Introduction
Female genital schistosomiasis (FGS) is a gender-specific manifestation of urogenital schistosomiasis caused by Schistosoma haematobium, and more rarely by Schistosoma mansoni. Infections are acquired through contact with contaminated freshwater bodies, where schistosome cercariae are present, as used by rural communities. Girls and women are particularly at risk due to their daily chores and livelihoods involving environmental water for domestic use. Over 53 million women in sub-Saharan Africa have or are at-risk of FGS. Several gynaecological complications arise from FGS, including infertility and adverse outcomes of pregnancy. Additionally, an association between FGS and HIV infection has been identified, and this link is now acknowledged by UNAIDS in their 2019 report (UNAIDS, 2019; Patel et al., Reference Patel, Rose, Kjetland, Downs, Mbabazi, Sabin, Chege, Watts and Secor2021). FGS exemplifies the experiences of women and girls who face multiple and intersecting health, sociocultural, environmental and economic vulnerabilities and challenges (Mbabazi et al., Reference Mbabazi, Del Pino, Ducker, Dean, Broekkamp, Prasetyanti, Jacobson, Krentel, Seunik, Bustinduy and Malecela2021; Williams et al., Reference Williams, Seunik and Meier2022). Indeed, FGS affects the same people who carry a disproportionate global burden of HIV, HPV and cervical cancer in Africa (Engels et al., Reference Engels, Hotez, Ducker, Gyapong, Bustinduy, Secor, Harrison, Theobald, Thomson, Gamba, Masong, Lammie, Govender, Mbabazi and Malecela2020). Known barriers include stigmatization from within healthcare services due to the symptoms often wrongly associated with sexually transmitted infections (STIs) that reduces patient treatment seeking behaviours (Jacobson et al., Reference Jacobson, Pantelias, Williamson, Kjetland, Krentel, Gyapong, Mbabazi and Djirmay2022).
All too few women benefit from a confident clinical diagnosis and coherent management of FGS and associated infections (Mazigo et al., Reference Mazigo, Samson, Lambert, Kosia, Ngoma, Murphy and Matungwa2021). Diagnosis of FGS is generally based on visual inspection by clinical colposcopy according to WHO guidelines and pocket atlas (World Health Organization, 2015). However, new studies question the consistency of diagnoses made by experts only based on visual inspection (Sturt et al., Reference Sturt, Bristowe, Webb, Hansingo, Phiri, Mudenda, Mapani, Mweene, Levecke, Cools, van Dam, Corstjens, Ayles, Hayes, Francis, van Lieshout, Vwalika, Kjetland and Bustinduy2023). Other methods, based on molecular biology, which have shown better accuracy can be used, but they are not yet validated, are costly and difficult to implement in remote locations (Sturt et al., Reference Sturt, Webb, Phiri, Mweene, Chola, van Dam, Corstjens, Wessels, Stothard, Hayes, Ayles, Hansingo, van Lieshout and Bustinduy2020). In Cameroon, urogenital and intestinal schistosomiasis, caused by S. haematobium and S. mansoni, respectively, affects the lives of millions of impoverished populations, and it is estimated that over 2 million people are infected. National parasitological surveys provided a full appreciation of the wide distribution of urogenital schistosomiasis in Cameroon, but few specific clinical studies have been conducted on the burden of FGS per se in Cameroon so far except one to our knowledge conducted in a highly endemic area in the West Province with more than half of the women investigated presenting FGS lesions (Masong et al., Reference Masong, Wepnje, Marlene, Gamba, Mengue, Kouokam, Stothard and Ekobo2021). Despite mass drug administration interventions which have led to a significant decrease of infection prevalence in all regions of Cameroon, the transmission of schistosomiasis remains high, and FGS remains a serious threat which has not been quantified explicitly.
