1. Introduction
Approximately 30% of patients diagnosed with schizophrenia experience auditory hallucinations (AH) that are resistant to treatment with antipsychotic medication and are associated with a significant decrease in the quality of life [Reference Shergill, Murray and McGuire1]. Non-invasive brain stimulation may provide a viable treatment option for this patient population. For example, transcranial magnetic stimulation (TMS) has reduced AH in some, but not all, clinical trials [Reference Cole, Green Bernacki, Helmer, Pinninti and O’Reardon2]. The reason for this heterogeneity in outcomes remains unknown. In addition, TMS is expensive and needs to be performed in the clinic. In contrast, transcranial current stimulation applies a weak electric current to the scalp and represents a potentially attractive alternative due to the low-cost and portability of the technology [Reference Philip, Nelson, Frohlich, Lim, Widge and Carpenter3]. Transcranial direct current stimulation (tDCS) significantly reduced AH symptoms measured by the Auditory Hallucination Rating Scale (AHRS) in a double-blind, sham controlled study [Reference Brunelin, Mondino, Gassab, Haesebaert, Gaha and Suaud-Chagny4]. In this study the anode (assumed to increase neural activity) and the cathode (assumed to decrease neural activity) were placed over the dorso-lateral prefrontal cortex (dl-PFC) to target hypoactivity and the temporo-parietal junction (TPJ) to target hyperactivity, respectively. However, in a recent study from our group with a similar design, active tDCS did not separate from sham on the AHRS score, due at least in part to a substantial placebo response to sham stimulation [Reference Frohlich, Burrello, Mellin, Cordle, Lustenberger and Gilmore5]. The reasons for this discrepancy are uncertain but it is notable that the outcomes of these two studies differ primarily in the magnitude of the placebo response. Other studies examining the efficacy of tDCS for the treatment of psychiatric disorders have found mixed results [Reference Kekic, Boysen, Campbell and Schmidt6, Reference Mervis, Capizzi, Boroda and MacDonald7].
Here, we pursued a novel approach and asked if targeting aberrant temporal organization of brain activity can modulate medication-refractory AH in schizophrenia patients. This approach was motivated by previous magnetoencephalography (MEG) and electroencephalography (EEG) studies that have noted changes in cortical oscillation patterns and functional connectivity specifically during AH [Reference Ropohl, Sperling, Elstner, Tomandl, Reulbach and Kaltenhauser8–Reference Lawrie, Buechel, Whalley, Frith, Friston and Johnstone13]. Transcranial alternating current stimulation (tACS) employs a weak electric current for non-invasive brain stimulation similar to tDCS. However, the stimulation current assumes a sine-wave waveform to target brain oscillations in a frequency-specific manner that engages and enhances naturally occurring cortical oscillations at the applied frequency [Reference Herrmann, Rach, Neuling and Struber14–Reference Vossen, Gross and Thut16]. It is hypothesized that alpha oscillations (8–12 Hz) are generated by thalamo-cortical and intra-cortical circuits [Reference Bollimunta, Mo, Schroeder and Ding17, Reference Hindriks and van Putten18] making this frequency band susceptible to cortical brain stimulation. For example, a study conducted by Herrmann et al. [Reference Zaehle, Rach and Herrmann19] successfully demonstrated the enhancement of alpha band oscillations using tACS and simultaneous EEG in healthy human participants.
In this study tACS was used to target alpha oscillations, given the deficits in resting state alpha band power in patients with chronic schizophrenia and first episode psychosis [Reference Goldstein, Peterson, Sanguinetti, Tononi and Ferrarelli20, Reference Sun, Tang, Lim, Wang, Tong and Li21]. To our knowledge, this represents the first study of tACS in psychiatric patients. This was a double-blind, sham controlled pilot study that compared tDCS, tACS, and sham stimulation in an across-participant design in patients with schizophrenia and persistent AH. Due to the novelty of tACS in schizophrenia, we performed this pilot feasibility study for which we formulated outcomes based on raw effect sizes. We hypothesized that tACS outperforms both tDCS and sham stimulation in terms of reduction of AHRS scores.
