1. Introduction
Many philosophers of science argue that disagreements are essential to scientific progress. However, it cannot be the case that all disagreement are equally productive – after all some disagreements are not genuinely epistemically motivated, but rather motivated by personal vendetta or profit. This paper will examine the disagreement over the timing of the origins of angiosperms (flowering plants) in order to reveal some deeper properties of disagreements which lead to their being more or less fruitful.
Section 2 provides an overview of perspectives on disagreement in science as understood by many prominent philosophers of science. Section 3 examines the debate over the timing of the origin of angiosperms as a case study of one prominent disagreement in paleobiology. Section 4 considers what we might learn about the conditions under which disagreement may be productive in the natural sciences. Ultimately, the purpose of this paper is not to disparage the importance of disagreement to science and scientific practices, but to help us delineate when such disagreements might be fruitful and to help us avoid disagreeing is ways that are likely to be fruitless. I point to issues of evidence quality and social epistemic structures which deserve more attention in understanding the productivity of disagreement.
2. Disagreements in Science
In the wake of the pluralist turn in the philosophy of science, diversity of opinion has come to be understood as key to a healthy science. Some pluralists have argued that scientific progress requires a variety of conceptual classifications which provide us with different perspectives on our world (e.g., Dupré, Reference Dupré1993; Cartwright, Reference Cartwright1999; Kitcher, Reference Kitcher2001; Chang, Reference Chang2012). The existence of these different versions of the world would seem to depend on some disagreement among practitioners. Thus, in some important ways, the popularity of pluralism in philosophy of science, is also an indication of the popularity of the importance of disagreement.
Feminist standpoint theory has been used to argue that since knowledge is shaped by the social positioning of actors and their experiences, having diverse voices within the scientific community is epistemically advantageous (Wylie, Reference Wylie, Figueroa and Sandra2003; Intemann, Reference Intemann2010). The point here is not that marginalized individuals necessarily “know better”, but that they can bring attention to neglected facts or questions. In this way, the many advantages conferred by incorporating diverse standpoints will be achieved through some level of disagreement among researchers.
Feminist empiricists have argued that values have an important role to play within science. Helen Longino has influentially argued that we ought to replace the value-free ideal with a set of regulatory norms which center around having genuine peer disagreement (1990). While debates have raged about which values and how to properly include them in science, following Longino, many still highlight the role of disagreement in productive science.
Agent-based models of scientific reasoning also suggest that epistemic diversity is important for the productivity of science. Zollman argued that transient diversity of scientists’ opinions provided an epistemic benefit because it amounted to a division a labor (Reference Zollman2009). Weisberg and Muldon compared populations of mavericks, who stubbornly pursue unpopular research, or conformists, who follow the most recently successful strategies, or mixed groups of both mavericks and conformists (Reference Weisberg and Muldoon2009). They found that mixed populations performed best (Reference Weisberg and Muldoon2009). Other modelers have found that continuing to follow a research path even in the face of disagreement can increase the likelihood of converging on a true theory (e.g. Douven, Reference Douven2010; De Langhe, Reference De Langhe2012).
Here I offer two points of clarification about what I mean by disagreement. While some philosophers are concerned with what happens when two theories in science contradict each other (see Parkkinen et al., Reference Parkkinen, Russo and Wallmann2017), scientists can disagree about different aspects of their work – their methods, the results, their theories, etc – without logically contradicting each other. This paper is concerned with a broader category of apparent disagreements, which occur when scientists believe they disagree with one another, regardless of the logical status of their claims.
Second, much ink has been spilled over the status of individuals as “epistemic peers”. For my purposes, I will consider individuals to be epistemic peers when they are cognitive and evidential equals – as in they possess similar cognitive capacities and have access to the same evidence with respect to whatever is at issue (Lackey, Reference Lackey, Gendler and Hawthorne2010; Christensen, Reference Christensen2007). I will consider that authors working on and publishing on similar issues in the scientific literature de facto epistemic peers.
