Introduction
Helminth parasites of amphibians and reptiles have been studied for a long time, particularly from South American hosts. Research on this topic includes inventories dating back to Rudolphi (Reference Rudolphi1819) and significant contributions from Travassos in the early 20th century. Additionally, species compilations were also presented by Baker (Reference Baker1987) and Vicente et al. (Reference Vicente, Rodrigues, Gomes and Pinto1993).
In recent years, research on helminth parasites of the herpetofauna has significantly increased. For instance, over the past decade, Ávila and Silva (Reference Ávila and Silva2010), Campião et al. (Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Da Silva2014), González and Hamann (Reference González and Hamann2015a), Castillo et al. (Reference Castillo, Acosta, González-Rivas and Ramallo2020) and Luque and Chero (Reference Luque and Chero2022) have made efforts to compile comprehensive checklists of helminths found in amphibians and reptiles. However, studies on helminth parasites still reveal gaps, including a lack of published manuscripts, local journals that disappeared, insufficiently detailed species descriptions and a scarcity of updated information sources (Laakso et al. Reference Laakso, Matthias and Jahn2021; Macedo et al. Reference Macedo, Willkens, Silva, Gardner, Melo and Santos2023). These challenges are particularly pronounced in studies cataloguing many species and/or those with poorly/insufficient morphological characters for the described species, with no clear diagnostic characters, leading to fragmented information and complicating data analysis.
Nematodes of the family Molineidae are known to parasitize the digestive tract of vertebrates worldwide. There are 9 genera of molineid nematodes that specifically infect amphibians and reptiles: Schulzia Travassos 1937; Batrachostrongylus Yuen 1937; Poekilostrongylus Schmidt and Whittaker 1975; Trichoskrjabinia Travassos 1937; Typhlopsia Baruš and Coy Otero 1978; Kentropyxia Baker 1982; Bakeria Moravec and Sey 1986; Ragenema Ben Slimane, Chabaud and Durette-Desset 1996; and Oswaldocruzia Travassos 1917 (Ben Slimane et al. Reference Ben Slimane, Chabaud and Durette-Desset1996a). However, in neotropics, only species of the genera Kentropyxia, Oswaldocruzia, Poekilostrongylus, Schulzia and Typhlopsia have been reported (Ben Slimane et al. Reference Ben Slimane, Chabaud and Durette-Desset1996a).
The genus Schulzia is characterized by a genital cone with small irregular digitations and by the absence of true cuticular ridges in the synlophe, with simple undulations instead. Males possess single-pointed, bifurcated or trifurcated spicules and caudal bursa with well-developed dorsal lobe, with rays 10 and 9 notably longer. Females possess a distinct ovojector, with a vestibule consisting of 3 muscular branches, the first being a prolongation of the vagina vera; the caudal spine is absent (Durette-Desset et al. Reference Durette-Desset, Baker and Vaucher1985; Bursey et al. Reference Bursey, Goldberg and Telford2006a; González and Hamann, Reference González and Hamann2015b).
Poekilostrongylus is a monospecific genus, lacking both synlophe and alae. It has a long, thick dorsal ray forming a pointed dorsal lobe, and spicules divided into 3 branches: 1 long external-lateral and 2 short internal (Schmidt and Whittaker, Reference Schmidt and Whittaker1975; Ben Slimane et al. Reference Ben Slimane, Chabaud and Durette-Desset1996a). Typhlopsia is also a monospecific genus and also present a prominent pointed dorsal lobe but differs by the presence of synlophe with numerous crests (Baruš and Coy Otero, Reference Baruš and Coy Otero1978).
In Oswaldocruzia, the corona radiata is absent and spicule morphology varies among biogeographical groups: (a) Oriento-Ethiopian, with 2–3 indistinct tips; (b) Neo-Ethiopian, with numerous indistinct tips; (c) Caribbean, with 3 main branches subdivided into numerous tips; and (d) Holarctic and (e) Neotropical, both with 3 branches forming a ‘fork’, ‘blade’ and ‘shoe’ (Ben Slimane et al. Reference Ben Slimane, Chabaud and Durette-Desset1996a). The genus Kentropyxia is morphologically similar to Oswaldocruzia, but it can be readily distinguished by having a corona radiata encircling the oral aperture and unique 3-branched spicules: 1 pointed and 2 fan-like with numerous tips (Baker, Reference Baker1982; Willkens et al. Reference Willkens, Furtado, Santos and Melo2021, Reference Willkens, Jesus, Borges, Ribeiro, Costa-Campos, Santos and Melo2023).