2. Methods
2.1 Participants
This study was conducted at the University of North Carolina at Chapel Hill (ClinicalTrials.gov NCT02360228) and was approved by the UNC Chapel Hill Institutional Review Board. Participants were recruited from local clinics both affiliated and unaffiliated to the university.
2.1.1 Inclusion criteria
Participants were diagnosed with schizophrenia or schizoaffective disorder (confirmed by the Structured Clinical Interview for DSM-IV Axis I Disorders); experience at least three AH per week (as determined by the frequency item in the AHRS); clinical stability as demonstrated by no hospitalizations for the past 3 months; stable dosing of antipsychotic medications (no changes in medication or doses for 1 month prior to enrollment); verified by chart review and/or discussion with the treating clinician to have treatment-persistent AH, defined as having ongoing AH during trials of at least 2 antipsychotic agents of adequate dose and duration; stable AH as demonstrated by having less than or equal to 20% change in AHRS scores across a 2 week interval during the screening period; ability to provide written informed consent.
2.1.2 Exclusion criteria
No concurrent anticonvulsant medications or daily treatment with benzodiazepines (limited as-needed use that was discontinued more than 48 h prior to a study session was allowed); no DSM-IV diagnosis of alcohol or substance abuse within the past month or DSM-IV diagnosis of alcohol or substance dependence within the past 6 months; no history of significant head injury or traumatic brain injury, prior brain surgery or any brain devices/implants, history of seizures, unstable medical illness, or pregnancy.
This study used a Data Safety Monitoring Board (DSMB), through the North Carolina Translational & Clinical Sciences Institute to ensure participant safety. Bi-annual reviews of blinded data and adverse events were submitted to the DSMB.
2.2 Study design
This study was a double blind, randomized, sham controlled pilot clinical trial, with three study arms (10 Hz 2 mA tACS, 2 mA tDCS, sham 10 Hz, Fig. 1A). The CONSORT diagram is included in the Supplementary materials. Participants were assigned to a code in chronological order based on the date of enrollment. Randomization was blocked such that all three groups had 8 participants. All authors and members of the research team were unaware of the group assignments until completion of the entire study. To administer stimulation in a double-blind fashion, we developed a custom built Matlab (Mathworks, Natick, MA) interface that controlled two Neuroconn DC Plus stimulators (Neuroconn Ltd., Ilmenau, Germany) via the “remote in” feature. This setup provided stimulation linked to the study code and recorded the applied waveform for subsequent verification by a group member not associated with this study.
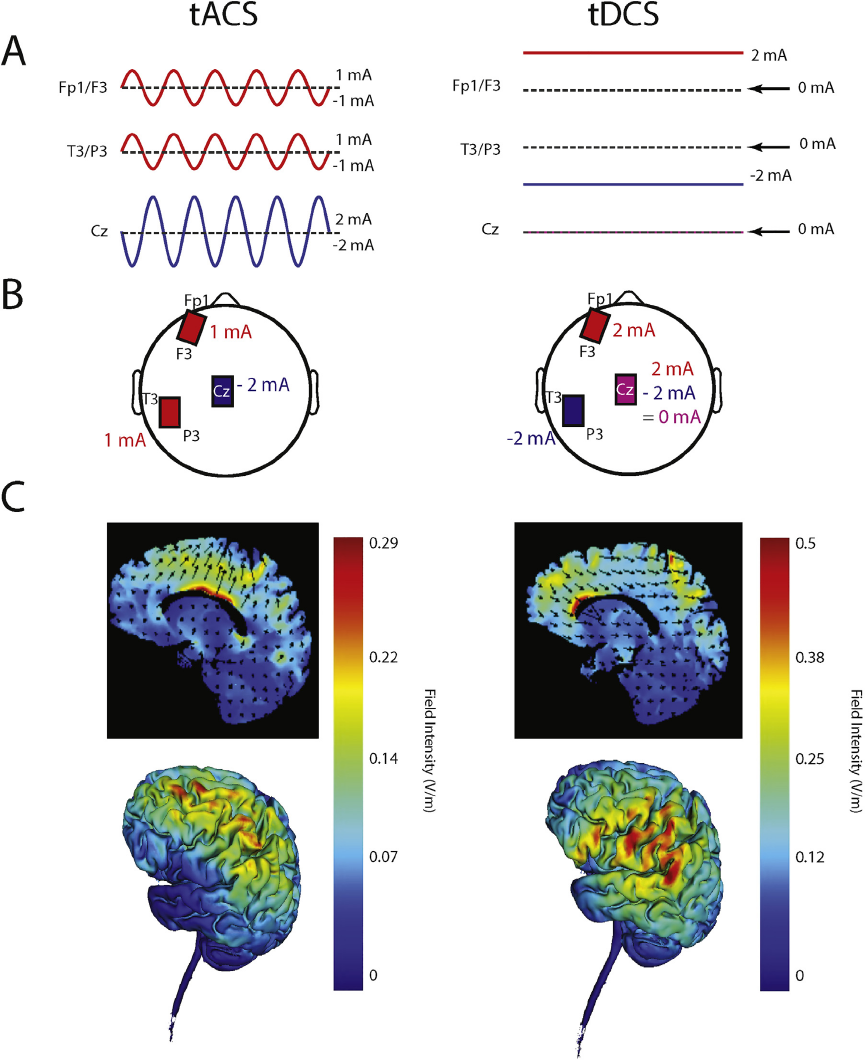
Fig. 1. A Symbolic representation of tACS (left) and tDCS (right) stimulation waveforms. B Location of electrodes on scalp. C Electric field simulation: 2D (top) and 3D (bottom) representation. Two stimulators were used, one connected to the electrode located over F3/Fp1, one connected to the electrode over T3/P3 and both connected to the Cz electrode.
