Introduction
The United Nations (UN) urges protection and restoration of global ecosystems (UN, 2015). However, the human population, predicted to exceed nine billion in 2050 (UN, 2017), requires feeding and c. 37% of the world's land area is used for agriculture (pasture and arable) (FAO, 2017). Some predictions indicate this will increase by 2050 (Öborn et al., Reference Öborn, Magnusson, Bengtsson, Vrede, Fahlbeck, Jensen, Westin, Jansson, Hedenus, Lindholm Schulz, Stenström, Jansson and Rydhmer2011); in less than a century, globally significant wilderness may not exist (Watson et al., Reference Watson, Shanahan, Di Marco, Allan, Laurance, Sanderson, Mackey and Venter2016).
Various estimates put Earth's carrying capacity for Homo sapiens at or below eight billion (UN, 2012), so choices are necessary. If human population growth is not controlled, semi-natural areas must be sacrificed to food production and urbanization, engendering ecosystem collapse and environmentally forced population reduction. An alternative is to create more living space, e.g., habitats orbiting Earth or on the Lunar or Martian surface. The concept of extraterrestrial (ET) nature reserves (ETNRs) arises, as envisaged in Douglas Trumbull's 1972 film ‘Silent Running’.
Such work is portended by the European Space Agency's (ESA's) MELiSSA project, envisaging closed, bioregenerative life-support for human space missions (e.g., Lasseur et al., Reference Lasseur, Brunet, de Weever, Dixon, Dussap, Godia, Leys, Mergeay and Der Straeten2010), and the contained ecosystems of Biosphere 2 (cf., Nelson, Reference Nelson2018). Plants will be critical to human survival outside Earth (Poulet et al., Reference Poulet, Fontaine and Dussap2016), as life-support systems, providing food, oxygen (O2) and water purification (Wolff et al., Reference Wolff, Coelho, Karoliussen and Jost2014). Therefore, space exploration requires establishing ecosystem services in ET environments, for pragmatic, selfish and altruistic reasons.
Terraforming Mars is considered (e.g., Sagan, Reference Sagan1973, McKay, Reference McKay1982, McKay et al., Reference McKay, Toon and Kasting1991, Birch, Reference Birch1992, McKay and Marinova, Reference McKay and Marinova2001, Beech, Reference Beech2009, Jakosky and Edwards, Reference Jakosky and Edwards2018, Pazar, Reference Pazar2018). Accepting long time scales, this offers security for Earth life threatened by astronomical or anthropogenic catastrophe. Eventually, Terran-type ecosystems (TTEs) might be assembled on a modified planet's surface, though contained communities may be achievable sooner and as a necessary step.
Mars is therefore proposed as a location for a contained TTE (CTTE). Assuming containment can provide tolerable conditions, Mars offers gravity, atmosphere, sufficient sunlight for photosynthesis (Table 1) and water (Rummel et al., Reference Rummel, Beaty, Jones, Bakermans, Barlow, Boston, Chevrier, Clark, de Vera, Gough, Hallsworth, Head, Hipkin, Kieft, McEwen, Mellon, Mikucki, Nicholson, Omelon, Peterson, Roden, Lollar, Tanaka, Viola and Wray2014), while proximity to Earth allows management.
Table 1. Relative characteristics of Earth and Mars
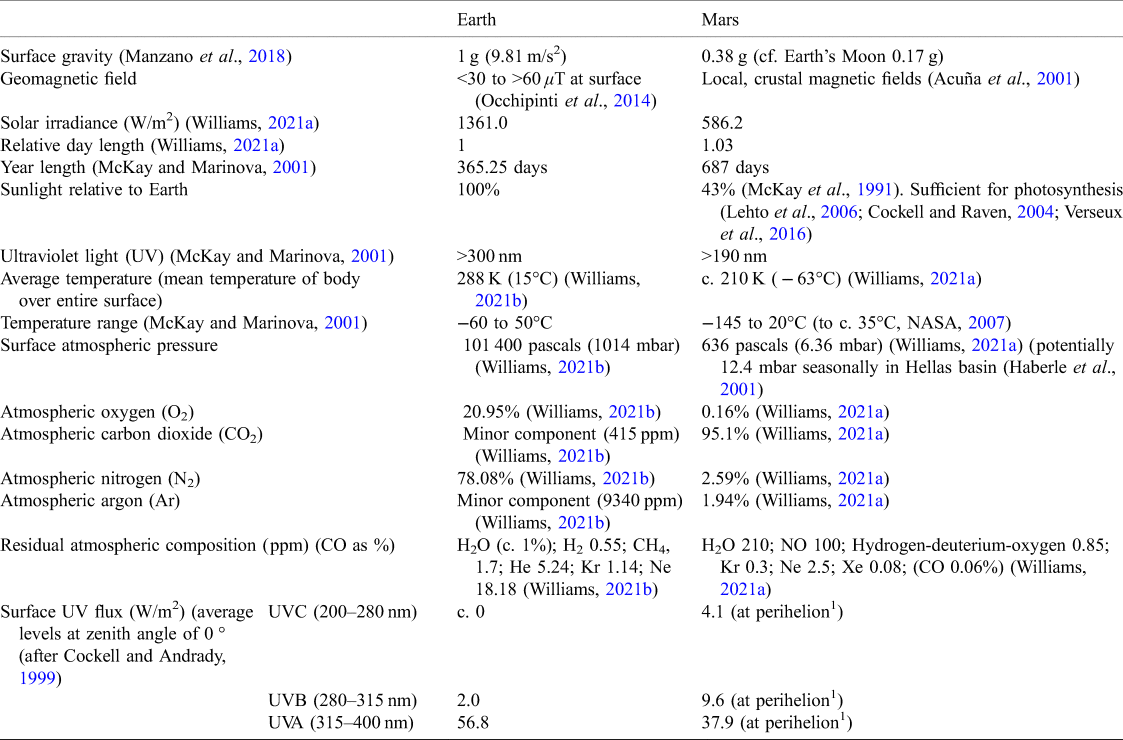
1Perihelion: closest point to sun during an elliptical orbit, therefore maximum incident UV flux.
A Martian CTTE would self-justify, offering refuge, retreat and ecosystem services for astronauts concerned with exploration, resource harvesting or colonization. It would provide wonder, inspiration, purpose and a psychological stepping-stone for more ambitious projects. Humanity's exploration of space will need a network of such oases, supporting terraforming resources and offering biotic refreshment. Forest ecosystems would be apposite.
Forest environments have health benefits (stress reduction, recovery from fatigue, rehabilitation) (Karjalainen et al., Reference Karjalainen, Sarjala and Raitio2009). Ancient forests have emotional, spiritual and cultural significance (e.g., Lowman and Sinu, Reference Lowman and Sinu2017). Threatened by human impact (Frank et al., Reference Frank, Finckh, Wirth, Wirth, Gleixner and Heimann2009; Laurance, Reference Laurance2015), they are archetypes, visions of arboreal majesty and biotic complexity (e.g., Wirth et al., Reference Wirth, Messier, Bergeron, Frank, Fankhänel, Wirth, Gleixner and Heimann2009). Such TTEs would offer relief from ET sterility.
Facsimile old growth forest could be established over a century on Earth (Smith, Reference Smith2018), but present-day Mars' surface is hostile to Earth-adapted life, with high radiation levels (Nixon et al., Reference Nixon, Cousins and Cockell2013), thin atmosphere and other stressors (e.g., Table 1). TTEs would need shielding.
This account assumes a semi-autonomous, contained environment could be created on Mars, large, strong and shielded enough to support a forest, hold positive atmospheric pressure, protect it from meteorites (e.g., Daubar et al., Reference Daubar, Banks, Schmerr and Golombek2019) and exclude harmful radiation (a ‘forest bubble’, McKay 2022 personal communication 20th August) (Fig. 1).