Unlike the above concerns about the rationality of disagreement, I am concerned with disagreement’s productivity. De Cruz and Dr Smedt have argued that peer disagreements are advantageous for three reasons: they generate new evidence, they lead to the re-evaluation of existing evidence and assumption, and they serve as an antidote to confirmation bias (Reference De Cruz and De Smedt2013). Cases like climate change have been used to highlight the problems with the simple narrative that dissent is necessarily productive (Biddle & Leuschner, Reference Biddle and Leuschner2015). Biddle and Leuschner argue that dissent is epistemically detrimental if it fulfills four criteria: 1. non-epistemic consequence are high, 2. Dissent violates conventional standards, 3. Dissent puts risk of false acceptance over risk of false rejection, and 4. risk of false acceptance and risk of false rejection fall on different parties (Reference Biddle and Leuschner2015). These have been important contributions to our understanding of what makes for productive science. Still, there have even been calls for further clarification of what types of dissent, disagreement, and diversity are desirable in scientific practice (e.g., Dellsén & Baghramian, Reference Dellsén and Baghramian2020; Intemann, Reference Intemann and Heidi2011; Leuschner, Reference Leuschner2018; Rolin, Reference Rolin2017). In what follows I will examine the disagreements about the origin of angiosperm, and what this disagreement can teach us about the productivity of disagreement in science.
3. The Origins of Angiosperms
During the Cretaceous Terrestrial Revolution (KTR) there was a massive diversification of various flora and fauna (Benton et al., Reference Benton, Wilf and Sauque2021). By the end of the KTR almost all crown lineages of angiosperms had evolved (Benton et al., Reference Benton, Wilf and Sauque2021). Some authors argue that the rise of angiosperms drove the macroecological revolution that occurred on land – the diversification of angiosperms provided new niches and opportunity for changes in many terrestrial species (Benton et al., Reference Benton, Wilf and Sauque2021). However, researchers have not been able to agree about when exactly angiosperms appeared on earth.
These days the disagreement about the origins of angiosperm centers on the conflicting dates provided by the fossil record and the molecular clock (van der Kooi & Ollerton, Reference van der Kooi and Ollerton2020). Despite a nearly world-wide search for highly preservable pollen fossils, paleontologists have yet to recover convincing evidence of pre-Cretaceous angiosperms (Coiro et al., Reference Coiro, Doyle and Hilton2019). By contrast, a great number of molecular dating analyses have suggested much