2.3 Electrode montage
All three study arms used the same electrode montage to ensure blinding of the research personnel to the stimulation condition. Three electrodes with ten20 paste (Bio-Medical Instruments, Clinton Township, Michigan) were applied to the scalp. One 5 × 5 cm electrode was placed between F3 and Fp1 (left dl-PFC) and one 5 × 5 cm electrode was placed between T3 and P3 (left TPJ). A third “return/reference” electrode (5 × 7 cm) was placed over Cz. The resulting electric field distribution is shown in Fig. 1C. These figures were created using the Soterix Medical HD-Targets™ software (Soterix, New York, NY). After specifying the brain region and stimulation electrode location, the resulting simulation depicted the current flow through the head. The location of the stimulation electrodes was determined using the 10–20 placement system. The choice of location for the stimulation electrodes was motivated by previous tDCS studies for auditory hallucinations in schizophrenia [Reference Brunelin, Mondino, Gassab, Haesebaert, Gaha and Suaud-Chagny4, Reference Frohlich, Burrello, Mellin, Cordle, Lustenberger and Gilmore5]. In order to maintain the double blind nature of the study, the location of the tACS electrodes had to necessarily be the same. The stimulation amplitudes for the tACS condition were chosen in a way that the peak amplitude at third electrode at Cz never exceeded 2 mA.
2.4 Stimulation paradigms
Each participant completed twice-daily 20 min stimulation sessions, separated by 3 h, over 5 consecutive days. The 10 Hz tACS stimulation waveform was a sine-wave with a peak-to-peak amplitude of 2 mA. Both stimulators delivered a 2 mA peak-to-peak amplitude current between the frontal site and Cz and between the temporo-parietal site and Cz, respectively. For tDCS, the stimulation paradigm was +2 mA at the frontal site (F3/Fp1) and −2 mA at the temporo-parietal site (T3/P3). Sham stimulation included 10 s of ramp-in to 20 s of 10 Hz tACS, with a ramp-out of 10 s for a total of 40 s of stimulation. Ramping up and down the stimulation amplitude is a common approach for transcranial current stimulation to reduce skin sensation at stimulation onset. All three conditions used this procedure. The brief stimulation delivered as part of the sham stimulation is unlikely to be biologically active, since duration of tACS appears to be an important variable in terms of the modulation of brain activity after discontinuation of stimulation. Recently, brief periods of tACS were shown to be ineffective in modulating alpha oscillations in healthy human participants [Reference Strüber, Rach, Neuling and Herrmann22].
During stimulation, all participants were kept in the same relaxed state. Each participant was seated comfortably upright with their eyes open and asked to focus on the ReefScapes video (Undersea Productions, Queensland, Australia) directly in front of them. This video also served the purpose of helping to disguise the phosphenes induced by tACS.