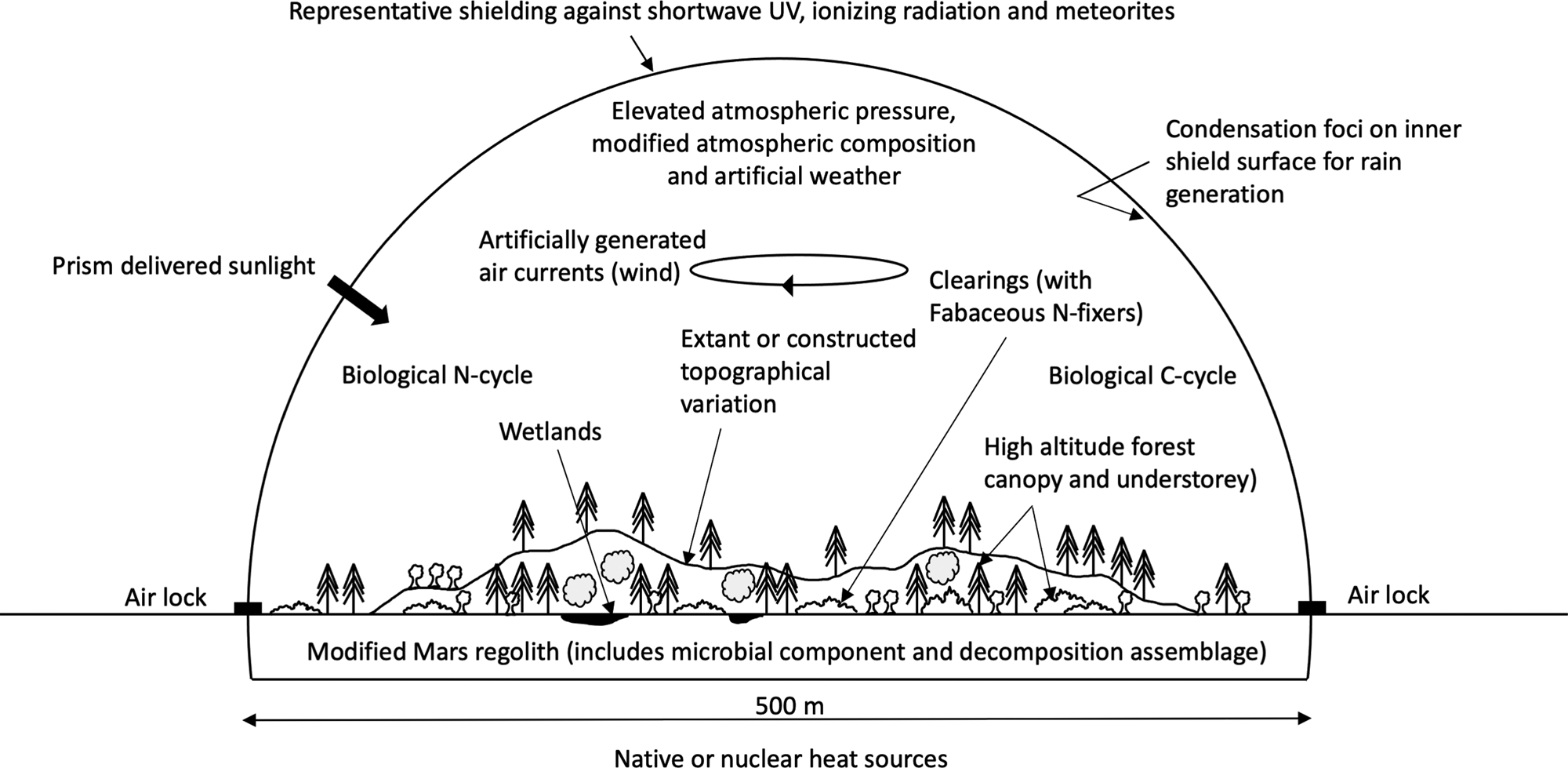
Fig. 1. Conceptual drawing of 20 ha footprint ‘forest bubble’ (location may constrain shape).
Challenges of constructing, large Martian ‘worldhouses’ have been discussed (Taylor, Reference Taylor1992; Reference Taylor1998). Acknowledging small containments' limitations (Taylor, Reference Taylor1998), and flaws in minimum habitat areas (e.g., van der Hoek et al., Reference van der Hoek, Zuckerberg and Manne2015), a hemispherical, environmentally controlled, enclosure of c. 0.25 km radius is envisaged (20 ha footprint). Many semi-natural Earth woods are smaller (e.g., Peterken, Reference Peterken1993) and Biosphere 2's designed rainforest occupies c. 0.2 ha (Nelson, Reference Nelson2018).
Outlining of constraints (cf. Table 1) leads to justification of a concomitant forest design with novel species complement (cf. Table 2). Discussion of ethics follows.
Table 2. Potential integrants for contained Martian TTE
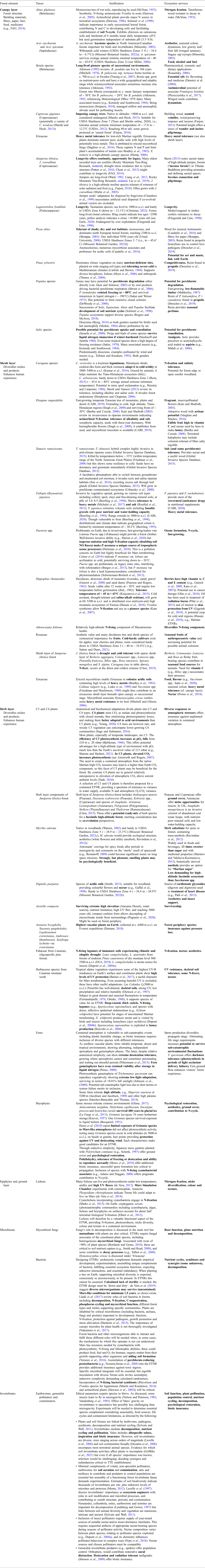
Non-human vertebrates omitted as ability to engage in natural behaviours is not ensured.
Environmental constraints
Ionizing particle radiation (IR)
Unlike Earth, Mars lacks a significant global magnetic field to exclude incoming charged particle radiation (e.g., Atri, Reference Atri2016), its surface subject to solar energetic particles and galactic cosmic radiation that damage living tissues (e.g., Nelson, Reference Nelson2013, Reference Nelson2016).
Artificially generated magnetic (e.g., Townsend, Reference Townsend2005; Battiston et al., Reference Battiston, Burger, Calvelli, Musenich, Choutko, Datskov, Della Torre, Venditti, Gargiulo, Laurenti, Lucidi, Harrison and Meinke2012; Bamford et al., Reference Bamford, Kellet, Bradford, Todd, Benton, Stafford-Allen, Alves, Silva, Collingwood, Crawford and Bingham2014; Ambroglini et al., Reference Ambroglini, Battiston and Burger2016), or electrostatic (Tripathi et al., Reference Tripathi, Wilson and Youngquist2008; Joshi et al., Reference Joshi, Qiu and Tripathi2013) fields, improved passive shielding, local crustal magnetic fields (e.g., Alves and Baptista, Reference Alves and Baptista2004) and/or thick layers of Mars regolith (e.g., Röstel et al., Reference Röstel, Guo, Banjac, Wimmer-Schweingruber and Heber2020) might provide solutions.