Previous experiences in cervical cancer screening in a rural nearby region showed that approaching women through mobile clinics resulted in a much better uptake compared to the static gynaecological clinic at the district hospital. Targeting HIV clinics is important for epidemiological reasons as HIV is associated with FGS in several studies and increases the probability of FGS finding lesions in endemic areas (UNAIDS, 2019; Patel et al., Reference Patel, Rose, Kjetland, Downs, Mbabazi, Sabin, Chege, Watts and Secor2021).
To develop the national strategy and properly tackle the issues of FGS in Cameroon, it is essential to have reliable data on feasibility, acceptability of a screening strategy based on gynaecological examination in settings where this is not routinely offered to women. In this study, two different strategies (static-clinics vs mobile pop-up clinics) for detection and care of affected women were compared in eastern Cameroon. We measured and compared the prevalence of typical lesions between the two groups.
Given the lack of knowledge about FGS, we evaluated the remote training of a general practitioner (GP) experienced in cervical cancer screening. The performance of the trained field clinician was assessed by the review of the cervical photos by an expert who identified the lesions blindly. Acceptability among younger sexually active women was specifically assessed as their precocious lesions usually respond better to praziquantel treatment.
The East region of Cameroon has been chosen as it is a relatively high schistosomiasis endemic area and there is no cervical cancer programme detection and no gynaecologic preventive care. This study gave the opportunity to offer a cervical cancer screening test to women with HIV who are at increased risk (World Health Organization, 2021b).
Materials and methods
Study design
We conducted a comparative cross-sectional study to evaluate feasibility and acceptability for FGS screening in two different settings:
• A ‘static’ reproductive health service in HIV clinics.
• An integrated reproductive health ‘mobile pop-up’ clinic, in villages selected based on a high schistosomiasis endemicity by precision mapping.
Study settings
The mobile clinic visited seven villages in the Dimako and Mandjou localities identified as highly endemic through precision disease mapping (see map) with a total number of women in reproductive age of 13,052. The static clinic was set up in the HIV services of the regional hospital of Bertoua (designated HIV care centre) and in a more peripheral district HIV Unit (HIV care unit) of Dimako. These facilities provide care for 2,739 and 205 women living with HIV (WLWH) of reproductive age, respectively.
Study population
The target population was the sexually active women aged from 13 to 50 years in the selected areas. Eligible women were excluded in case of pregnancy, bleeding, hysterectomy or if not able to provide parental consent if less than 21 years old (as for Cameroonian law on majority age). Every woman was offered an HIV screening test if not already known positive.
Study procedures
The study team was composed of a physician (GP) and a nurse. They were both experienced in cervical cancer screening by cervical inspection and management of precancerous cervical lesions. For the purpose of the study, they both participated in a 2-day online training on FGS with a clinical expert from Madagascar (Bodo Randrianasolo). The team was completed by HIV care local staff at the static clinic. The local staff was trained for basic knowledge on FGS during a 1-day training session and their progression validated by a test. In the community, the team was completed by community health workers who were also trained by the study team. In the HIV clinic, women were informed through group sessions and individually proposed for participation if eligible. Visit procedures and management of FGS were the same as in the mobile clinics.
Once the communities with high urogenital schistosomiasis endemicity were identified upon a precision mapping performed by the parasitology team, local authorities were met, and the study introduced and discussed. Participants were recruited using a convenient sampling method. On established dates, women in the targeted area were invited to come to the nearest health centre or another appropriate location for consultation following awareness-raising efforts carried out through door-to-door visits and communication provided in churches, mosques, associations (both men’s and women’s), markets and other community settings. After a group information session on cervical cancer and FGS, the study information notice was presented individually, and participation was offered. Any woman aged 13–50 years, who was not pregnant, not experiencing bleeding and had not undergone a hysterectomy was included in the study after signing the informed consent form (and, if under 21, was able to provide parental consent), and agreed to the collection and storage of cervical images.