earlier origins for the angiosperm clade, with typical estimates being 70+ Million years earlier than the earliest fossil appearances (Magallón et al., Reference Magallón, Gómez-Acevedo, Sánchez-Reyes and Hernández-Hernández2015). Li et al. (Reference Li, Yi, Gao, Ma, Zhang, Yang, Gitzendanner, Fritsch, Cai, Luo, Wang, van der Bank, Zhang, Wang, Wang, Zhang, Fu, Yang, Hollingsworth, Chase, Soltis, Soltis and Li2019) have named the gap between molecular dates and the fossil record the “Jurassic gap” after the fact that the different origin dates seem to skip over the entire Jurassic period.
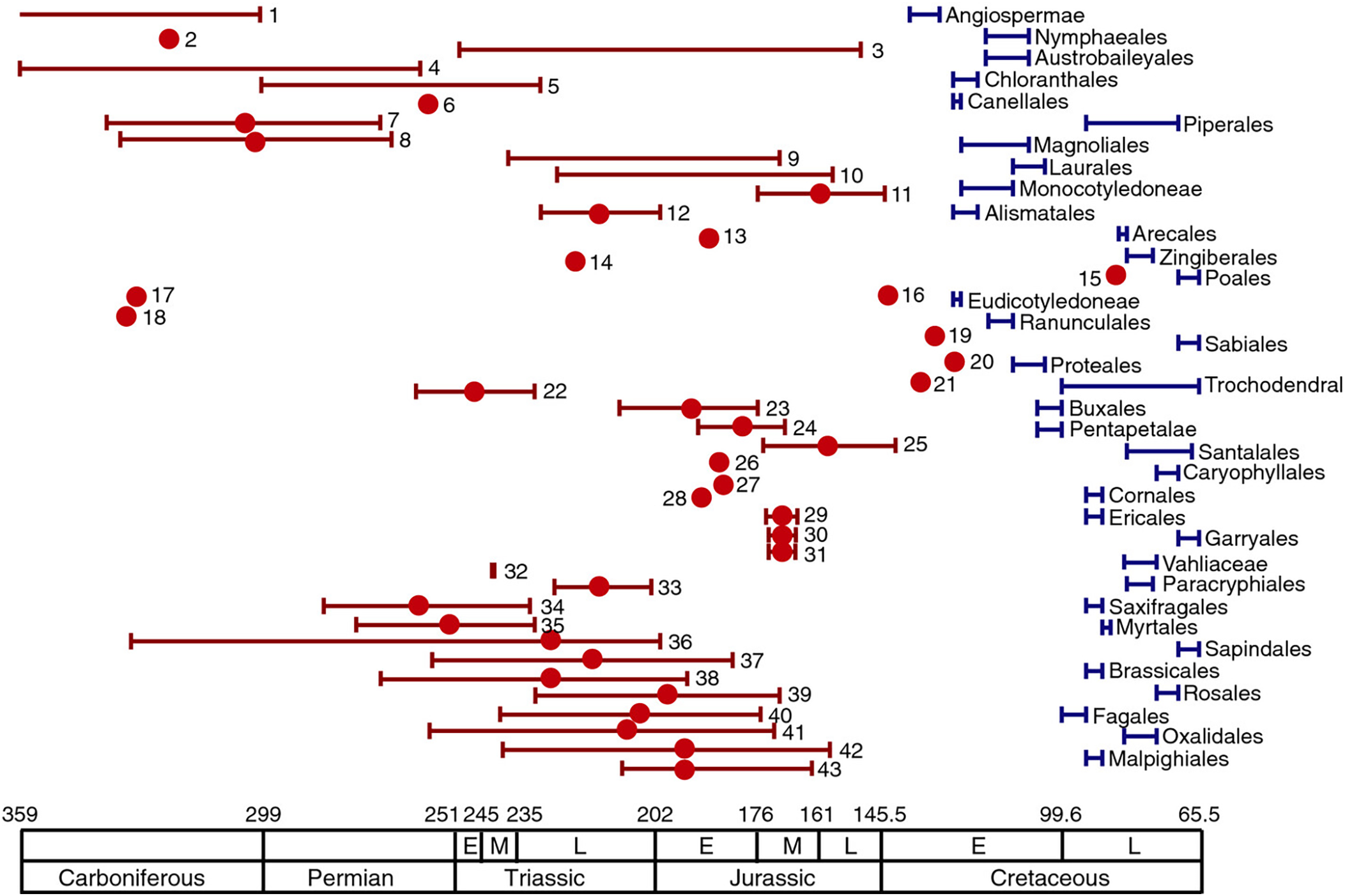
We might wonder just how confident these different groups are in their disparate findings. The answer is very. In the past three decades, a great number of molecular dating analyses, based on the divergence of DNA sequences of living plants, have supported a pre-Cretaceous origin of crown-group angiosperms. Magallón et al. (Reference Magallón, Gómez-Acevedo, Sánchez-Reyes and Hernández-Hernández2015) compiled results from 43 molecular clock studies of almost every order within the angiosperm clade (figure 1).

Figure 1. As found in Magallón et al. (Reference Magallón, Gómez-Acevedo, Sánchez-Reyes and Hernández-Hernández2015). Blue bars indicate the stratigraphic interval from which the oldest fossil belonging to angiosperm orders and major clades is known. The name of the order or major clade is shown next to each bar. Red dots and bars indicate the age and/or range of angiosperm crown age estimated in molecular clock studies.
As figure 1 demonstrates the Jurassic gap is found in almost all of those studies (Magallón et al., Reference Magallón, Gómez-Acevedo, Sánchez-Reyes and Hernández-Hernández2015). Further, the Jurassic gap has persisted as methodologies have improved. For example, Li et al. (Reference Li, Yi, Gao, Ma, Zhang, Yang, Gitzendanner, Fritsch, Cai, Luo, Wang, van der Bank, Zhang, Wang, Wang, Zhang, Fu, Yang, Hollingsworth, Chase, Soltis, Soltis and Li2019) used gene sequences from chloroplast genomes belonging to species from 85% of extant flowering-plant families, time-calibrated using 62 fossils, to date the origin of angiosperms. Li et al. were able to corroborate much of the conventional phylogeny of the angiosperm clade, which many take to be a positive indicator for their methods. Beyond the headline late Triassic date, Li et al.’s work suggests that major radiations occurred in the Late Jurassic and Early Cretaceous (approximately 165 to 100Ma) (Reference Li, Yi, Gao, Ma, Zhang, Yang, Gitzendanner, Fritsch, Cai, Luo, Wang, van der Bank, Zhang, Wang, Wang, Zhang, Fu, Yang, Hollingsworth, Chase, Soltis, Soltis and Li2019), again much earlier than the fossil record suggests.