Dohm et al. (Reference Dohm, Miyamoto, Ori, Fairn, Davila, Komatsu, Mahaney, Williams, Joye, Di Achille, Oehler, Marzo, Schulze-Makuch, Acocella, Glamoclija, Pondrelli, Boston, Hart, Anderson, Baker, Fink, Kelleher, Furfaro, Gross, Hare, Frazer, Ip, Allen, Kim, Maruyama, McGuire, Netoff, Parnell, Wendt, Wheelock, Steele, Hancock, Havics, Costa, Krinsley, Garry and Bleacher2011) consider cavern refuges. One existing skylight accesses a void at least 37 m deep (Cushing, Reference Cushing2012). Such places might protect CTTEs from IR. Prisms could refract sunlight into underground chambers (cf. Luxfer prisms, Neumann, Reference Neumann1995), IR bypassing them.
Sunlight (including UV)
Martian day length resembles Earth days, and CTTEs can exploit local sunlight (Table 1). Subterranean situations might use mirror/fibre optic delivery, augmented by light emitting diodes (Nakamura et al., Reference Nakamura, Monje and Bugbee2013). Electric light requires maintenance but sunlight collection is vulnerable to dust storms (e.g., Fernández, Reference Fernández1998; Martínez et al., Reference Martínez, Newman, De Vicente-Retortillo, Fischer, Renno, Richardson, Fairén, Genzer, Guzewich, Haberle, Harri, Kemppinen, Lemmon, Smith, de la Torre-Juárez and Vasavada2017). Self-cleaning, light-harvesting surfaces are needed. Superomniphobic materials (Sun and Böhringer, Reference Sun and Böhringer2019) augmented by electrodynamic technology (Mazumder et al., Reference Mazumder, Stark, Heiling and Liu2016) or ‘plasma brooms’ (Ticoş et al., Reference Ticoş, Scurtu and Ticoş2017) might provide solutions but surface micro-/nano-coatings are short-lived (Sun and Böhringer, Reference Sun and Böhringer2019). Vertical light-harvesting surfaces might shed dust, while light-transmitting plants (e.g., Duckett and Ligrone, Reference Duckett and Ligrone2006) might inspire materials.
Besides diffuse illumination, forest understoreys experience sunflecks (short periods of direct irradiance when sun penetrates canopies) (e.g., Pallardy, Reference Pallardy2008). Their nature depends on canopy physiognomy, solar declination and solar time (Chazdon and Pearcy, Reference Chazdon and Pearcy1991). Sunflecks are energy sources, significant to small organisms (including seedlings) and potential stressors (e.g., Leakey et al., Reference Leakey, Scholes and Press2004). Photosynthesis during them may provide 30–60% of daily C gain (Chazdon, Reference Chazdon1988), plants' responses involving UVB photoreceptors (Moriconi et al., Reference Moriconi, Binkert, Costigliolo, Sellaro, Ulm and Casal2018).
Static, unidirectional lighting in windless CTTEs would not engender sunflecks' dynamic chiaroscuros (cf. Way and Pearcy, Reference Way and Pearcy2012), so lighting manipulation, exposure to natural solar ecliptic and/or leaf-animating wind is needed.
Mars' harsh surface UV flux (Table 1) is sterilizing due to thin atmosphere and lack of significant ozone (Cockell et al., Reference Cockell, Catling, Davis, Snook, Kepner, Lee and McKay2000; Kminek et al., Reference Kminek, Rummel, Cockell, Atlas, Barlow, Beaty, Boynton, Carr, Clifford, Conley, Davila, Debus, Doran, Hecht, Heldmann, Helbert, Hipkin, Horneck, Kieft, Klingelhoefer, Meyer, Newsom, Ori, Parnell, Prieur, Raulin, Schulze-Makuch, Spry, Stabekis, Stackebrandt, Vago, Viso, Voytek, Wells and Westall2010). UV has positive (Juzeniene and Moan, Reference Juzeniene and Moan2012) and negative (e.g., Lee et al., Reference Lee, Wei, Hong, Yu and Wei2013, Rettberg et al., Reference Rettberg, Rabbow, Panitz and Horneck2004) effects on organisms. Fortunately glass/plastic combinations can exclude harmful wavelengths whilst transmitting beneficial UV and visible light (e.g. (Duarte et al., Reference Duarte, Rotter, Malvestiti and Silva2009; Tuchinda et al., Reference Tuchinda, Srivannaboon and Lim2006), so flux in CTTEs can be controlled.
Some UV is necessary for vitamin D synthesis and other mechanisms in animals (e.g., Juzeniene and Moan, Reference Juzeniene and Moan2012; Wilson et al., Reference Wilson, Moon and Armstrong2012; Baines et al., Reference Baines, Chattell, Dale, Garrick, Gill, Goetz, Skelton and Swatman2016) and necessary irradiances may be determined (Cockell and Andrady, Reference Cockell and Andrady1999). Many, non-human animals have vision in the UV spectrum (e.g., Bennett and Cuthill, Reference Bennett and Cuthill1994, Cronin and Bok, Reference Cronin and Bok2016) including honeybees (Apis mellifera) (Reverté et al., Reference Reverté, Retana, Gómez and Bosch2016). Some pollinators use UVA for navigation (Cockell and Andrady, Reference Cockell and Andrady1999). Human wellbeing and ecosystem function will therefore require modulation, not total exclusion, of Mars' UV flux.
Magnetic fields (MFs)
Life evolved within Earth's geomagnetic field (GMF) (Maffei, Reference Maffei2014). Magnetic guidance mechanisms exist in some microorganisms, and many animals (Frankel, Reference Frankel1984) and MF changes might impact plant growth and development (Wolff et al., Reference Wolff, Coelho, Karoliussen and Jost2014). So, some behaviours might be compromised on Mars due to the lack of a GMF. This may affect CTTEs.
Climate, temperature and pressure
Mars' mean surface temperature is −63°C. While periods above freezing occur, surface atmospheric pressure is so low (Table 1), any water ice that melts usually sublimes to vapour (Lewis, Reference Lewis2003). The limit for higher plant tissue growth may be 5°C, little occurring at 6–7°C (Körner, Reference Körner2008). ‘Biologic zero’ relates to soil temperatures when microorganisms and or plants become inactive, sometimes considered 5°C (Rabenhorst, Reference Rabenhorst2005). CTTEs must therefore maintain elevated internal air temperature and pressure.
Mars' equator might offer a thermal advantage over other Arean locations for a CTTE. However, other factors apply. Haberle et al. (Reference Haberle, McKay, Schaeffer, Cabrol, Grin, Zent and Quinn2001) discussed regions where ground temperature and surface pressure can be favourable for the existence of liquid water, including the Hellas basin. The base of this 9 km deep impact crater (Ali and Shieh, Reference Ali and Shieh2014) potentially experiences 12.4 mbar surface pressure during the northern summer (Haberle et al., Reference Haberle, McKay, Schaeffer, Cabrol, Grin, Zent and Quinn2001). Such locations might facilitate contained pressure differentials.
CTTEs will require heating and dispersal of excess heat. Inevitably, heat will be lost to the external environment over time. No insulation is perfect, seals must allow ingress/egress, and dust storms (cf. Fernández, Reference Fernández1998) will reduce solar benefits but ‘intelligent’ computer-controlled structures (Taylor, Reference Taylor1998) might maintain suitable environments. Biosphere 2's complex control systems indicate engineering challenges and power needs (Nelson, Reference Nelson2018).
Solar power has potential (e.g., Delgado-Bonal et al., Reference Delgado-Bonal, Martín-Torres, Vázquez-Martín and Zorzano2016; Vincente-Retorcillo et al., Reference Vincente-Retorcillo, Martínez, Renno, Newman, Ordonez-Etxeberria, Lemmon, Richardson, Hueso and Sánchez-Lavega2018) but due to dust storms (Fernández, Reference Fernández1998), hybrid power generation, with rechargeable batteries and/or nuclear thermoelectric technology (e.g., LaMonica, Reference LaMonica2012; NASA, 2019a), may be needed. Geothermal options might exist (Morgan, Reference Morgan and Badescu2009; Sori and Bramson, Reference Sori and Bramson2019).