Demographical data, gynaecological history and living habits including water exposure were collected from all enrolled women. Subsequently, a gynaecological visual inspection of the native cervix was done in order to identify suspect FGS lesions. During the whole process, several pictures of the native cervix were taken to be reviewed by the distant FGS expert. Women with FGS suspect lesions were treated with praziquantel 40 mg/kg single dose on site according to the current existing recommendations.
Data and pictures were collected with REDCap® and stored on a secured server. All native cervix pictures were blindly revised by an FGS experienced clinician who gave the final FGS diagnostic. All procedures, including treatment of sexually transmitted diseases in case of clinical symptoms, were provided for free to study participants.
Period of the study
Data collection took place from March to December 2021.
Outcome assessment
Feasibility was assessed by the number of screenings, diagnosis and treatment conducted in a defined period in the target populations. Acceptability was assessed by the number of eligible women who accepted to participate in the study and their adherence to all steps of study procedures. Acceptability was assessed by age and specifically analysed among minors (13–21 years) as this population benefits more from FGS treatment. Informal feedback from local staff and clinicians provided information on the reasons for patient refusal to participate in the study and procedures. Clinical performance of the field clinician after distance training was measured by the proportion of concordance between the field GP and the expert. Static and mobile pop-up strategies were compared for feasibility, acceptability and FGS lesions prevalence.
Data analysis