2.5 Assessment of side effects
We administered an adverse effects stimulation questionnaire at the end of each 20 min stimulation session. This assessment was a Likert Scale and measured patient-reported headache, neck pain, scalp pain, tingling, itching, ringing/buzzing noise, burning sensation, local redness, sleepiness, trouble concentrating, improved mood, worsening of mood, dizziness, and flickering lights on a scale from 1 (absent) to 4 (severe). After the final stimulation session, participants were asked whether they thought they had received stimulation over the past week, and whether they thought their symptoms (AH) had improved.
2.6 Screening procedures
At the initial session, data was collected for each participant regarding demographics, handedness, their belief in the treatment (to understand susceptibility to placebo effect), and current medications which was verified with medical records or treatment providers. All available information from participants, medical records and providers was used to assess whether the AH met study criteria for medication-refractory. All assessments were administered by a researcher blind to the group assignment.
2.7 Analysis
For analysis, custom written scripts in R (R Foundation for Statistical Computing, Vienna, Austria) and SPSS software version 24.0 (IBM, Armonk, NY) were used. Libraries used in R included lme4 [Reference Bates, Machler, Bolker and Walker23] and pbkrtest [Reference Halekoh and Hojsgaard24]. Differences in demographics and characteristics of the three study arms and the severity of adverse effects were assessed with a one-way ANOVA. Pearson’s Correlation was used to assess possible susceptibility to placebo response using the Hunter Beliefs About Treatment Questionnaire, (used with the permission of the UCLA Laboratory of Brain, Behavior and Pharmacology,© 2005, 2017 UC Regents). Pearson’s Correlation was also used to examine correlation between age of participant and amount of symptom improvement. We used a linear mixed model analysis with fixed factors of “session” (baseline at day 1 of stimulation, day 5 of stimulation, 1 week follow up, and 1 month follow up) and “condition” (10 Hz tACS, tDCS, active sham 10 Hz), with random factor “participant” to account for repeat measures within participants. The interaction between “session” and “condition” is defined as the effects of “session” on “condition”. Kenward-Roger approximations were used to calculate P-values and perform F-tests for each factor and their interaction in the mixed model. Post-hoc analyses included paired t-tests to compare the four sessions, with a Bonferroni correction to account for multiple comparisons.
2.8 Outcome measures
The primary outcome measure was defined as the change in AH severity measured by the Auditory Hallucination Rating Scale (AHRS) from baseline (day 1 of stimulation) to day 5 of stimulation. The AHRS was administered before the 1st stimulation session on day 1 of stimulation and after the 10th stimulation on day 5 of stimulation. The AHRS was also administered at the one-week and the one-month follow up. We also included changes in the oscillatory structure of the resting state EEG data which was collected at 4 time points throughout the study (day 1, day 5, one week follow up and one month follow up). The EEG data will be reported in a separate manuscript.
Secondary outcomes included change in overall symptoms as measured by the Positive and Negative Syndrome Scale (PANSS) and changes in cognitive function as assessed by the Brief Assessment of Cognition in Schizophrenia (BACS) from baseline (day 1 of stimulation) to day 5 of stimulation. Both the PANSS and the BACS were administered before the 1st session of stimulation, after the 10th session of stimulation, and the one month follow up.
Data was also collected to determine whether participants believed their symptoms (AH) had improved after the 5 days of stimulation with a self-rating questionnaire. Participants were asked at day 5 of stimulation, the one week and one month follow up. During the stimulation week, questionnaires were administered immediately after stimulation, typically with a brief delay of less than 10 min to give the participant the chance to rinse out electrode paste from their hair.
3. Results
3.1 Study sample
Twenty-five clinically stable participants with a diagnosis of schizophrenia or schizoaffective disorder were randomized to one of three treatment arms (tACS, tDCS, or active sham). One participant randomized to the tACS group withdrew due to unrelated health concerns (instability of diabetes related symptoms not well controlled by the medication regimen during their participation in the study), a total of 24 participants completed the study (schizophrenia: 15, schizoaffective disorder: 7; 15 men, 7 women). Two participant datasets were not included in analysis due to instability of AH symptoms at baseline and non-adherence to antipsychotic medication during study participation that was unknown to the study team at the time of stimulation. In this paper, we present the analysis of the remaining 22 participants.