Seasons
Biomes change seasonally, so CTTEs require seasons. Temporality determines critical developmental stages, individual physiologies and interspecific relationships, while timing of abiotic events influences global nutrient fluxes (Forrest and Miller-Rushing, Reference Forrest and Miller-Rushing2010). Photoperiod and winter chilling are involved in temperate plants' phenology (Richardson et al., Reference Richardson, Keenan, Migliavacca, Ryu, Sonnentag and Toomey2013). Development of many insects is seasonally synchronized, enabling tolerance of adversity (Danks, Reference Danks2007). Phenological cycles are fundamental to ecosystem function (e.g., Stucky et al., Reference Stucky, Guralnick, Deck, Denny, Bolmgren and Walls2018) and climate changes can desynchronize critical interactions (Thackeray et al., Reference Thackeray, Henrys, Hemming, Bell, Botham, Burthe, Helaouet, Johns, Jones, Leech, Mackay, Massimino, Atkinson, Bacon, Brereton, Carvalho, Clutton-Brock, Duck, Edwards, Elliot, Hall, Harrington, Pearce-Higgins, Høye, Kruuk, Pemberton, Sparks, Thompson, White, Winfield and Wanless2016). Seasons also imbue characteristics critical to psychological restoration, e.g., autumn colour, winter silence, spring flowers and summer leafiness.
Mars has four seasons, approximately twice duration of Earth's (e.g., ESA, 2019). These vary in length due to its elliptical orbit, spring in the northern hemisphere (autumn in the southern) being the longest (NASA, 2019b). Whether Earth organisms can adapt to Mars seasons, even in containment is unknown.
Conditions on Earth have not selected for tolerance of seasons of such asymmetry and length (Taylor, Reference Taylor1998). So, CTTEs need artificially controlled seasons (diverging from semi-autonomy) or to be assembled from species tolerant of seasonal aberrance, the latter if relying heavily on passive sunlight delivery.
Lunar cycle
Most Earth organisms have circadian clocks, endogenous, molecular timing systems, allowing anticipation of Earth's 24-h light-dark cycle and maintenance of behavioural cycles (Bollinger and Schibler, Reference Bollinger and Schibler2014). Similarity of day length of Mars and Earth (Table 1) suggests adaptation may occur in CTTEs, processes according with the same temporal cues (zeitgebers).
However, the lunar cycle is also relevant. Earth's moon, Luna, is a zeitgeber for many ecological processes, some pertaining to monthly or half monthly cycles, others to shorter periods (e.g., Raible et al., Reference Raible, Takekata and Tessmar-Raible2017). Examples include animals (e.g., Raible et al., Reference Raible, Takekata and Tessmar-Raible2017; Sinclair, Reference Sinclair1977; Dixon et al., Reference Dixon, Dixon, Bishop and Pettifor2006) and plants (e.g., Barlow, Reference Barlow2015; Ben-Attia et al., Reference Ben-Attia, Reinberg, Smolensky, Gadacha, Khedaier, Sani, Touitou and Boughamni2016). Lunisolar tidal force may also influence plant growth (Barlow and Fisahn, Reference Barlow and Fisahn2012).
Mars' two moons, Phobos and Deimos, have maximum radii of c. 13.5 km (NASA, 2019c) and c. 7.5 km (NASA, 2019d) respectively, small compared to Luna's radius of c. 1737 km (cf. Williams, Reference Williams2021c). Rao (Reference Rao2015) speculates there are parts of Mars from which the moons are never visible due to orbital proximity and Mars' curvature.
Evidence for organisms' responses to Earth's lunar cycle varies from well substantiated to speculation but inevitably Terran species, translocated to Mars, would experience a different lunar influence, the effects hard to predict.
Soil
TTEs require suitable organic substrate. Freighting constraints require local development. Mars has a basaltic upper crust, with variable abundances of other materials (Ehlmann and Edwards, Reference Ehlmann and Edwards2014). Basalt-derived soils with volcanic ash are good agricultural soils (e.g., Olowolafe, Reference Olowolafe2002). Crushed basalt can increase soil pH, while its dissolution releases beneficial nutrients, including phosphorus (P) (Shamshuddin and Che Fauziah, Reference Shamshuddin and Che Fauziah2010).
Martian substrate probably contains nutrients to sustain plant growth (e.g., Jordan, Reference Jordan2015). ‘Mars regolith simulant’, supports angiosperms (Wamelink et al., Reference Wamelink, Frissel, Krijnen, Verwoert and Goedhart2014) and, with added organic matter, earthworms (Wamelink et al., Reference Wamelink, Schug, Frissel and Lubbers2022).
Plants require 16 essential elements, C, hydrogen, O2, nitrogen (N), P, potassium, calcium, magnesium, sulphur, iron, zinc, manganese, copper, boron, molybdenum and chlorine (Uchida, Reference Uchida, Silva and Uchida2000). These are all reported from Mars or Mars meteorites (Jordan, Reference Jordan2015). Cobalt and nickel (e.g., Brown et al., Reference Brown, Welch and Cary1987, López and Magnitskiy, Reference López and Magnitskiy2011) are also relevant, being involved in biological N-fixation. Nickel has been detected in Martian substrate (Gellert et al., Reference Gellert, Rieder, Brückner, Clark, Dreibus, Klingelhöfer, Lugmair, Ming, Wänke, Yen, Zipfel and Squyres2006; Yen et al., Reference Yen, Mittlefehldt, McLennan, Gellert, Bell, McSween, Ming, McCoy, Morris, Golombek, Economou, Madsen, Wdowiak, Clark, Jolliff, Schröder, Brückner, Zipfel and Squyres2006) and cobalt in putative Martian meteorites (Lodders, Reference Lodders1998).
Plant growth requires reactive N, predominantly nitrate (NO3−); 40–60 ppm NO3− advised for vegetable crops (Cantisano, Reference Cantisano2000). Evidence suggests up to c. 1100 ppm of NO3− in Mars' sedimentary deposits (Stern et al., Reference Stern, Sutter, Freissinet, Navarro-González, McKay, Archer, Buch, Brunner, Coll, Eigenbrode, Fairen, Franz, Glavin, Kashyap, McAdam, Ming, Steele, Szopa, Wray, Martín-Torres, Zorzano, Conrad and Mahaffy2015).
Phosphates are essential for Earth life (Tirsch and Airo, Reference Tirsch, Airo, Gargaud, Irvine, Amils, Claeys, Cleaves, Gerin, Rouan, Spohn, Tirard and Viso2014). Evidence indicates Mars is 5–10 times more phosphate rich than Earth, mineralogical studies (Adcock et al., Reference Adcock, Hausrath and Forster2013) suggesting biological accessibility.
So Martian regolith may contain necessary nutrients for a CTTE, while low organic C, water holding capacity and cation accessibility might be improved by microbiological weathering (Cockell, Reference Cockell2011).
Cyanobacteria are proposed for in situ resource processing (Verseux et al., Reference Verseux, Baqué, Lehto, de Vera, Rothschild and Billi2016). Photosynthetic, N-fixing Nostoc, will grow on Martian regolith simulant (Arai et al., Reference Arai, Tomita-Yokotani, Sato, Hashimoto, Ohmori and Yamashita2008) and early successional cyanobacterial communities improve soil moisture retention (Danin et al. (Reference Danin, Dor, Sandler and Amit1998).
