their strengths and weaknesses, as detailed elsewhere(Reference Booth and AL Rajaba51). However, measures of plasma or tissue menaquinone concentrations are needed to isolate the effects of menaquinones from those of phylloquinone on human health. Other markers of vitamin K status, which include urinary metabolites of vitamin K(Reference AL Rajaba, Peterson and Choi52, Reference Harrington, Booth and Card53), coagulation times and uncarboxylated Gla proteins, cannot differentiate the effects of menaquinones from phylloquinone. Therefore, differences between menaquinones and phylloquinone can only be determined through the use of study designs that directly compare the response of individual biomarkers with the intakes of individual forms of vitamin K.
Under controlled conditions of dietary intake, circulating menaquinone concentrations increase in response to the high intake of menaquinones and decline over time when the dietary source of menaquinones is removed(Reference Schurgers, Teunissen and Hamulyak13, Reference Gijsbers, Jie and Vermeer26). However, data are limited, since the HPLC and MS techniques are limited to a few qualified laboratories and the long-chain menaquinones are often below the detection limit in the circulation when measured in the general population. Only a few studies have measured plasma menaquinones in response to the intake of individual menaquinones, and these have been limited to MK-7 or MK-4 supplementation(Reference Schurgers, Teunissen and Hamulyak13, Reference Sato, Schurgers and Uenishi27, Reference Bruge, Bacchetti and Principi54). For plasma MK-7, two studies showed a clear dose–response effect on circulating MK-7 concentrations after supplementation with doses ranging between 45 and 420 μg(Reference Schurgers, Teunissen and Hamulyak13, Reference Bruge, Bacchetti and Principi54). In contrast, MK-4 was not detected in the circulation following a single dose of 420 μg(Reference Sato, Schurgers and Uenishi27).
Only two studies have investigated the response of vitamin K urinary metabolites to single oral doses of menaquinones. Both studies showed a good response of urinary menadione(Reference Thijssen, Vervoort and Schurgers12) or side-chain catabolite(Reference Harrington, Soper and Edwards55) excretion to relatively high doses of 15 or 45 mg of MK-4 or 1 mg of MK-7. Both studies included a direct comparison of menaquinones with phylloquinone, and showed similar results for both vitamin K forms. Harrington et al. (Reference Harrington, Booth and Card53) showed that the excretion of the 5- and 7-carbon side-chain metabolites responds to the depletion and repletion of phylloquinone. A similar response to menaquinones would be expected, but no studies of similar design have been conducted.
Prothrombin time (PT), also expressed as an international normalised ratio, is a test of coagulation that can reflect clinical deficiency of vitamin K due to frank deficiency or the antagonism of vitamin K. However, PT is non-specific because abnormal values are also indicative of diseases unrelated to vitamin K deficiency. PT changes only when prothrombin concentrations drop below 50 % of normal, demonstrating its low sensitivity for detecting the deficiency of vitamin K(Reference Suttie56). To date, only two studies(Reference Schurgers, Teunissen and Hamulyak13, Reference Schurgers, Shearer and Hamulyak57) have reported the effects of MK-7 and MK-9 on coagulation parameters. In these studies, the antidotal effect of single doses of MK-7 and MK-9 was studied in volunteers stabilised on oral vitamin K antagonists. Both studies showed that MK-7 and MK-9 decreased the international normalised ratio and the concentrations of coagulation factors, and this effect was stronger for MK-7 than phylloquinone(Reference Schurgers, Teunissen and Hamulyak13, Reference Schurgers, Shearer and Hamulyak57). However, in one study(Reference Schurgers, Shearer and Hamulyak57), menaquinones were provided as different food sources with differing doses and bioavailability, which may have influenced the results. Although these studies are informative in the clinical context of the reversal of oral anticoagulation, they are not suitable for determining the amount of menaquinones required to prevent deficiency or maintain optimal body stores. Although the effects of coagulation factors on depletion or repletion with menaquinones have not been investigated to date, sustained intakes as low as 10 μg/d of phylloquinone for several weeks do not prolong PT in otherwise healthy adults(7, Reference Frick, Riedler and Brogli58–Reference Udall60).