3.2 Safety and tolerability
Participants in all treatment arms tolerated stimulation well (Table 1). Mild tingling, itching and burning were reported by some participants. Some participants in both tACS and sham treatment groups reported the appearance of flashing lights, likely related to phosphenes that result from retinal stimulation or, potentially, stimulation of visual cortex by tACS. Group-averaged side-effect scores did not exceed a value of 2 (on a scale from 1 to 4) and there were no statistically significant differences between groups for the averaged total score (p =.31). There were no significant adverse events reported throughout the entirety of the study. All participants who completed the study were able to sit through all 10 stimulation sessions during the treatment week. Participants who decided not to continue with their participation in the study withdrew for reasons unrelated to the stimulation itself, as reported to study personnel.
Table 1 Stimulation Side Effect Scores.

tACS: transcranial alternating current stimulation; tDCS: transcranial direct current stimulation.
3.3 Auditory Hallucination Rating Scale (AHRS)
Effect size calculations of baseline to day 5 of stimulation resulted in the largest effect size for tACS (1.31), followed by the sham and the tDCS arms (1.06 and 0.17, respectively, Supplementary Table 1). The tACS group displayed a mean improvement of 15% (mean: −3.75 points, SD 2.87), the tDCS group displayed a mean improvement of 5% (mean: −1.14 points SD 6.15), and the sham group had a mean improvement of 10% (mean: −2.29 points SD 2.14) after 5 consecutive days of twice daily stimulation (Fig. 2, Table 2; individual trajectories in Fig. 3). An exploratory F-test with Kenward-Roger approximation analysis was conducted examining factors “session”, “condition” and the interaction between “session” and “condition”. These factors were compared across time points “baseline”, “day 5 of stimulation”, “1 week” and “1 month”. The interaction analysis examined whether there was an impact of “session” on “condition” for the AHRS scores. Factor “session” was found to be significant (F3,63 = 5.05, P <.01). Analyses conducted with a Bonferroni correction resulted in a difference between “baseline” and “1 month”. Factors “condition” and the interaction were not significant (Table 3).
Table 2 AHRS, PANSS and BACS scores.

tACS: transcranial alternating current stimulation; tDCS: transcranial direct current stimulation; AHRS: Auditory Hallucination Rating Scale; PANSS: Positive and Negative Syndrome Scale; BACS: Brief Assessment of Cognition in Schizophrenia.

Fig. 2. Normalized AHRS scores for tACS, tDCS and sham groups at baseline (before first stimulation), Day 5 (after the last stimulation), at the one week follow up (F1), and at the one month follow up (F2).

Fig. 3. Change in Auditory Hallucination Rating Scale (AHRS) score for each participant in transcranial alternating current stimulation (tACS) arm, transcranial direct current stimulation (tDCS) arm, or sham arm. AHRS scores were collected at baseline (before first stimulation), after stimulation (after last stimulation, at the one week follow up (F1), and the one month follow up (F2).
3.4 Positive and Negative Syndrome Scale (PANSS)
Effect size calculations of baseline to day 5 of stimulation show the largest effect size for tDCS (1.13), with tACS and sham having a small effect size (0.42 and 0.39, respectively, Supplementary Table 2). Effect size calculations were also conducted for the PANSS Hallucinations question. Results of this analysis showed that tACS had the largest effect size at baseline to day 5 of stimulation (0.48), while tDCS also had a small effect size (0.30) and sham had no effect size (0.00, Supplementary Table 3). An exploratory analysis of the PANSS total scores with a linear mixed model showed that factor “session” was significant (F-test with Kenward-Roger approximation with time points “baseline”, “1 week”, and “1 month”; F2,42 = 4.95, P =.01). We found no significant effects for the factor “condition” or the interaction for the PANSS total score. No significant effect was found for factors “session”, “condition”, or the interaction for the positive symptom subscale. No significant effect was found for “condition” or the interaction for the negative symptom subscale. However there was a significant effect found for factor “session” (F2,42 = 6.87, P =.003) for the negative symptom subscale. No significant effect was found for the factors “session”, “condition”, or the interaction for the general psychopathology subscale. No significant effect was found for the factors “session”, “condition”, or the interaction for the hallucination question in the positive symptom subscale. Results are presented in Table 2.