Toxicity
Martian substrate contains perchlorates (ClO4−) at concentrations much higher than typically found on Earth (Davila et al., Reference Davila, Wilson, Coates and McKay2013). These affect thyroid function (e.g., Srinivasan and Viraraghavan, Reference Srinivasan and Viraraghavan2009) and some plant growth experiments with regolith simulant assume remediation (Gibbens, Reference Gibbens2017). Other oxidants present at Mars' surface include hydrogen peroxide and iron oxides (e.g., Lasne et al., Reference Lasne, Noblet, Sopa, Navaro-González, Cabane, Poch, Stalport, François, Atreya and Coll2016). Mars has over twice as much iron in its outer layers as Earth (Peplow, Reference Peplow2004) and, though an essential plant nutrient, it can accumulate to become toxic (Connolly and Guerinot, Reference Connolly and Guerinot2002). In combination, iron oxides, hydrogen peroxide, perchlorates and Mars' UV flux, are highly deleterious to living cells (Wadsworth and Cockell, Reference Wadsworth and Cockell2017). Extreme salinity is another potential stressor (e.g., Ramírez et al., Reference Ramírez, Kreuze, Amoros, Valdivia-Silva, Ranck, Garcia, Salas and Yactayo2017).
Tolerance of such parameters will be desirable in ETNRs, though CTTEs allow remediation. Many perchlorate-reducing bacteria exist (e.g., Coates and Achenbach, Reference Coates and Achenbach2004) and bacterial enzymes have potential to detoxify hydrogen peroxide (Nóbrega and Pauleta, Reference Nóbrega and Pauleta2019). Perchlorate is also highly soluble in water (Davila et al., Reference Davila, Wilson, Coates and McKay2013), allowing biotic and/or abiotic decontamination.
Water
Present Mars is a cold desert (McKay, Reference McKay2010). However, the freshwater content of Mars' permanent north polar ice cap is c. 100 times that of the Laurentian Great Lakes (Rummel et al., Reference Rummel, Beaty, Jones, Bakermans, Barlow, Boston, Chevrier, Clark, de Vera, Gough, Hallsworth, Head, Hipkin, Kieft, McEwen, Mellon, Mikucki, Nicholson, Omelon, Peterson, Roden, Lollar, Tanaka, Viola and Wray2014). Liquid water may even exist beneath the southern polar ice (Orosei et al., Reference Orosei, Lauro, Pettinelli, Cicchetti, Coradini, Cosciotti, Di Paolo, Flamini, Mattei, Pajola, Soldovieri, Cartacci, Cassenti, Frigeri, Giuppi, Martufi, Masdea, Mitri, Nenna, Noschese, Restano and Seu2018) and ‘recurring slope lineae’ may be active surface brine flows (e.g., Ojha et al., Reference Ojha, Wilhelm, Murchie, McEwen, Wray, Hanley, Massé and Chojnacki2015).
Evidence indicates sufficient water reserves for CTTEs (toxin removal possible). Conifer needles collect cloud drops (Unsworth and Wilshaw, Reference Unsworth and Wilshaw1989) suggesting delivery options. Atmospheric temperature gradients with dew points (Lu and Ho, Reference Lu and Ho2019) and microstalactite ceiling materials (condensation foci) merit exploration for artificial rain.
Di-oxygen (O2) and di-nitrogen (N2)
Mars' atmosphere is CO2 rich with little O2 or N2 compared to Earth (cf. Table 1). O2 is essential for aerobic TTEs, while relatively inert N2 is useful in bulking atmospheric pressure. Reactive N is present in proteins and nucleic acids, so sufficient atmospheric N2 must be available for biological N-fixation (McKay and Marinova, Reference McKay and Marinova2001) and cycling. CTTEs on Mars therefore require increased atmospheric O2 and N2.
Fortunately, Mars' resources include oxygen bound in perchlorate, carbonate (Bridges et al., Reference Bridges, Hicks, Treiman, Filiberto and Schwenzer2019) and nitrate (the latter providing fixed N) that might be harvested. Davila et al. (Reference Davila, Wilson, Coates and McKay2013) propose enzymic release of O2 from perchlorate and N2 might be liberated by bacterial denitrification (e.g., Hart et al., Reference Hart, Currier and Thomas2000). Technologies are also developing for mining Martian atmosphere (Finn et al., Reference Finn, McKay and Sridhar1996; Sridhar et al., Reference Sridhar, Finn and Kliss2000) and CTTEs do not necessitate duplication of Earth's mean atmospheric pressure and composition; atmospheric pressure varies altitudinally and species' tolerances vary.
Klingler et al. (Reference Klingler, Mancinelli and White1989) showed some bacteria capable of N-fixation from partial pressures of N2 down to 5 mbar (25 times current Mars levels). Some plants can utilize O2 levels well below, and tolerate CO2 levels above, current Earth values. Photosynthesis can be enhanced at O2 concentrations below ambient (e.g., Downes and Hesketh, Reference Downes and Hesketh1967), due to reduction in photorespiration (e.g., Hagemann et al., Reference Hagemann, Weber and Eisenhut2016). Some show higher photosynthetic rates under elevated CO2 (e.g., Ainsworth and Rogers, Reference Ainsworth and Rogers2007), benefitting from the ‘CO2 fertilization effect’ (Zheng et al., Reference Zheng, Li, Hao, Shedayi, Guo, Ma, Huang and Xu2018).
Green plant photosynthesis might generate elevated O2 levels in a CTTE. Fogg (Reference Fogg1995) considered root respiration demand could limit plant growth on Mars until atmospheric O2 was raised to 20–100 mbar (>3000 times current levels) but levels in containment could be primed.
Modification of contained Martian atmosphere is therefore conceivable and may be less demanding than anticipated. As initial O2 levels rise, and biological N-cycle initiates, photosynthetic eukaryotes may mediate further atmospheric modification, ultimately achieving conditions tolerable by invertebrates.
Gravity
Terran life evolved within Earth's gravitational field (1 g) and CTTE success depends on development and function under Mars' lower gravity (Table 1).
Light and gravity modulate plant development (Vandenbrink et al., Reference Vandenbrink, Kiss, Herranz and Medina2014). Experiments indicate 0.3 g (< Mars) sufficient to trigger gravitropic responses, but that meristematic competence can be lost under lunar-like (0.17 g) gravity (Manzano et al., Reference Manzano, Herranz, den Toom, te Slaa, Borst, Visser, Javier Medina and van Loon2018). Nevertheless, plants will grow and photosynthesize even in microgravity (e.g., Monje et al., Reference Monje, Stutte and Chapman2005; Wolverton and Kiss, Reference Wolverton and Kiss2009). Though some biochemical (Cowles et al., Reference Cowles, Lemay and Jahns1988) and anatomical (Hoson et al., Reference Hoson, Soga, Wakabayashi, Kamisaka and Tanimoto2003) changes may occur, results conflict (Stanković, Reference Stanković2001).
From such evidence, it is conceivable that some plants (and fungi, cf. Kern, Reference Kern1999) will tolerate Mars' gravity. However, forest function is also influenced. Leaf and propagule fall, leaping, flight, deadwood collapse, raindrop impact and drainage of water contribute dynamism. On Mars, things weigh 38% their Earth weight, potentially benefitting trees etiolated by low light, or lacking wind-induced reaction wood (Groover, Reference Groover2016) (cf. Biosphere 2, Nelson, Reference Nelson2018).
Many organisms reproduce and disperse by airborne propagules. If these develop normally, greater dispersal capacity under lower gravity may not be problematic, provided they can disperse. This may require vectoring to avoid intergenerational competition, so, CTTEs need wind. However, lower gravity means lighter propagules and thermal gradients might be exploited to generate air currents.
Some plants and fungi exploit ‘splash cups’ from which propagules are dispersed by raindrop impact (Brodie, Reference Brodie1951). Such structures evolved in response to rain falling under 1 g, their functionality on Mars unknown but testable.
Potential to leap, climb or fly on Mars with less effort will influence TTE function and some animals may benefit from positive energy budgets. Some insects learn to fly in microgravity (Nelson and Peterson, Reference Nelson and Peterson1982; Vandenberg et al., Reference Vandenberg, Massie, Shimanuki, Peterson and Poskevich1985), so potential exists. Capacity of most animals to adapt is unknown but 0.38 g is not zero g.