It might be easy to suggest (as many have) that the conflict between fossil and molecular clocks must then reflect profound flaws in the fossil record or our interpretation of it. After all, fossil record is notoriously incomplete and the probability that any individual becomes a fossil is low. Yet many paleobotanists who play close attention to the Jurassic gap remain skeptical of such explanations. In other words, fossil folks are just as confident in the reliability of their findings as the molecular clock folk. Researchers Mario Coiro, James Doyle, and Jason Hilton conducted an extensive review of the known fossil record in 2019 and argue that there are two key pieces of evidence supporting the hypothesis that the fossil record is largely accurate in the case of angiosperms.
First, there are many sites with good pollen fossil preservation before the Cretaceous, and researchers have looked extensively for angiosperm fossils at these sites. Pollen serves as an extremely important fossil indicator in the Angiosperm origin conversation. Unlike botanical megafossils (leaves or flowers), pollen fossils are both well distributed and well preserved in the fossil record. While angiosperm megafossils only appear near the Barremian-Aptian boundary in two or three locations, there is an extensive pre-Aptian age pollen record of Angiosperms distributed all over the world (Coiro et al., Reference Coiro, Doyle and Hilton2019). Despite claims to the discovery of pre-Cretaceous angiosperm fossils, a careful analysis of the claims reveals that these interpretations result from “misunderstandings of the basic plant morphology” (Coiro et al., Reference Coiro, Doyle and Hilton2019, p.95). A full analysis of the pre-Cretaceous fossil record would require a careful consideration of plant morphology, anatomy, and taphonomy, and is beyond the scope of this paper. The important point here is that despite the existence and extensive survey of the pollen fossil record, there have been no definitive pre-Cretaceous angiosperm fossils found.
The second piece of evidence that Coiro, Doyle, and Hilton point out is that when we do see definitive angiosperm pollen, and the diversification of that pollen, these events occur in a precise order. This order is the same in different location around the globe, and it is the same order predicted by molecular analyses of the phylogenetic relationship among angiosperm groups (Coiro et al., Reference Coiro, Doyle and Hilton2019). If this “stratigraphic succession” were not an accurate reflection of angiosperm emergence, we would have to explain why plants were waiting around for millions of years before appearing in the fossil record in a precise order.
This view of angiosperm origins has been termed the “short-fuse” hypothesis because the suggestion is that angiosperms diversified fairly quickly after their initial appearance, and it suggests that it is our molecular clock estimates that are wrong rather than the fossil record. While molecular clock experts readily admit that their estimates are not precise and can only provide ranges of high probability for the emergence of angiosperms, many are quite confident they can reject a post-Jurassic emergence for angiosperms (e.g. Barba-Montoya et al., Reference Barba-Montoya, dos Reis, Schneider, Donoghue and Yang2018; Li et al., Reference Li, Yi, Gao, Ma, Zhang, Yang, Gitzendanner, Fritsch, Cai, Luo, Wang, van der Bank, Zhang, Wang, Wang, Zhang, Fu, Yang, Hollingsworth, Chase, Soltis, Soltis and Li2019). These researchers instead suspect the “long-fuse” hypothesis is correct – they believe that crown group angiosperm evolved in the Jurassic or earlier leaving either little fossil evidence of this long tail or leaving poorly understood fossil evidence.
4. What Can We learn
As it currently stands, the angiosperm debate appears to be at an impasse: no evidence could be provided that would settle the debate in a way that convinces all parties. Cleland (Reference Cleland2002) calls evidence that clinches the case for a particular story a “smoking gun”. In the case of the origins of angiosperms, there are several hypothetically possible smoking guns. Most obviously, upon the invention of time-travel researchers could visit the Jurassic to discover the presence or absence of angiosperms. If DNA evidence from the fossil preserves were available, this might also provide some sort of adjudication between the competing hypotheses. Unfortunately, with our current constraints both suggestions are impossible. We are left with needing to work only with the fossilized megafauna and pollen specimen which have been preserved in combination with DNA and protein extracted from currently living angiosperms.