Thus far, conformation-specific tests have been developed for prothrombin, OC and MGP to evaluate the extent to which the various Gla proteins are carboxylated in healthy subjects. Advantages of measuring uncarboxylated vitamin K-dependent proteins are that insufficiencies measured in circulating forms theoretically reflect what occurs at the tissue level. Undercarboxylated prothrombin, also known as PIVKA-II, detects abnormalities in prothrombin before the prolongation of PT, but does not have the sensitivity to detect the variability of usual vitamin K intakes observed in normal healthy populations. PIVKA-II has been used as a marker of vitamin K status in healthy people and has been shown to respond to both dietary depletion and subsequent repletion with phylloquinone(Reference Booth, Lichtenstein and O'Brien-Morse61, Reference Booth, Martini and Peterson62). However, only one study(Reference Furukawa, Nakanishi and Okuda63) investigated the effect of a single intravenous dose of 10 mg MK-4 in vitamin K-deficient cancer patients, and showed a decrease in PIVKA-II levels 1–3 d after ingestion.
The effect of menaquinones on the proportion of OC that is uncarboxylated (ucOC) has been more frequently studied. ucOC is highly responsive to supplementation with either MK-4 or MK-7 in doses ranging from 45 μg/d to 45 mg/d (MK-4 only)(Reference Schurgers, Teunissen and Hamulyak13, Reference Bruge, Bacchetti and Principi54, Reference Emaus, Gjesdal and Almas64–Reference van Summeren, Braam and Lilien66). Only a low dose of 45 μg MK-7/d did not lead to a significant reduction in ucOC(Reference Bruge, Bacchetti and Principi54). A direct comparison of phylloquinone with MK-7 supplementation indicated that MK-7 is more effective in carboxylating OC than phylloquinone(Reference Schurgers, Teunissen and Hamulyak13). Assays to measure desphospho-uncarboxylated MGP only recently became available. Since that time, several intervention studies have shown clear dose–response effects of desphospho-uncarboxylated MGP to MK-7 supplementation with doses ranging between 10 and 360 μg/d(Reference Cranenburg, Koos and Schurgers67–Reference Westenfeld, Krueger and Schlieper70). However, a direct comparison between menaquinones and phylloquinone in the response of desphospho-uncarboxylated MGP to supplementation by the individual vitamin K forms has not been made.
The amount of menaquinones required to prevent chronic diseases
Menaquinones, coronary calcification and CVD
Coronary artery calcification is an important predictor of CVD(Reference Greenland, Bonow and Brundage71). MGP is an inhibitor of vascular calcification(Reference Shanahan, Proudfoot and Farzaneh-Far72). Through carboxylation of MGP, vitamin K may help reduce coronary calcification and thereby reduce the risk of CVD. Observational studies have indeed shown that a high intake of vitamin K is associated with reduced coronary calcification and a reduced risk of CVD(Reference Beulens, Bots and Atsma2, Reference Geleijnse, Vermeer and Grobbee3, Reference Gast, de Roos and Sluijs23). The results from some studies suggest that this is mainly due to menaquinones(Reference Beulens, Bots and Atsma2–Reference Spronk, Soute and Schurgers4, Reference Gast, de Roos and Sluijs23). Thus far, three cross-sectional studies(Reference Beulens, Bots and Atsma2, Reference Geleijnse, Vermeer and Grobbee3, Reference Maas, van der Schouw and Beijerinck73) investigated the associations of menaquinone intake and coronary calcification, as summarised in Table 1. In the Rotterdam Study, intakes of menaquinones were lower in participants with severe aortic calcifications (25·6 μg/d) than in participants with moderate or mild calcifications (28·6 and 28·8 μg/d, respectively; P= 0·001)(Reference Geleijnse, Vermeer and Grobbee3). A strong inverse relationship between menaquinone intake and severe calcification was found in the mid and upper tertiles of menaquinone intake compared with the lowest tertile, reaching significance in the highest tertile with a menaquinone intake of more than 32·7 μg/d. Using breast arterial calcification as a measure of arterial calcification, the prevalence of breast arterial calcification was less common in the highest (9 %) quartile of menaquinone intakes, compared with the lowest quartile (13 %)(Reference Maas, van der Schouw and Beijerinck73). This study showed a similar association to that of Geleijnse et al. (Reference Geleijnse, Vermeer and Grobbee3) with an OR of 0·7 (95 % CI 0·5, 1·1), although it did not reach significance. Similarly, a high menaquinone intake over 48 μg/d was associated with reduced coronary calcification among 600 middle-aged women(Reference Beulens, Bots and Atsma2). We are not aware of any randomised trials to date that investigated the effect of menaquinones on the progression of arterial calcification.