Forest design
Species complement dictates forest appearance, physiognomy and functioning. Limited by the abiotic environment sustained, this will perforce include an unusual assemblage of species (integrants), tolerant of prevailing conditions, comprising a novel ecosystem. On Earth, species niches are limited by competition and availability. Local environmental parameters in CTTEs will lead to new fitnesses, species occupying different roles where niche requirements are provided.
It would be counterproductive to plan replication of a specific forest biome. Earth's forests owe their assemblages to environmental and evolutionary pressures that will differ to those in Martian CTTEs. No single forest food web has been fully mapped, canopies themselves potentially comprising over 100 000 trophic links (Nakamura et al., Reference Nakamura, Kitching, Cao, Creedy, Fayle, Freiberg, Hewitt, Itioka, PinKoh, Ma, Malhi, Mitchell, Novotny, Ozanne, Song, Wang and Ashton2017), challenging duplication. Lack of GMF, reduced sunlight, aberrant seasons, variant lunar cycle, reduced gravity and pedological peculiarities will engender novel ecosystem function.
Significant seasonal differences make it unlikely the same palettes of synchronized mutualisms, which define Earth's forests, could be established on Mars, though dormancy traits might prove useful (e.g., Taylor, Reference Taylor1998) and potentially some species would adapt. If so, a forest might be established but it would only consist of those organisms than can adapt. Design must therefore include planned redundancy, allowing for unknowns.
Mitsch and Jørgensen (Reference Mitsch and Jørgensen2003) indicate that if enough organisms and propagules are delivered, local conditions will select out the best-adapted assemblage. In Odum's (Reference Odum1983) terminology, ecosystems self-organize from the available (Smith, Reference Smith2018 discusses) and designers must allow ‘self-organization’ since active assembly of complex species networks would demand unattained prescience.
ETNR designers should consider species as ecological cogs that might be assembled into functional ecosystems. Replication of Earth forests is currently unfeasible but development of new ecosystems, functioning in unexpected ways, is conceivable. Mars' forests would not resemble or function exactly like Earth's forests but could still deliver wonder; autumn at 0.38 g offering dreamlike leaf fall.
Early ETNRs may be relatively oligospecific, freighting considerations, even for seeds, restricting initial complement. Selection must acknowledge survivability and ecosystem function, while expedience requires instrumental value, species producing wood, fibre and important secondary metabolites (vitamins, flavours, perfumes, colours, mood enhancers). Species diversity must be built incrementally, over time, by assisted colonization, monitoring, adjustment and replenishment.
The proposed forest is intended as an expansion of Earth's ecosystem, a utilitarian botanic garden and restorative sanctuary. Arboreal communities that can be ‘entered’, offering a sense of ‘escape’ support the latter, several tree taxa proposed as canopy. The design incorporates some organisms considered problematic on Earth but exhibiting potential ET adaptability or terraforming value. Heretical recombination is incited, selecting species from various forest biomes to exploit useful traits, fulfilling essential roles. On Earth, ecosystems rely on co-existence for services including N-fixation and mineral breakdown but in a CTTE all needs must be met either artificially or by integrants. Experimentation will be necessary, knowledge accruing, anticipating subsequent ecosystem modification.
Varietal forests adapted to extreme ambient parameters offer templates of resilience. High-altitude forests tolerate low atmospheric pressures and temperatures. Early successional forests exploit soils low in nutrients and sometimes high in heavy metals. Attempted duplication of a specific high-altitude ecosystem has merit in that species complement can be determined, co-evolution satisfied and incompatibilities minimized. However, this does not allow selective assembly, failing to acknowledge precedent diasporic Earth forest (Smith, Reference Smith2018) and unique Martian exigencies.
Species complement
Mars' forest complement is designed with reference to local constraints, instrumental value and survivability (Fig. 2). The assemblage is broadly justified below and detailed in Table 2.
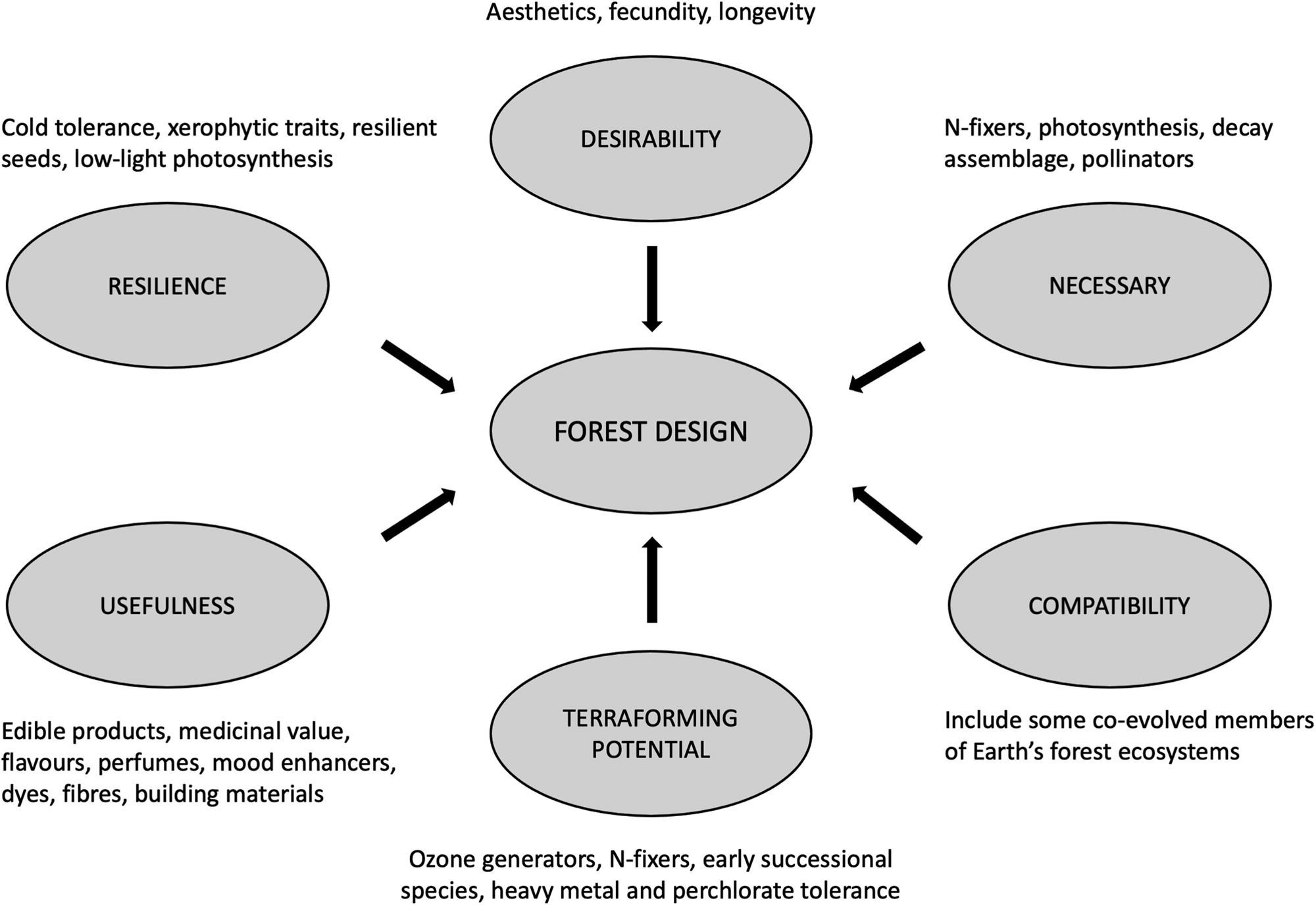
Fig. 2. Selection factors for Mars’ forest species complement based on local constraints, instrumental value and survivability.