Variables are presented with median and interquartile ranges. For proportions comparison and for continuous variables’ comparison, Fisher’s exact test and Kruksal–Wallis test were used respectively.
Results
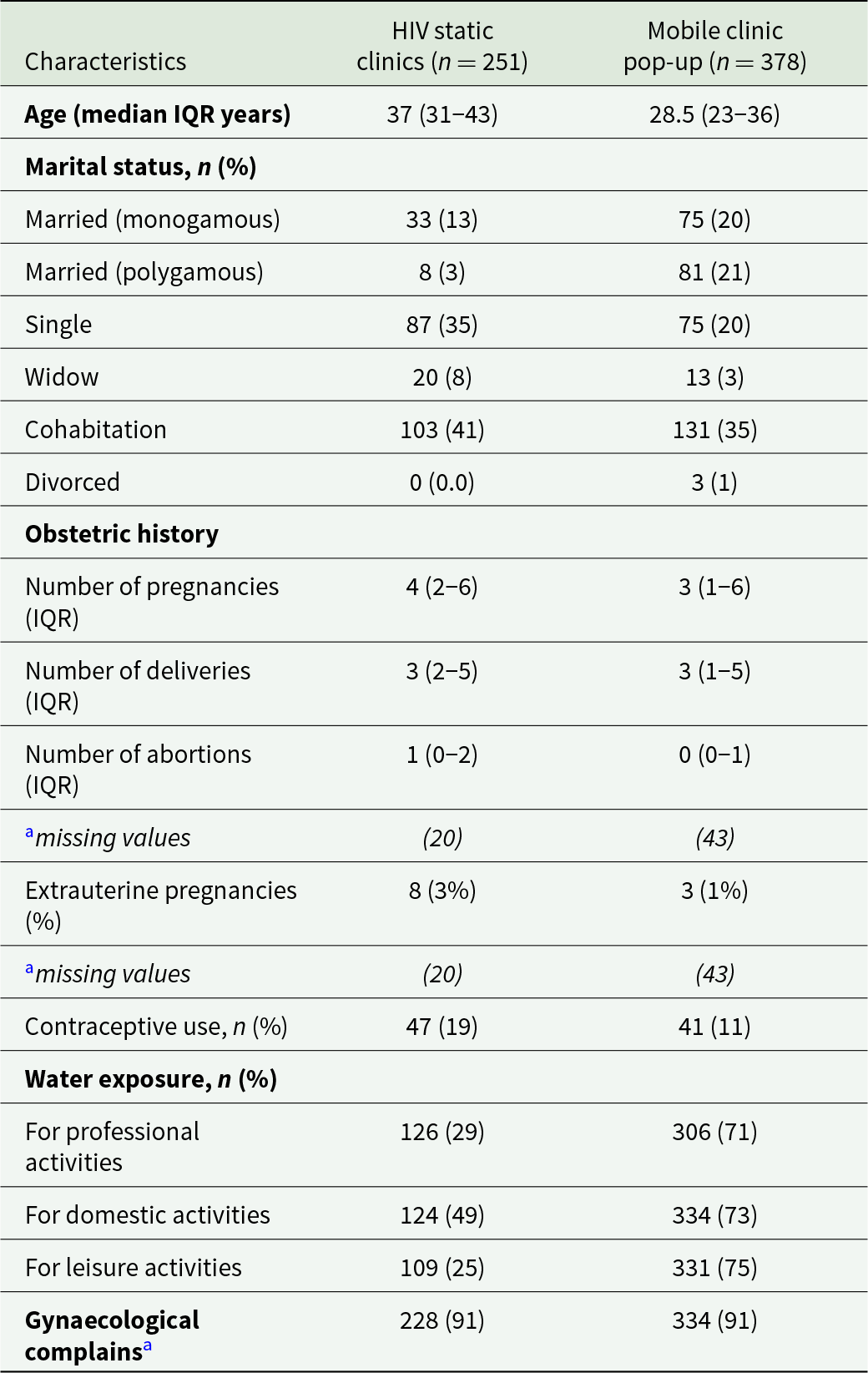
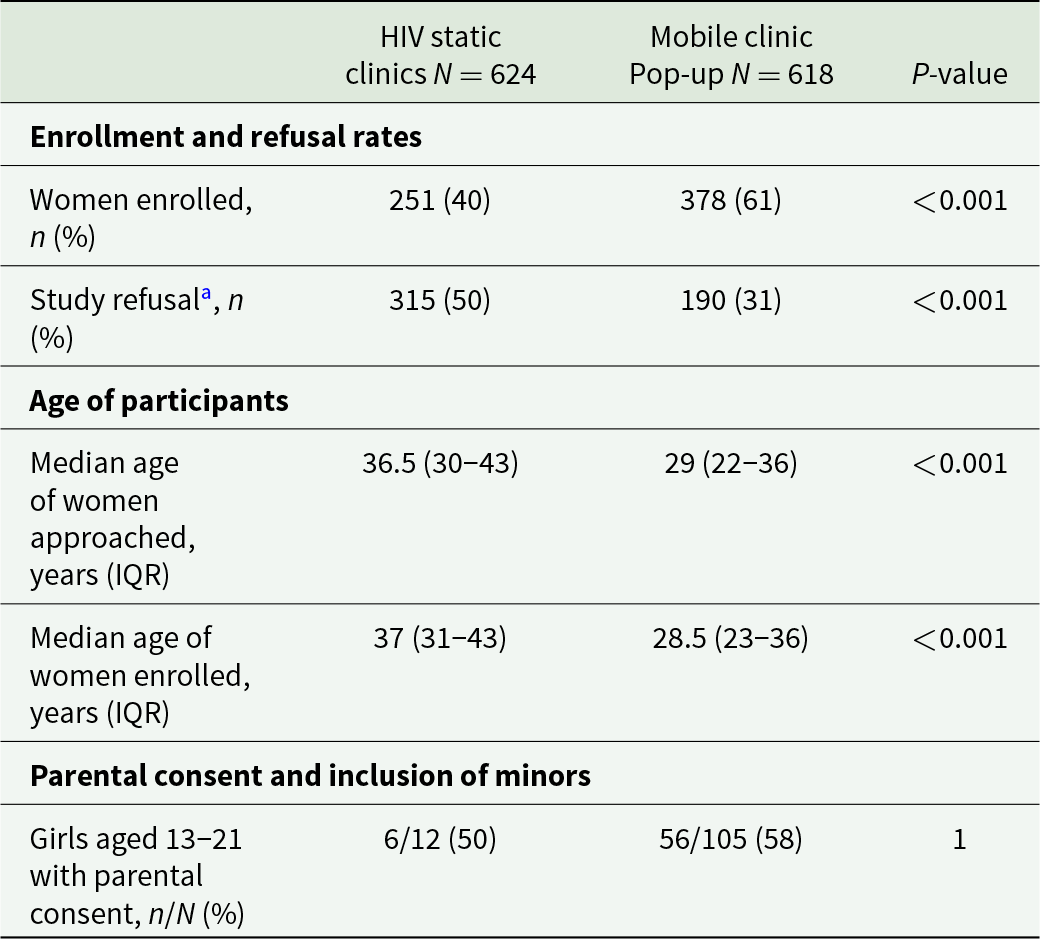
From 1st March to 21st December 2021, 1242 women were approached and offered to participate, 624 in the HIV static clinics and 618 in the community mobile pop-up clinics, respectively. A total of 505 women refused to participate while 108 women were not included for predefined exclusion reasons (pregnancy, bleeding, not in the target age group) (Figure 1). A remaining 629 women were enrolled, 251 in the HIV clinics and 378 in mobile pop-up clinics. Population characteristics are presented in Table 1. We can observe that enrolled women in mobile clinics were younger, more often married and more exposed to contact with water. More than 90% of women in the 2 groups reported symptoms that can be associated with FGS and other reproductive health issues (Table 1).

Figure 1. Flowchart of study design.
Table 1. Main demographical descriptors of enrolled women

a Includes abnormal bleeding, itching, pelvic pain, urinary urgency or stress incontinence.
IQR, interquartile range.
Feasibility
Despite the restrictions due to the SARS-CoV2 epidemic, the study team accounted for 84 days of field work, provided training for 43 health workers and approached more than 1200 women. The conducted 629 clinical visits with gynaecological examination and FGS screening and providing care for all who needed it. The mean daily screening capacity including all study procedures was 7.5/day, with a minimum of 1 and a maximum of 22. The activities in the HIV services were delayed due to administrative procedures which were unexpectedly complicated. In the community, the shortage of praziquantel hindered the screen and treatment approach for the last 12 diagnosed women.