Not only is no smoking gun currently available, but it also seems implausible to think one will arrive. Both streams of available evidence are highly incomplete, and most of our ways for dealing with their incompleteness make them also interdependent. As Derek Turner argued out, the historical science often has episodes of “local underdetermination” (Reference Turner2005). He argues that local underdetermination occurs when four criteria are met: 1) two incompatible hypotheses are genuine rivals, 2) both hypotheses are equally well supported by currently available evidence, 3) the hypotheses have roughly equal non-empirical virtues, and 4) background theories give us some reason to think the hypotheses are equivalent (Turner, Reference Turner2005). One might think that impasses such as this one, therefore, is as an example of fruitless disagreement—where there is no existent evidence that can resolve the controversy, and it seems likely that (barring significant technological revolutions) there is no further promise such ‘tie-breaking’ evidence is on the horizon either.
As Turner’s suggestion that we would never know the color of dinosaurs didn’t survive the test of time, we perhaps ought not be too hasty to dismiss the future of the angiosperm origin debate (Turner, Reference Turner2016). Still, the angiosperm case may tell us something about how continued disagreement can be (and has been) fruitful. For example, if we recall the three ways De Cruz and Dr Smedt argued that peer disagreements are advantageous (1. generating new evidence, 2. leading to the re-evaluation of existing evidence, and 3. serving as an antidote to confirmation bias), it is clear that the angiosperm disagreement has fulfilled all three. The controversy has spurred the creation of new molecular clock models and the search for pre-Cretaceous angiosperm fossils (1). For example, in 2021, Silvestro and colleagues attempted to create a Bayesian model based on the fossil record alone. These models are important because they are independent of the assumptions of molecular clock models, and thus represent a different sort of input to the conversation about angiosperm origins. Strikingly, Silvestro et al.’s fossil-based model estimated most families to appear between 254.8 and 153.7 Million years ago (before the Cretaceous).
This debate has also caused the continued re-evaluation of evidence (2). In reply to Silvestro et al.’s (Reference Silvestro, Bacon, Ding, Zhang, Donoghue, Antonelli and Xing2021) work, Budd and colleagues (including Coiro, Doyle, and Hilton who conducted the 2019 review of extant pollen fossils) argue that the models and results developed by Silvestro et al. are “unsound” (Reference Silvestro, Bacon, Ding, Zhang, Donoghue, Antonelli and Xing2021). Budd et al.’s reply has two prongs: first, it attacks the mathematical choices Silvestro et al. made in their model. Budd et al. simulated clades similar to those within the angiosperms category, including simulated fossil sampling, but they made each originate at 120 Million years ago (i.e. within the cretaceous). Budd et al. then used Silvestro et al.’s model to estimate the ages of their simulated clades and found that simulated clades had a probability of 0.99996 for emerging before the Cretaceous, despite the fact that all of their clades actually emerged within the Cretaceous (Reference Silvestro, Bacon, Ding, Zhang, Donoghue, Antonelli and Xing2021). They argued that this is evidence for the “unsoundness” of Silvestro et al.’s model – thus contributing to a clear re-evaluation of the newly asserted evidence.
The second prong of Budd et al.’s response also continued the evaluation of the fossil record. Silvestro et al.’s results suggest that many of the eudicot lineages (which are currently united morphologically by their tricolporate pollen) emerged early in the Jurassic (Reference Silvestro, Bacon, Ding, Zhang, Donoghue, Antonelli and Xing2021). Budd et al. assert that “this would conflict strongly with the sequential appearance of monosulcate, tricolpate and tricolporate pollen in the Cretaceous dispersed pollen record” (Reference Budd, Mann, Doyle, Coiro and Hilton2021, p. 3). They look not merely at the presence of absence of fossils, but at fossil morphology and how that morphology links up to our understandings of the phylogenetic relationships among angiosperms. If Silvestro et al.’s assertion is to be correct, it might mean that there was a long pre-Cretaceous history of eudicots, and that the modified their pollen within the clade. This continual reevaluation of the fossil record is the result of the disagreement about angiosperm origins.
Finally, the controversy has continuously demanded researchers be exposed to evidence that does not conform to their preferred version of events (3). This literature is not filled with continual affirmations of a preferred hypothesis, but rather it is a back and forth between differing interpretations of the evidence which conform to different versions of events. This sort of disagreement does not guarantee the sort of standpoint diversity that authors like Wylie (Reference Wylie, Figueroa and Sandra2003) and Intemann (Reference Intemann and Heidi2011) were interested in, but there is still difference in perspective within the field such that it is difficult to become isolated within an echo chamber. If we’re merely looking for enough difference to prevet confirmation bias, the angiosperm origin research community meets that standard.