Also, two of the previously mentioned cohort studies investigated the relationship between menaquinone intake and the risk of CHD (Table 1). In the Rotterdam cohort(Reference Geleijnse, Vermeer and Grobbee3), the relative risk of incident CHD was reduced in the upper tertile of menaquinone intake compared with the lowest tertile (0·43; 95 % CI 0·24, 0·77). In the Prospect-EPIC cohort(Reference Gast, de Roos and Sluijs23), the investigators also observed an inverse association between the intake of menaquinones and the risk of CHD with a hazard ratio of 0·91 (95 % CI 0·85, 1·00, P= 0·08) per 10 μg/d of menaquinone intake. In order to compare these results with previous studies using categories, a menaquinone intake of 35 μg/d would lead to a hazard ratio of about 0·7, which compares nicely with previous studies. The association between menaquinone intake and the incidence of stroke has not been investigated to date. Of note, several of these studies also investigated the relationship between phylloquinone intake and coronary calcification or the risk of CHD, but could not detect significant associations(Reference Beulens, Bots and Atsma2, Reference Geleijnse, Vermeer and Grobbee3, Reference Gast, de Roos and Sluijs23). Whether this is due to biological differences between menaquinones and phylloquinone or perhaps lower validity of the FFQ to estimate phylloquinone intake is currently unclear(Reference Beulens, Bots and Atsma2). Finally, these associations have only been investigated in Dutch populations and generalisability of these results should be studied in different populations.
Menaquinones and bone
In bone, three vitamin K-dependent proteins have been isolated: protein S; MGP; OC. The anticoagulant protein S is synthesised by osteoblasts (bone-forming cells), but its role in bone metabolism is unclear. MGP has been found in bone, dentine, cartilage and soft tissue, including blood vessels, and is associated with the organic matrix and mobilisation of bone Ca. The results of animal studies suggest that MGP prevents the calcification of soft tissue and cartilage, while facilitating normal bone growth and development(Reference Booth74). OC is a protein synthesised by osteoblasts. The synthesis of both OC and MGP is regulated by calcitriol and retinoic acid. Higher ucOC concentrations, indicating a low vitamin K status, were associated with a higher hip fracture risk and lower bone mineral density (BMD) in adults(Reference Booth, Tucker and Chen75–Reference Vergnaud, Garnero and Meunier85) and children(Reference Kalkwarf, Khoury and Bean86–Reference van, Braam and Noirt89). Unfortunately, the proportion of ucOC does not differ between phylloquinone and menaquinones in terms of the form responsible as an enzyme cofactor. However, serum menaquinone levels were lower in patients with osteoporosis, osteopenia and osteoporotic fractures compared with controls(Reference Hodges, Pilkington and Stamp90–Reference Tamatani, Morimoto and Nakajima93). In addition, an inverse association was found between circulating MK-7 levels and the incidence of vertebral fractures in Japanese women, although this association was stronger for phylloquinone and fracture risk(Reference Tsugawa, Shiraki and Suhara94).
Intervention studies using pharmacological doses of MK-4 showed beneficial effects on bone parameters(Reference Cockayne, Adamson and Lanham-New1). However, these intervention studies used very high doses of MK-4 (generally 45 mg) that cannot be obtained from the habitual diet. Since this paper is focused on dietary doses that are relevant for nutritional requirements, we will focus on intervention studies that used doses that can be nutritionally obtained. Although natto contains more than 100 times as much menaquinones as various cheeses, studies on natto or its equivalent amount of MK-7 are considered within the dietary intake range.