Table 3 F-test with Kenward-Roger Approximation Analysis Results.

AHRS: Auditory Hallucination Rating Scale; PANSS: Positive and Negative Syndrome Scale; BACS: Brief Assessment of Cognition in Schizophrenia.
3.5 Brief Assessment of Cognition in Schizophrenia (BACS)
Effect size calculations of baseline to day 5 of stimulation show the largest effect size for tDCS (1.50), with sham having a medium effect size (0.57) and tACS having a small effect size (0.26, Supplementary Table 4). In an exploratory analysis of the BACS total scores with a mixed linear model, we found a significant effect for factor “session” (F-test with Kenward-Roger approximation with the time points “baseline”, “1 week”, and “1 month”; F2,42 = 4.31, P =.02). No significant effect was found for factor “condition” or the interaction. Results can be seen in Table 2.
*Results from Participant Demographics, Participant Expectation of Outcomes, Self-Rating of Improvement, and Participant Age and AHRS Improvement can be found in Supplementary materials, along with the corresponding tables and figures.
4. Discussion
4.1 tDCS efficacy
Although several studies have examined tDCS, conclusive results have not emerged as to whether it represents an effective treatment of AH. Studies examining once daily tDCS, either over 5 consecutive days [Reference Brunelin, Mondino, Gassab, Haesebaert, Gaha and Suaud-Chagny4] or 3 consecutive weeks (15 total stimulation sessions) [Reference Fitzgerald, McQueen, Daskalakis and Hoy25], did not find significant changes in severity of AH. Studies conducted by Brunelin et al. [Reference Brunelin, Mondino, Gassab, Haesebaert, Gaha and Suaud-Chagny4] and Mondino et al. [Reference Mondino, Jardri, Suaud-Chagny, Saoud, Poulet and Brunelin26] looked at twice daily tDCS for the treatment of AH in patients with schizophrenia, both of which had positive results. In fact, one study found that the improvement in AH remained apparent through the 3 month follow up [Reference Brunelin, Mondino, Gassab, Haesebaert, Gaha and Suaud-Chagny4]. Interestingly, although the treatment duration used in our current study mirrored the treatment duration in [Reference Brunelin, Mondino, Gassab, Haesebaert, Gaha and Suaud-Chagny4], there were no sustained improvement in AH past the one week follow up in our study. Although the current trial was smaller, each study was double blinded and randomized, with similar study inclusion/exclusion criteria. It is not clear why these studies differ substantially in response to tDCS in the same population. These inconsistent findings indicate that the benefits of tDCS for persistent AH remain uncertain and will require further study.
4.2 tACS dosage
Although the stimulation paradigm mirrored the twice daily treatment in the Brunelin study [Reference Brunelin, Mondino, Gassab, Haesebaert, Gaha and Suaud-Chagny4] which found improvement in AH symptoms up to 3 months after the week of stimulation, the present study did not find benefits of tACS past the one week follow up in terms of raw effect sizes when compared to sham stimulation. The tACS and sham arms had similar effect sizes for the difference from “Day 1” to “1 Week Follow-Up”, while the sham arm had the largest effect size for difference from “Day 1” to “1 Month”. It is possible that the duration of treatment may need to be extended past the five day mark in order to sustain symptom improvement. Early TMS studies for depression administered stimulation in short periods for a total of two weeks [Reference Daskalakis, Levinson and Fitzgerald27], while it has become common practice for treatments to last in durations of four to six weeks [Reference Carpenter, Janicak, Aaronson, Boyadjis, Brock and Cook28]. Future studies may consider increasing the frequency or duration of treatment.