Acceptability
Refusal of the screening procedures was significantly higher in the HIV clinics than in the community (50% vs 31%) (Table 2), but all the women who consent to participate in the study accepted the gynaecological visit and the proposed treatment. Enrolled women in the community were significantly younger than those in HIV clinics with 28.5 years (interquartile range [IQR]: 23–36) vs 37,0 years (IQR: 31–43) respectively. Acceptability was similar in the 2 groups in the minor population, but a higher number of minors were reached in community settings.
Table 2. Comparison of key features between static and pop-up clinics

a Refusal rate includes only women who actively declined participation, excluding ineligible or unreachable individuals.
IQR, interquartile range.
Women who refused did not differ in age from those enrolled in the study with 37.0 years (IQR: 31–43) vs 36.5 years (IQR: 30–43) neither in HIV clinic nor in the community with 29 years (IQR: 22–36) vs 28.5 years (IQR: 23–36) (Table 2).
FGS prevalence in HIV clinics and community
All consenting women in each group were screened for FGS lesions. Typical lesions have been identified in more than half of the screened women: 51% (129/251) in the HIV clinic and 56% (212/378). The difference in prevalence was not statistically significant.
We have not found any relevant statistical associations between residency, water contacts, symptoms and positive FGS diagnostic.
HIV tests and results in both settings
In the HIV clinics, 251 women, of whom 238 were HIV positive (13 were family or health staff) were included in the study. Among women included in the mobile pop-up clinics, 18 women (5%) were known HIV positive and 285 (79%) underwent an HIV test, 8 (3%) resulted positive and were referred to the nearest HIV service. The other women either refused or reported recent testing.