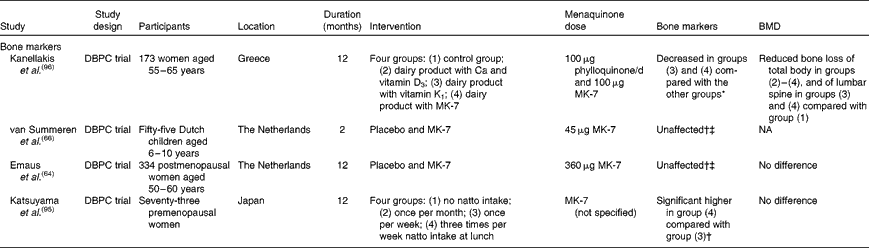
Intervention studies investigating the effect of menaquinone supplementation on bone markers are shown in Table 2. Whereas MK-7 at low doses did not affect bone formation(Reference Emaus, Gjesdal and Almas64, Reference Tsukamoto, Ichise and Kakuda65), intake of natto three times per week increased bone-specific alkaline phosphatase when compared with once-per-week natto intake(Reference Katsuyama, Ideguchi and Fukunaga95). Only one study showed decreased bone resorption due to a combination of vitamin K forms, vitamin D3, Ca and lifestyle recommendations(Reference Kanellakis, Moschonis and Tenta96). This finding was independent of the form of vitamin K taken, although on a molecular basis, the daily intake of MK-7 (0·154 μmol) in this study was about 30 % less than that of phylloquinone (0·221 μmol).
Table 2 Intervention studies on the dietary levels of vitamin K, bone markers and bone mineral density (BMD)

DBPC, double-blind placebo-controlled; MK-7, menaquinone-7; NA, not applicable.
* Urinary deoxypyridinoline.
† Bone-specific alkaline phosphatase.
‡ Degradation products of C- or N-terminal telopeptides of type I collagen.
A few cross-sectional studies investigated the association between menaquinone intake and bone maintenance. For example, two Japanese studies showed that the usual dietary intake of natto was effective in maintaining bone stiffness(Reference Katsuyama, Ideguchi and Fukunaga97) and was positively associated with a 3-year change in BMD at the femoral neck(Reference Ikeda, Iki and Morita98). Within Norwegian individuals, no linear association was found between dietary menaquinones and BMD of the total hip; however, fractional polynomial regression analyses for the detection of non-linear associations showed a small, positive association between dietary menaquinones and BMD among women(Reference Apalset, Gjesdal and Eide99). Of note, these studies described a proposed effect of dietary menaquinones via natto consumption. Although MK-7 is an important nutrient of natto, natto also contains other ingredients that have been postulated to promote bone health. Hence MK-7 may be a surrogate marker for other bone-promoting ingredients in these studies.
As shown in Table 2, three intervention studies on menaquinones and BMD used doses of menaquinones that are attainable with the diet. Only one study(Reference Kanellakis, Moschonis and Tenta96) observed a beneficial effect of vitamin K on BMD of the lumbar spine. The two other studies did not show an effect on bone stiffness or the rate of bone loss. The main differences between these three studies are the inclusion of vitamin D as part of the treatment and the regional differences in the prevalence of vitamin D deficiency(Reference Lips100). These vitamin D-related disparities in study designs may have influenced the effect of menaquinones.
Only one observational study specifically examined the association between dietary menaquinone intake and fracture risk, reporting that a low intake of phylloquinone, but not menaquinones, was associated with an increased risk of hip fractures in Norwegian individuals(Reference Apalset, Gjesdal and Eide101). To date, no intervention study has evaluated the efficacy of menaquinones in doses attainable in the diet in reducing fracture risk.
Only a few studies have made a direct comparison between menaquinones and phylloquinone for bone health. These studies indicated no differences(Reference Kanellakis, Moschonis and Tenta96) or showed stronger effects for phylloquinone than menaquinones(Reference Tsugawa, Shiraki and Suhara94, Reference Apalset, Gjesdal and Eide101).