Trees of high altitudes provide the foundation. Earth has elevational limits beyond which trees cannot grow (Körner, Reference Körner2012). About 100 species worldwide form trees at the climatic treeline, reducing to c. 20 (Pinaceae and Betulaceae) at the arctic equivalent, (Körner, Reference Körner2012). Miehe et al. (Reference Miehe, Miehe, Koch and Martin2003, Reference Miehe, Miehe, Vogel, Co and La2007) discuss high-altitude Tibetan forests, including the sacred Reting Forest, where Juniperus tibetica (Cupressaceae) grows in an open shrub layer of J. pingii var. wilsonii, Potentilla fruticosa, Lonicera spp. and Caragana spp. Two junipers, Juniperus convallium (3500–4570 m a.s.l) and J. tibetica (4100–4850 m a.s.l.), are widespread in southern Tibet (Miehe et al., Reference Miehe, Miehe, Koch and Martin2003). They form small forests, sometimes amongst Picea and in open stands of Cupressus torulosa var. gigantea (Farjon, Reference Farjon2010). J. tibetica forms the highest northern hemisphere treeline (e.g., Miehe et al., Reference Miehe, Miehe, Vogel, Co and La2007), some stands being pilgrimage sites (Miehe et al., Reference Miehe, Miehe, Koch and Martin2003) or religious landmarks (Miehe et al., Reference Miehe, Miehe, Will, Opgenoorth, Duo, Dorgeh and Liu2008).
Climatic limits of cold timberlines relate to isotherms of 10°C for warmest month's mean temperature (Daubenmire, Reference Daubenmire1954). Similar mean growing-season temperatures of c. 6.7°C, at climatic, high-elevation treelines worldwide indicate temperature control (Hoch and Körner, Reference Hoch and Körner2005). Rainfall data suggest the drought limit of juniper trees correlates with annual precipitation of 200–250 mm and Miehe et al. (Reference Miehe, Miehe, Koch and Martin2003) argue the high mountain deserts of southern Tibet could be reforested without irrigation, even lacking a high groundwater table.
Mars' soils will shape the contained forest, so, early successional colonizers, common to high altitude environments, e.g., pines (Pinus spp.) and birches (Betula spp.) are included. Substrate pH constrains species choice. Contained soils may be modified but minimal intervention is expedient. Phoenix Mars Lander measured an alkaline pH of 7.7 ± 0.5 for substrate (Hecht et al., Reference Hecht, Kounaves, Quinn, West, Young, Ming, Catling, Clark, Boynton, Hoffman, DeFlores, Gospodinova, Kapit and Smith2009) whereas Opportunity rover found evidence of slightly acidic to circum-neutral pH (Arvidson et al., Reference Arvidson, Squyres, Bell, Catalano, Clark, Crumpler, De Souza, Fairén, Farrand, Fox, Gellert, Ghosh, Golombek, Grotzinger, Guinness, Herkenhoff, Jolliff, Knoll, Li, McLennan, Ming, Mittlefehldt, Moore, Morris, Murchie, Parker, Paulsen, Rice, Ruff, Smith and Wolff2014), so soils of varying pH could be developed allowing species diversity.
Juniperus tibetica grows on rocky soils derived from siliceous and calcareous materials, experiencing extremes of solar radiation and frost (Farjon, Reference Farjon2010) but CTTEs might also incorporate canopies from high altitude heaths, i.e., Erica arborea or E. trimera (e.g., Beentje, Reference Beentje2006). Ericaceous plants' tolerance of acidic, metalliferous soils (Bradley et al., Reference Bradley, Burt and Read1982) may be useful. N-fixing plants will also be essential. The N-fixing shrub Caragana versicolor, tolerant of Himalayan cold aridity (Kumar et al., Reference Kumar, Adhikari and Rawat2016), is proposed for clearings and margins.
Creation of ETNRs as biotic insurance against planetary disaster requires human redundancy, currently unachievable. A biocentric ideal would be a self-monitoring, self-repairing containment able to support a living ecosystem even following human abandonment. However, this would represent a pyrrhic legacy if the containment survived an event, its ecosystem extirpated. CTTEs should include some species resilient to sub-catastrophic containment failure, providing opportunity for re-establishment in the manner of Terran ecosystems post adversity.
Plants categorized in USDA Hardiness Zones 1a to 7b (USDA, 2012) offer potential for survival of unplanned temperature drops, these covering species able to survive average, annual winter temperatures of −51.1 to −12.2°C. Choice of range is subjective but −12.2°C must offer ‘some’ insurance against partial containment failure (increasing levels below that). This provides significant opportunity for the development of boreal forest types, dominated by conifers but with associated broad-leaved plants.
Long-lived seeds, able to withstand deep cold, O2 starvation, fire and/or decompression and to subsequently germinate, would contribute to recovery from temporary containment failure. Ability to resprout from hypogeal structures (e.g., James, Reference James1984) would also be useful. A mixture of species, resilient to periods of reduced atmospheric pressure, darkness, desiccation or other extremes, facilitates biotic insurance, i.e., however severe life support failure is, something will survive.
Individual longevity is desirable in certain integrants. Initiating ancient tree development would provide visionary opportunity. Intangible though such issues are, ancient trees may ultimately engender historical connection between CTTE designers, facilitators and future visitors, potential for legacy, a harnesser of political and financial support.
Junipers are long-lived. J. communis can live c. 200 years (Ward, Reference Ward1982), J. pingii var. wilsonii >300 years (Liang et al., Reference Liang, Lu, Ren, Li, Zhu and Eckstein2012). Dendrochronological analysis provides ages >2000 years for J. occidentalis (Rocky Mountain Tree-Ring Research, undated) and >2230 and 1265 years for J. przewalskii and J. tibetica respectively (Liu et al., Reference Liu, Yang and Lindenmayer2019). By vegetative reproduction, some trees persist as clonal organisms for centuries. A Populus tremuloides clone in North America, extending over 43.6 hectares (DeWoody et al., Reference DeWoody, Rowe, Hipkins and Mock2008), is potentially of great age (Rogers and McAvoy, Reference Rogers and McAvoy2018).
Fecundity is important in future proofing. Rapidly reproducing plant species can repopulate in the event of non-critical losses. Less fecund species may be vulnerable to vicissitudes of population decline, entering extinction debt in the event of partial catastrophe. Rapidly propagating species (e.g., Betula spp.) should therefore be included.
ET ecosystem design philosophy is nascent. Just as some plants develop winter hardiness, surviving freezing (e.g., Vitasse et al., Reference Vitasse, Lenz and Körner2014), unexpected phenotypic adaptations to other stressors might be expressed in ETNRs. Such unpredictable phenomena make the difference between success and failure, so adaptability is important and ‘invasiveness’ on Earth may be a valuable trait in ET environments.
To facilitate ecosystem construction, some understorey species are selected with consideration to natural occurrence alongside canopy integrants. Necessity to avoid allelopathic incompatibility motivates this, though, notably, some invertebrates require multiple plant species for life cycle completion. Such niche requirements could be difficult to fulfil but experiment may reveal unknown tolerances and suitabilities, while selection of some naturally co-occurring plant species may assist.
Use of extremophiles from Mars-like, high-altitude deserts on Earth is not emphasized. Though replication of cold desert ecosystems might be easier to achieve on Mars than forests and would offer biotic insurance, it is arguable whether similar psychological and inspirational benefits would ensue. Recreation of non-forest ecosystems on lifeless planets is laudable, a responsibility inherent to humanity's burgeoning space-faring ability, but the creation of wonder and incitement to pilgrimage require a physically imposing plant community; this demands trees.