Concordance between trained field clinician and FGS expert on FGS lesions identification
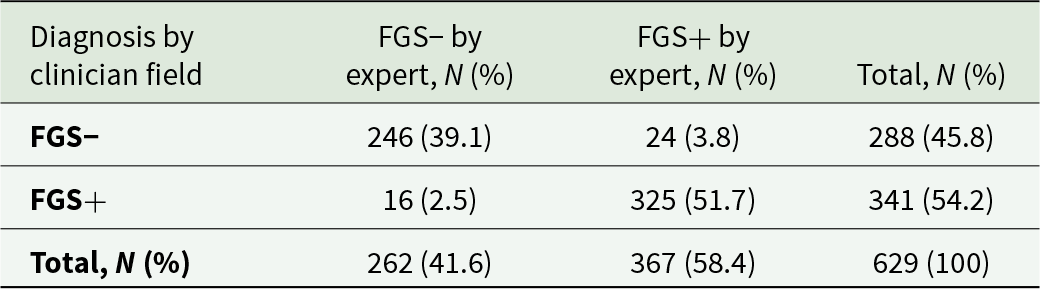
In order to assess the training efficacy, 1258 images (2 per women) have been taken during the visit and reviewed by the expert blindly. We recorded 91% of concordance on FGS lesion identification between the expert and the field clinician. FGS rate of detection by the expert was slightly higher than the proportion detected by field clinician (Table 3).
Table 3. Diagnostic agreement between field clinician and expert for FGS (N and proportions)

Discussion
In our study, we assessed feasibility and acceptability of screening, diagnosis and treatment of FGS in two different settings with 2 different populations and two different recruitment methods in a schistosomiasis endemic area of East Province Cameroon. One setting was integrated in a static HIV clinical care services (district hospital or smaller clinic) and the other setting consisted of mobile pop-up clinics in the rural area identified by micromapping by a parasitological survey, otherwise known as precision disease mapping.
In terms of feasibility, both strategies could be quickly implemented by a small and flexible external team composed of a nurse and a GP, trained on FGS in primary care by telemedicine. The implementation of both models was successfully achieved with a minimal, flexible team – consisting of a nurse and a GP – trained remotely via telemedicine. This low-resource approach aligns with recent WHO recommendations promoting integration of FGS care into existing sexual and reproductive health services, particularly in endemic regions with overlapping HIV burden (World Health Organization, 2021a). Importantly, the integration of FGS screening into HIV clinics capitalizes on existing infrastructure and patient flow, facilitating streamlined access to care for women already engaged in health services. On the other hand, mobile pop-up clinics significantly enhanced geographical reach and accessibility, particularly in underserved rural communities. This dual-model approach mirrors strategies used effectively in other public health programmes, such as cervical cancer and HIV testing, where mobile outreach has been shown to improve early detection and equity in access (UNAIDS, 2019; Goldstein et al., Reference Goldstein, Williams, Haynes and Coyne-Beasley2020).
Knowledge and awareness about FGS diseases were very limited among health workers, as it was demonstrated in other setting. This can limit their engagement in providing education and care to their patients (Mazigo et al., Reference Mazigo, Samson, Lambert, Kosia, Ngoma, Murphy and Matungwa2021). Nevertheless, these gaps can be rapidly filled with short training sessions focusing on basic information about the disease and practical exercises of health communication (Jacobson et al., Reference Jacobson, Pantelias, Williamson, Kjetland, Krentel, Gyapong, Mbabazi and Djirmay2022). Collaboration with and among health staff was important for women information and mobilization and the patients’ flow organization. Moreover, collaboration with local health personnel, particularly community health workers, was critical to ensure patient mobilization, flow coordination and cultural acceptability – factors often overlooked but essential to feasibility and sustainability. Our findings reinforce the importance of leveraging existing health networks and training models to scale up FGS screening and care in endemic regions.