Requirements across the life cycle
Dietary intake has historically been considered the primary determinant of vitamin K status(Reference Booth and Suttie11). However, other non-dietary factors are emerging as determinants, such as age and ethnicity(Reference Booth and AL Rajaba51). To develop recommendations for dietary intakes(8), sufficient data are needed to evaluate requirements across the life cycle. The data for phylloquinone are sparse(7), but there are even less data for menaquinones. Currently, data on the intake or supplementation of menaquinones are limited to the assessment in children and teenagers in the UK National Dietary and Nutrition Survey(Reference Bresson, Flynn and Heinonen25).
The only clinically indicated use of vitamin K is as a prophylactic against vitamin K deficiency bleeding in otherwise healthy-appearing neonates(Reference Shearer102). The low content of vitamin K in breast milk, low placental transfer of vitamin K, low levels of clotting factors at birth and a sterile gut are all contributing factors to the risk of vitamin K deficiency bleeding in the first few months of life. Prevention of vitamin K deficiency bleeding by oral or intramuscular administration of vitamin K at birth is standard practice in many countries. Whereas most countries use phylloquinone, certain Asian countries, including Japan, use MK-4 prophylactically(Reference Shearer102). At no other point in the life cycle is frank deficiency of vitamin K a concern among an otherwise healthy population.
Safety of high vitamin K intake
There is no documented case of toxicity for phylloquinone or menaquinones(7, Reference Bresson, Flynn and Heinonen25). The European Food Safety Authority's safety assessment of menaquinones as a source of vitamin K added for nutritional purposes concluded that low doses of menaquinones presented no safety concerns(Reference Bresson, Flynn and Heinonen25). Similarly, an animal study reported no toxicity associated with synthetic MK-7 administered in a single oral dose up to 2000 mg/kg or for 90 d of oral administration of 10 mg/kg per d(Reference Pucaj, Rasmussen and Moller103).
It is often postulated that excessive vitamin K may result in overcoagulation, i.e. increased thrombosis risk. However, vitamin K-dependent proteins have a limited number of Glu residues capable of γ-carboxylation per molecule, beyond which there can be no further γ-carboxylation or excessive coagulation. Despite this, it is critical to demonstrate that a high intake of menaquinones does not increase thrombosis risk. It was shown in rats that thrombosis risk is not increased at doses up to 250 mg/kg of MK-4(Reference Ronden, Groenen-van Dooren and Hornstra104). In human subjects, the endogenous thrombin potential, which is the most sensitive marker to evaluate thrombosis risk in plasma(Reference Hemker, AL Dieri and De Smeat105), was not affected by MK-7 intakes as high as 360 μg/d for 6 weeks(Reference Westenfeld, Krueger and Schlieper70). The only exception to this is observed in individuals on coumarin-based oral anticoagulants, for whom dietary supplementation with vitamin K can influence the stability of the international normalised ratio(Reference Suttie, Mummah-Schendel and Shah59, Reference Holmes, Hunt and Shearer106). MK-7 has the potential to interfere with oral anticoagulants at doses greater than 50 μg/d(Reference Schurgers, Teunissen and Hamulyak13). However, there is little collective experience on the potential toxicity or adverse events associated with sustained menaquinone supplementation among individuals with normal coagulation.
In Asia, MK-4 is routinely used for osteoporosis treatment in doses of 45 mg/d without reported toxicity. Reported adverse effects associated with these high doses are limited to skin rashes that subside with cessation of the MK-4 dosing(Reference Bunyaratavej, Penkitti and Kittimanon107). As concluded by the European Food Safety Authority(Reference Bresson, Flynn and Heinonen25), phase I clinical trials have not yet been designed to test the safety of menaquinones nor has any form of vitamin K been adequately tested for mutagenicity. However, it is biologically implausible to attain such high levels of intake and sufficient bioavailability from menaquinones obtained from food sources to present a risk to health.