Thus, if we were to evaluate the debate on De Cruz and Dr Smedt’s criteria, it would seem that the angiosperm debate should be considered an advantageous disagreement. Further, the angiosperm debate clearly fails to fulfil the criteria for “epistemically detrimental dissent” proposed by Biddle and Leuschner (Reference Biddle and Leuschner2015). The non-epistemic consequences are not high and the dissent does not violate conventional standards. Since Biddle and Leuschner (Reference Biddle and Leuschner2015) require all four of their criteria to be considered epistemically detrimental dissent, and the angiosperm debate easily fails at least two of the criteria, the angiosperm debate would not be considered “detrimental” on their standards.
Still, I would not be so quick to declare victory for the angiosperm debate. It is not merely the impasse researchers appear to be at, but also the way they are engaged in the debate that might be making it less fruitful than it could possibly be. If we look closely at some of the exchanges that have been discussed, we frequently see that researchers seem to be talking past one another. For example, Coiro, Doyle, and Hilton assert that “Most molecular studies have not addressed these conflicts directly, but recently Barba-Montoya et al. (Reference Barba-Montoya, dos Reis, Schneider, Donoghue and Yang2018) argued that they reflect deep flaws in interpretation of the fossil record” (2019, p. 84). However, upon closer inspection of the cited Barba-Montoya et al. paper one finds that they do not truly argue for the falseness of the fossil record. Instead, Barba-Montoya et al. conclude that “In large part, the discrepancy between these approaches is an artefact of false precision on both sides” (Reference Leuschner2018, p. 831). In this case, and others like it, researchers disagree, but not in a way that is merely pinning their work as responses to one another, rather than through deep thoughtful engagement.
We can see something similar in the frequent references to Darwin’s remarks about angiosperms in this literature. In a letter to friend and colleague J.D. Hooker, Charles Darwin described the sudden appearance and diversification within angiosperms as “an abominable mystery” (Davies et al., Reference Davies, Barraclough, Chase, Soltis, Soltis and Savolainen2004). Even in the contemporary literature, many papers on the origin of angiosperms open with reference to Darwin’s abominable mystery (e.g. Coiro et al., Reference Coiro, Doyle and Hilton2019; van der Kooi and Ollerton, Reference van der Kooi and Ollerton2020; Sauquet et al., Reference Sauquet, Ramírez-Barahona and Magallón2022; Silvestro et al., Reference Silvestro, Bacon, Ding, Zhang, Donoghue, Antonelli and Xing2021). Yet our contemporary mystery is not analogous to Darwin’s. Darwin’s concerned the apparent rapid diversification of angiosperms because he preferred a gradualist picture of evolutionary change (Friedman, Reference Friedman2009). While Darwin did acknowledge the possibility of the rapid diversification of angiosperms, he also speculated that there might be a “long, gradual, and undiscovered pre-Cretaceous history of flowering plants on a lost island or continent” (Friedman, Reference Friedman2009, p. 5). Many, if not most, of the authors who invoke Darwin’s name today take for granted that there was a rapid diversification of angiosperms in the Cretaceous. The “abominable mystery” these modern authors refer to are varied (see Friedman, Reference Friedman2009), but are rarely the actual mystery Darwin had in mind.
What is interesting about both examples is that the appearance of disagreement is being utilized by authors, but not accurately or productively. One could speculate about the reasons for holding up a mystery as “Darwin’s” when it is not really Darwin’s, or contrasting your work with already published work when there is no material disagreement. Perhaps our modern angiosperm mysteries are ancestrally related to those that puzzled Darwin. Perhaps there are meaningful disagreements between colleagues that are being shorthanded for ease of writing. Perhaps the social conditions we have created around publication incentivize the creation of disagreement over the creation of convergence – certainly many more articles have been published about the origins of angiosperms than if there were no mystery. In fact, while most of the articles in the area open with references to profound disagreement, many close with the same suggestion of how to dissolve the mystery. Even in the context of apparent disagreement, we can have incentives that funnel research in a particular direction.