4.3 Effect sizes
Due to the small sample size used in this study, statistically significant results were not expected. Raw effect sizes were used to understand the effect that tACS, tDCS and sham had on AH symptoms. As shown, tACS had the largest effect size for the AHRS. However, tDCS had the largest effect sizes for the PANSS and BACS assessments. The divergence of improvement on these different scales may be explained by the multifaceted nature of the disease. Just as not all antipsychotics produce the same improvement in a specific symptom, the different stimulation paradigms may not affect the same symptom clusters. The large effect of for the BACS in the tDCS group is intriguing given the emerging literature of anodal tDCS applied to left prefrontal cortex for remediating cognitive deficits in patients with schizophrenia [Reference Mervis, Capizzi, Boroda and MacDonald7, Reference Hoy, Arnold, Emonson, Daskalakis and Fitzgerald29]. We performed an exploratory analysis to understand to what extent this effect on cognition was a result of the significantly different mean ages across the three groups. Across all participants, we found no significant correlation between age and the difference in BACS scores from day 1 to day 5 (r = 0.004, n = 22, p =.78). This suggests that the effect of tDCS on cognition is not uniquely an artefact of the uneven age distribution across the three study arms.
4.4 Blinding
In contrast to tDCS, tACS can induce the appearance of flashing lights, or phosphenes, that are caused by stimulation of the optical nerve [Reference Kar and Krekelberg30]. Several steps were taken in this study to ensure the participants were unable to distinguish whether they had been assigned to the tACS, tDCS or sham group. The sham was designed to mimic the skin sensations of the tACS group in order to blind participants assigned to this group (sometimes referred to as “active”). All participants were asked to sit still with their eyes open while a ReefScapes video was played on a projector screen in front of them which displayed underwater sceneries with tropical fish. The shifting sunlight of the water and the flashing colors of the fish served as a method of disguising the phosphenes induced by tACS and the sham. The blinding of this study was successful, with only one participant believing that they had not received stimulation.
4.5 Electric field distribution
We emphasize that the two groups that received active stimulation differed in the waveform of the stimulation used and also in terms of the spatial targeting. In designing this study, we prioritized the full blinding of study participants and researchers to the assigned stimulation condition. In this decision, we have accepted this limitation of the study.
4.6 Limitations
There are several limitations to this study that should be addressed. This was an exploratory study with a low number of participants. Due to this small sample size and 3 separate arms, the study was only powered to detect very large effect size changes. Future studies should examine tACS with a fully powered sample size. As this was a randomized treatment study with no blocking for age, the average age of the tACS treatment arm was significantly higher than the tDCS and the sham group. It is possible that the age of the tACS group diminished the effects of the stimulation, as neuroplasticity can decrease with age [Reference Spriggs, Cadwallader, Hamm, Tippett and Kirk31, Reference Goh and Park32]. As a result, our study may underestimate the effect of tACS. Future studies should examine whether a younger population may demonstrate enhanced effects with tACS. The choice of tACS was motivated by targeting long-range functional interactions between the targeted cortical sites. Future analysis of the EEG data and subsequent studies with more targeted neuroimaging to examine changes in structural and functional connectivity will be needed to delineate to what extent clinical improvement is indeed driven by connectivity changes. Lastly, we decided to position the electrodes on the same scalp locations for all groups to allow for successful blinding of the research personnel. As a result, the spatial electric field distribution for the tACS and tDCS group is not exactly identical.
5. Conclusions
In this first study to examine the effects of tACS on persistent auditory hallucinations in patients with schizophrenia, the results indicate a difference in symptom response between tACS and tDCS. Further research is needed with a larger sample size and longer treatment duration to better understand the treatment possibilities with tACS and the effects on auditory hallucinations in patients with schizophrenia.
Disclosures of interest
Flavio Frohlich is the lead inventor of an IP filed by UNC. The clinical studies performed in the Frohlich Lab have received a designation as conflict of interest with administrative considerations. Flavio Frohlich is the founder, CSO and majority owner of Pulvinar Neuro LLC. No devices from Pulvinar Neuro LLC were used and Pulvinar Neuro LLC played no role in this study.
Acknowledgements
The authors thank Kristen Sellers and Zhe Charles Zhou for their help with patient randomization for this study. Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number 1R21MH105574-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also partially supported by the UNC Department of Psychiatry and UNC School of Medicine. Caroline Lustenberger was supported by the Swiss National Science Foundation, grant P2EZP3-152214. The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001111. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eurpsy.2018.01.004.








Comments
No Comments have been published for this article.