Conclusions
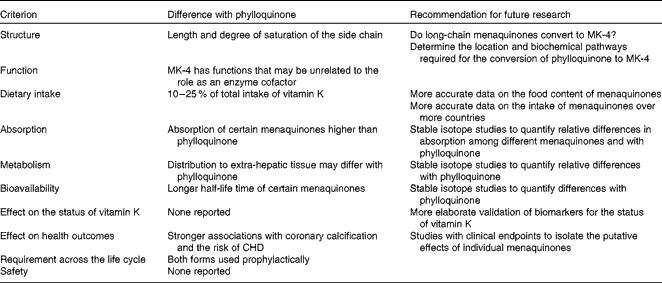
There is growing speculation that certain dietary menaquinones, while consumed in lower quantities than phylloquinone, may have unique and important contributions to the role of vitamin K on human health. However, present DRV for vitamin K are exclusively based on phylloquinone. In recognition of this emerging paradigm shift in vitamin K nutrition research, we have reviewed existing literature to evaluate the current state of knowledge on menaquinones that would be needed for inclusion in the DRV for vitamin K. It was concluded that differences in the chemical structure of menaquinones compared with phylloquinone may lead to differences in absorption and bioavailability (Table 3). Several studies have shown that certain forms of menaquinones may be more bioavailable and effective in carboxylating particular extra-hepatic Gla proteins than phylloquinone. The intake of menaquinones accounts for up to 25 % of the total intake of vitamin K, and should there be a higher bioavailability, menaquinones would be important to consider in their contribution to human health. Indeed, certain observational studies have indicated that high intakes of menaquinones may be associated with greater reductions of vascular calcification and the risk of CVD than comparable amounts of phylloquinone. Such effects have not been observed for bone health. However, these data are limited to observational studies conducted among Dutch or Japanese populations. Moreover, food composition tables for menaquinones are limited and available only for a few countries. In addition, studies investigating the bioavailability of menaquinones using stable isotope techniques are lacking. Therefore, research is warranted to compile more elaborate food composition data of menaquinones and more accurate data on the intake of menaquinones, at different stages of the life cycle (Table 3). These data should be used to investigate the relationship with disease incidence in populations other than the Dutch or Japanese. Stable isotope studies are required to quantify differences in absorption, bioavailability and distribution over the body between individual menaquinone forms and phylloquinone. Finally, intervention studies with clinical endpoints and a more elaborate validation of biomarkers for vitamin K status are required to quantify how the bioavailability and tissue distribution of menaquinones affect vitamin K status and health outcomes.
Table 3 Summary and recommendations for future research

MK-4, menaquinone-4.
Clearly, significant gaps in the current knowledge on menaquinones exist. However, there is merit for considering both menaquinones and phylloquinone when developing future recommendations for vitamin K intake.
Acknowledgements
The present study was commissioned by the Addition of Nutrients to Foods Task Force and the Nutrient Requirements Task Force of the European Branch of the European branch of the International Life Sciences Institute (ILSI Europe). Industry members of these task forces are Barilla G. & R. Fratelli, BASF SE, Bunge Europe, Coca-Cola Europe, Danone, DSM, Kellogg Europe, Nestlé, Red Bull, Royal FrieslandCampina, Ulker Bisküvi and Unilever. This publication was coordinated by Athanasia Baka, Evangelia Grammatikaki and Christophe Matthys, Scientific Project Managers at ILSI Europe. For further information about ILSI Europe, please email info@ilsieurope.be or call +32 2 771 00 14. The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies. A. B. is employed by ILSI Europe. J. W. J. B., S. L. B. and C. V. received an honorarium from ILSI Europe for their participation in this publication and/or reimbursement of their travel and accommodation costs for attending the related meetings. J. W. J. B. was supported by a personal Dr Dekker postdoctoral grant (2008T062) from the Netherlands Heart Foundation. S. L. B. was supported by the USDA, Agricultural Research Service under Cooperative Agreement no. 58–1950-7-707. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. VitaK collaborates with a variety of industries in the nutrition area and performs contract research on both vitamin K1 and K2.
J. W. J. B., S. L. B., E. G. H. M. v. d. H. and C. V. wrote the manuscript. All authors participated in the discussions about the content of the manuscript. E. S. and A. B. critically reviewed the manuscript for important intellectual content. All authors read and approved the final manuscript.