Acknowledging the issues above, Table 2 presents a forest-like assemblage incorporating biodiversity, resilience and functionality. Human psychological restoration requires interest and distraction, variation in colour, hue and form, species variety and opportunity for haptic exploration, so these are integrated. Ecosystem services that integrants could supply, relating to human life support and life quality are listed.
ET forest (ETF) survival requires future proofing against system failures. Political support dwindles less if such failure is only partial. Trial and error will shape the species palette, but that presented plans for unmitigated success and limited catastrophe. Since all photosynthetic plants provide O2 and absorb CO2, this major ecosystem service is common throughout. Designers must note that dioecy sometimes demands two genders.
Decomposition
Decomposition must occur in CTTEs. Without breakdown of dead biological material, nutrients become sequestrated, atmospheric CO2 depleted and ecosystem cycling ceases (e.g., Chapin et al., Reference Chapin, Matson, Mooney, Chapin, Matson and Mooney2002). Table 2, therefore includes decomposers.
Organic litter fall is crucial in biogeochemical cycling (e.g., Krishna and Mohan, Reference Krishna and Mohan2017). Arthropod communities mediate its degradation (e.g., Berg et al., Reference Berg, Kniese, Bedaux and Verhoef1998). Litter and deadwood are also C sources for forest soil microbes (e.g., Lladó et al., Reference Lladó, López-Mondéjar and Baldrian2017). Kjøller and Struwe (Reference Kjøller, Struwe, Teller, Mathy and Jeffers1992) discuss microfungi's key role in degrading diverse complex molecules. Bacteria are also important, especially in the soil N-cycle (e.g., Takai, Reference Takai2019).
CTTE designers must provide suitable biota able to carry out decomposition processes (disassembly, fragmentation, trituration, digestion, solution and N-cycle steps from ammonification to denitrification). The risk of N-cycle dysfunction requires monitoring and proactive correction technology may be necessary, diverging from ideals of human redundancy. With Earth's functional biogeochemical cycles, creation of a forest ecosystem is readily conceivable but incorporation of such support into CTTEs poses challenges.
Ethics
Whether an ETNR is ecologically effective depends on scale. Optimal size of Earth nature reserves is debated (e.g., Diamond and May, Reference Diamond, May and May1981; Higgs, Reference Higgs1981) and ETNRs demand similar scrutiny. Size of CTTEs may be limited by engineering constraints, but ‘minimum-area requirements’ and ‘minimum viable population’ sizes will be relevant, as per the SLOSS debate (i.e., whether a single large reserve will conserve more species than several small, e.g., Tjørve, Reference Tjørve2010).
To provide biotic insurance ETNRs require assemblages from all Kingdoms of living things including animals. This raises ethical issues, ecosystem dysfunction potentially leading to suffering through system failures, unsuitable design or intolerances.
In facilitating psychological recovery of space workers, animals would be beneficial. Woodland without birdsong or butterflies is a poor TTE. Such lack may exacerbate homesickness. However, when creating habitats on Earth, many animals can elect to inhabit or leave by their own volition. This choice is denied in a CTTE. Introduction of species unable to engage in natural behaviours should be avoided, consideration of the ‘five freedoms’ (Farm Animal Welfare Council, 1993; Webster, Reference Webster2016) will be necessary and human management may be essential.
Consequences of contact between biospheres is also a consideration, as reflected in the UN's Outer Space Treaty of 1967 (United Nations, 1967) and the International Council for Science's Committee on Space Research (COSPAR) Planetary Protection Policy (COSPAR, 2002 (amended 2011), Rummel et al., Reference Rummel, Beaty, Jones, Bakermans, Barlow, Boston, Chevrier, Clark, de Vera, Gough, Hallsworth, Head, Hipkin, Kieft, McEwen, Mellon, Mikucki, Nicholson, Omelon, Peterson, Roden, Lollar, Tanaka, Viola and Wray2014). Creation of contained biospheres reduces risk of ecosystem contamination but, since no containment is perfect, protection of Mars' ‘Special Regions’ (Rummel et al., Reference Rummel, Beaty, Jones, Bakermans, Barlow, Boston, Chevrier, Clark, de Vera, Gough, Hallsworth, Head, Hipkin, Kieft, McEwen, Mellon, Mikucki, Nicholson, Omelon, Peterson, Roden, Lollar, Tanaka, Viola and Wray2014) influences location choice.
Conclusions
Creating a contained ETF is more complex than establishing woodland plants in a protected environment. Even gardens rely on natural nutrient cycling, soil disturbance and irrigation. CTTEs should be almost self-sustaining with propagule dispersal vectors, internal weather and replication of the myriad changes that forests exploit. The designers' task is daunting but, if survival of Earth life is to be ensured, challenges must be overcome.
Humanity does not know if life exists elsewhere in the universe. Mars may support native organisms, but even if it does, Earthly life may be endemic to Earth. Perhaps, life only exists on Earth. In either of which cases, Homo sapiens as the local sentient, technologically empowered species, has responsibility.
From a biocentric perspective, world leaders should be concerned about the future of life in the Universe and humanity's role in its protection and promulgation. On a planet of limited habitability, this is a significant duty. The survival of life, in any form, is the ultimate biocentric priority.
The global ecosystem changes and its conservation requires imagination. Evidence indicates that a contained ETF could be established on Mars. A partially human-redundant protection system would be needed but, like the juniper forests of Tibet, the forest's existence would incite pilgrimage, emboldening efforts for space travel. It is easier enter a desert, knowing it contains an oasis.
This paper does not consider economics. Sending humans into environments without ecosystem services adds to space travel's cost (e.g., Glenn Smith and Spudis, Reference Glenn Smith and Spudis2015). ESA's MELiSSA project indicates that humans should not think of travelling alone but with a supporting biosphere. We travel through space every moment, sustained by Earth's biodiversity. Our planet carries a self-supporting, bioregenerative ecosystem that, accepting Lovelock's (Reference Lovelock1979) Gaia hypothesis, modifies and sustains its own life supporting qualities. So, spacecraft should be reimagined as symbiotic communities.
The sailing ships of past explorers were not sterile. They carried animals for food and as living cargo (e.g., Blancou and Parsonson, Reference Blancou and Parsonson2007), for companionship (Mäenpää, Reference Mäenpää2016) and as pests (e.g., Atkinson, Reference Atkinson1973). Sometimes, animals were released or escaped onto foreign shores where some thrived or became problematic (e.g., Campbell and Donlan, Reference Campbell and Donlan2005; Harper and Bunbury, Reference Harper and Bunbury2015), examples of accidental, incidental and deliberate dispersal of Terran species. Goats were once purposely liberated on remote islands by mariners, as a self-renewing food resource (Dunbar, Reference Dunbar1984). Such attitudes may become necessary during space exploration, creating oases on barren but habitable planets. Spacecraft will carry multiple species complements, contributing life support for long journeys and on arrival at lifeless destinations.
ETNR design will be inspired by human dependency on ecosystem services, even in purely utilitarian fashion, because, despite technology, that dependence cannot be shed. We need plants as chemical factories, producing secondary metabolites with greater ease and more autonomy than industry. Ultimately, humans must take Earth's ecosystem with them, acting as the medium through which it colonizes the planetary archipelago of space. We will not travel alone because we did not evolve in isolation. Homo sapiens was shaped, over aeons, by other species and will travel with a mutually supportive system of Terran organisms amongst which we fit, exchanging metabolites as we have evolved to do. It is not humanity that is reaching out from Earth, it is life, with all its diverse capabilities for colonization, humanity the ineluctable vector.
Acknowledgements
Thanks to Carol Jenner, Edward Hornibrook, David Gledhill, Yu Kan, Rafael Rosolem, Martin Schrön, Andrew Carr, Roger Chittock, Gareth Griffiths and Chris McKay.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The author reports no conflict of interest.