Acceptability was lower in the static HIV clinics than in the mobile pop-up ones. Age was not a factor associated with study acceptability. Community screening through mobile pop-up clinics provided better access to women younger than 21 years old which is important to target early lesions more responsive to treatment and possibly prevent HIV infection. The lower acceptability observed in HIV clinics mirrors trends reported in other sub-Saharan African settings, where women living with HIV have shown reluctance to engage in preventive gynaecologic care, even when services are free of charge (Mbatha et al., Reference Mbatha, Maharaj and Ndlovu2017; Baisley et al., Reference Baisley, Chisenga, Nyblade, Kaseba-Sata, Musonda, Silumesii and Vermund2022). One commonly cited barrier is the fear of receiving an additional diagnosis, which can lead to psychological distress and healthcare avoidance. Our hypothesis is that the lack of knowledge about a paucisymptomatic disease in women experimenting other health issues may also hinder the willingness to participate. Further qualitative research should be conducted in this setting to better understand this recurrent phenomenon. This could help improve screening uptake of a highly exposed population. Community-based outreach via mobile clinics may help overcome these barriers by offering screening in less formal, more familiar environments – reducing stigma and improving trust. In addition, mobile units allow for more targeted engagement strategies, such as involving community health workers in mobilization and follow-up, which has been shown to significantly enhance participation in other screening programmes (Goldstein et al., Reference Goldstein, Williams, Haynes and Coyne-Beasley2020; Jacobson et al., Reference Jacobson, Pantelias, Williamson, Kjetland, Krentel, Gyapong, Mbabazi and Djirmay2022). The acceptability in the mobile pop-up clinics was surely improved by the communication done by local community workers, who were very active in reaching and informing the population during the precision mapping and thereafter motivated to mobilize concerned women. On the contrary, in HIV clinics, health staff perceived FGS screening activities as extra work and were therefore reluctant to engage. These findings underscore the need for context-adapted strategies that consider not only biomedical feasibility but also social and behavioural dimensions of acceptability. Scaling up FGS screening will therefore require not only training and infrastructure but also efforts to address fears, improve health literacy and foster trust in both clinical and community-based settings.
The prevalence of FGS observed in this study is alarmingly high in both healthcare settings – static HIV clinics and mobile pop-up clinics – with over 50% of the women screened receiving a positive diagnosis. This finding not only confirms that FGS remains a major public health concern in endemic areas, but also suggests that the condition may be underdiagnosed and underreported due to the lack of routine screening services and low awareness among healthcare providers. The high prevalence we observed is consistent with previous studies conducted in Cameroon, notably the work by Masong et al. (Reference Masong, Wepnje, Marlene, Gamba, Mengue, Kouokam, Stothard and Ekobo2021) in the West province, which similarly reported a substantial burden of disease. This convergence of findings across regions highlights the systemic and widespread nature of FGS in the country. These findings align with other studies in sub-Saharan Africa which emphasize that community-based and decentralized approaches can significantly enhance detection rates for neglected tropical diseases like FGS (Christinet et al., Reference Christinet, Lazdins-Helds, Stothard and Reinhard-Rupp2016; Kayuni et al., Reference Kayuni, Ball, Chipeta, Kabole, Makaula, Juziwelo, Kalua, Stanton and Stothard2019). The high FGS prevalence in both settings raises additional concern and underscores the need for urgent integration of FGS screening into existing sexual and reproductive health services. FGS interventions are to be put in place, praziquantel must be placed on the essential drug list and be routinely available in health services as it was not available outside the national programme of mass drug administration. An increase in acceptance in HIV services could probably reach even more affected women, while coverage of young girls in the community may provide early diagnosis and early treatment to this population and eventually prevent negative consequences such as infertility, stigma, extrauterine pregnancies and HIV acquisition.