I want to be clear that the problems in the angiosperm origins debate do not arise due to a lack of convergence. Karen Kovaka argues that “relative frequency controversies”Footnote 1 are productive even when though may not be resolved (Reference Kovaka2021). While the angiosperm debate might be an instance of a relative frequency controversy, but that is not the point. The point is that even unresolved debates can be productive, so it would be a mistake to assume that the lack of resolution necessarily renders this controversy unproductive. If it were the eventual convergence of disagreements that made them fruitful, we might assume the outputs to be knowledge or progress. However, if genuine disagreement may have positive contributions toward generating convergence, this is not necessarily the case. Adrian Currie distinguished between epistemic goods which are “knowledge-tracking” vs “knowledge-promoting” (Reference Currie2019). While the lack of convergence might highlight that we have not achieved a tracking of the phenomenon in question (“knowledge-tracking”), we have evidence that the angiosperm disagreement independently increases the likelihood that some gains will be made (“knowledge-promoting”). Yet saying the debate is “knowledge-promoting” seems to miss something important in the same way that identifying it as “advantageous” or “not detrimental” did.
Luckily, there are other ways, beyond a lack of convergence, that we can characterize how the angiosperm debate is, at times, unproductive. First, there is the issue of the types of evidence available in the domains concerned. All of the evidence available comes from highly imperfect systems, which leads researchers to rely on what Currie called “traced-based reasoning” (Reference Currie2019). While trace-based reasoning can often appear to be “unreasonably effective”, our inferences are only as strong as our knowledge allows them to be. In the case of angiosperms there is so much inherent ambiguity in the system, there is little hope of total agreement (a point made by Barba-Montoya et al., Reference Barba-Montoya, dos Reis, Schneider, Donoghue and Yang2018, even if ignored by Coiro et al., Reference Coiro, Doyle and Hilton2019). While such cases might not be hopeless, there is certainly something importantly different about disagreements in cases with imperfect evidence. Focusing on the disagreement in cases where evidence is inherently imperfect, seems to make the conversation less productive. In such cases, we need to operate with different priors about agreement or convergence. If we expect some disagreement, we can more carefully evaluate the sources of disagreement, and what those mean for the state of our knowledge. This is, in fact, the constant solution offered by the researchers on both sides of the angiosperm controversy. In cases where researchers ignore these basic characteristics, we end up with lost opportunity for higher quality conversation.
In the case of angiosperm origins, there do seem to be good theoretical reasons to expect some level of disagreement between our different trace generating systems. We need not assume that the concepts involved in the two measurement systems neatly overlap with each other. The morphological species concept at use in the biostratigraphic clock is not necessarily the same as the genomic species concept at use in the molecular clock. In fact, there are prima facie reasons to suspect that these concepts should come apart. Since molecular changes are quite likely to evolve before large scale fossilizable changes in a lineage, one would not necessarily expect that the two dating methods would report the same date of change. A similar point has been made by Matt Haber when he argues that genealogical discordance complicates the taxonomic and phylogenetic pictures (Reference Haber2019). While the importance of hybridization and incomplete lineage sorting in angiosperms is not fully understood, there are indications that both have played important roles in the evolution of the lineage (see, Goulet et al., Reference Goulet, Roda and Hopkins2016 or Cai et al., Reference Cai, Xi, Lemmon, Lemmon, Mast, Buddenhagen, Liu and Davis2020), both of which are among the key factors leading to genealogical discordance. The assumption of tidy species concepts in early angiosperm evolution is misplaced, and more careful consideration of the nature and scope of disagreement would be more productive than a mere statement of disagreement.
Second, there might be reason to think that the requirement of disagreement is itself somewhat pernicious. Both the repeated use of Darwin, and the uncharitable treatment of colleagues’ work, could result from a pressure to frame one’s own work in terms of disagreement. Perpetuating the mystery means more opportunities for publication for all – a system which greatly benefits the scholars involved. Yet this system is no guarantee that the disagreement is fruitful. One might read this as a result of malicious intent, but I think that is unlikely. Individual scientists are doing their best, and it is more likely that the incentives set up by our scientific systems do not always incentivize behavior that will be maximally productive.
5. Conclusion
In general, the lesson from the angiosperm debate is a simple one: It is important that we not romanticize disagreement in science as always being productive, as this may blind us from the way the structures, incentives, and norms of our scientific practices may inadvertently be promoting less fruitful disagreement. There is more work to be done in characterizing what it is about disagreements that can make them less productive, but we must also be cognizant of the ways even well-intentioned genuine disagreement can undermine our goals.
Declaration
I have nothing to declare.