This study has several limitations. Acceptability has been evaluated only with quantitative methods, while qualitative assessment may be more informative to better understand the reasons of refusal and improve uptake. There are unidentified recruitment biases. Most women had symptoms, and this may have influenced their participation in the study. Regarding the prevalence of FGS in our study, the only method used was visual inspection, which is debated in terms of accuracy (Sturt et al., Reference Sturt, Bristowe, Webb, Hansingo, Phiri, Mudenda, Mapani, Mweene, Levecke, Cools, van Dam, Corstjens, Ayles, Hayes, Francis, van Lieshout, Vwalika, Kjetland and Bustinduy2023). This method lacks both sensitivity and specificity, as many FGS lesions resemble those caused by STIs or cervical precancerous changes. These visual overlaps can lead to misclassification and diagnostic discrepancies (Kjetland et al., Reference Kjetland, Kurewa, Ndhlovu, Midzi, Gwanzura, Mason, Gomo, Sandvik, Mduluza, Friis and Gundersen2008). In our study, HPV testing and cervical cancer screening via visual inspection were performed, but STI testing was not included – this represents a limitation that may have contributed to inconsistent diagnoses. Incorporating point-of-care STI diagnostics in future studies would help distinguish FGS from similar conditions and improve accuracy. Molecular tests for egg identification in the genitals are developed; this will allow a more confident diagnosis (Sturt et al., Reference Sturt, Webb, Phiri, Mweene, Chola, van Dam, Corstjens, Wessels, Stothard, Hayes, Ayles, Hansingo, van Lieshout and Bustinduy2020). Generalizability of results is limited by the specificity of the chosen study population.
In the current state, prevention strategies should be based on the general treatment of girls at a preschool age in this context of high endemicity. A new paediatric formulation of praziquantel currently submitted for validation to the European Medicines Agency will help address this underappreciated need of preventing FGS.
Conclusion
It is feasible for a small flexible team of a nurse and a GP to integrate an HIV clinic and to set up mobile pop-up clinics in order to screen FGS with cervical examination. Most women accepted to undergo a gynaecological examination for FGS screening. Proceeding through HIV clinics and through mobile pop-up clinics in endemic villages has been highly effective to identify FGS typical lesions. Targeting HIV clinics is an effective way to identify a high number of older women affected by FGS, whereas community screening gives access to younger women which may provide early diagnosis and early treatment to this population which are known to be more effective, and which could prevent HIV acquisition and other complications. Rapid distant training on FGS lesion identification is effective to train a GP already experimented in cervical examination. Both screenings (FGS and cervical cancer) could be integrated to increase efficacy.
Acknowledgements
Professor Amaya Bustinduy and Dr Amy Sturt gave us precious advice on FGS diagnostic methods and indicators to design our proposal. Mireille Gomes from Ares Trading S.A. (an affiliate of Merck KGaA, Darmstadt, Germany), the health workers and the patients.
Author contributions
Vanessa Christinet, Laura Ciaffi, John Russell Stothard, Jutta Reinhard-Rupp and Louis-Albert Tchuem Tchuente conceived and design the study. Sandrine Nyotue, Aimé Assigui conducted the clinical work and collected the data. Bodo Randraniasolo reviewed the images. Vanessa Christinet, Louis-Albert Tchuem Tchuente and Laura Ciaffi supervised the study, with collaboration of Sandrine Nyotue. Vanessa Christinet and Sandrine Nyotue cleaned the data and analyzed it. All authors discussed the results and contributed to the final manuscript.
Financial support
Funding Source: Department for International Development (DFID), UK Through The Task Force for Global Health, Inc (TFGK) 325 Swanton Way, Decatur, GA 30030, USA. These funds were used to set up and conduct study: personnel costs, Logistics/Supplies/Consumables, Transportation, training, laboratory tests, equipment and communication.
Competing interests
We didn’t have any competing interests in this case.
Ethical standards
The National Ethics Committee for Human Health Research (CNERSH) in Cameroon, in its extraordinary session of July 29, 2020, examined the research project entitled: ‘Concerted Action on Female Genital SCHISTOSOMIASIS AND Gynecological Diseases in Cameroon’ and delivered the clearance NO2020/07/1280/EC/CNERSH/SP. The study was conducted in strict compliance with ethical standards.