To date, only a single identification key is available for nematode species within these genera (Ben Slimane et al. Reference Ben Slimane, Chabaud and Durette-Desset1996a). This key covers only 1 of the 3 known species of Kentropyxia and 1 of the 4 described species of Schulzia. For Oswaldocruzia, the genus with the greatest number of described species, the key includes 74 of the 90 species currently recognized worldwide and only 29 of the 43 species known from the Neotropical and Panamanian realms. Also, recent studies on these genera have focused primarily on species descriptions, morphology and taxonomy. Information regarding their distribution and host range remains scattered and incomplete, molecular data are absent for most species, and their phylogenetic relationships are yet unknown.
This paper reviews the nematodes of the family Molineidae from amphibians and reptiles. We also provide a checklist of nematodes from the Caribbean, Panamanian and Neotropical regions. Additionally, we compile data about molineid nematodes’ diversity, host range, available molecular data and known geographic distribution.
Materials and methods
Literature research and review
We conducted a bibliographic survey using information from various scientific databases (BioOne, ISI, Jstor, PubMed, Scielo, Scopus and Web of Science) and published monographs. Incomplete records, data of unpublished theses or scientific meetings were not included.
Our review focused on all publications that referred to molineid parasites of amphibians and reptiles, specifically the genera Kentropyxia, Oswaldocruzia, Poekilostrongylus, Schulzia and Typhlopsia (along with their synonyms). We examine literature of molineid taxa from Neotropical and Panamanian regions published between 1819 and 2025. It included original species descriptions, checklists (Ávila and Silva, Reference Ávila and Silva2010; Campião et al. Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Da Silva2014; González and Hamman, Reference González and Hamann2015a), taxonomic keys (Ben Slimane et al. Reference Ben Slimane, Chabaud and Durette-Desset1996a) and other publications with locality reports for all molineid taxa from amphibians and reptiles. Records not identified at species level were not included.
We also searched the published literature and the GenBank database for molecular data corresponding to each molineid taxon included in this study. Sequence data, when available, are listed by their accession numbers, including information regarding their respective hosts, localities and specific genomic region. Records not identified to species level were excluded.
Taxonomy and terminology
The taxonomy of molineid species and terminology used here for anatomical structures follows Ben Slimane et al. (Reference Ben Slimane, Chabaud and Durette-Desset1996a), Anderson et al. (Reference Anderson, Chabaud and Willmott2009) and Gibbons (Reference Gibbons2010). The host taxonomy follows Frost (Reference Frost2025) and Uetz et al. (Reference Uetz, Freed, Aguilar, Reyes and Hošek2025). Whenever the nomenclature of nematodes or hosts has changed and is not as in the original reference, the synonyms are given within parenthesis and discussed in the remarks. Some publications lack or do not specify information about hosts or localities; in these cases, it is also discussed in the remarks if the data is missing or given in posterior references.
We did not update the nomenclature for host species Rhinella marina (Linnaeus) and Rhinella margaritifera (Laurenti) groups which present cryptical diversity. Thus, for records in hosts of these species’ groups, we updated their nomenclature to sensu lato.
Biogeographical division and localities
We followed the biogeographical division proposed by Holt et al. (Reference Holt, Lessard, Borregaard, Fritz, Araújo, Dimitrov, Fabre, Graham, Graves, Jønsson, Nogués-Bravo, Wang, Whittaker, Fjeldså and Rahbek2013) and used the Panamanian and Neotropical realms. However, since the Caribbean molineid species constitute a very particular morphological group (see Ben Slimane et al. Reference Ben Slimane, Chabaud and Durette-Desset1996a), we present the Caribbean species as a separate group. Geographical records are given to the most specific level (municipalities and/or collection sites) according to the locations provided in the papers. When specific localities are absent, the geographical records are given only in the state/province/department or the country. The abbreviations for all localities cited in this paper are as follows:
Argentina (ARG): Astilleros (AST), Concepción del Bermejo (CON), Corrientes (COR), Ingeniero Juárez (INJ), Itaú River (ITR) and Jujuy Province (JUY), Las Lomitas (LOM) Taco Pozo (TAC).
Bahamas (BHS): Bahamian Field Station (BFS).
Bonaire (BES)
Brazil (BRA): Acre State (AC), Amazon State (AM) Alter do Chão (ALT), Angra dos Reis (ANG), Belém (BEL), Corcovado (CVD) Bocaina (BOC), Bodoquena (BOD), Botucatu (BOT), Cachimbó (CHB), Camisão Municipality (CAM), Carreiro Castanho (CCA), Caseara Municipality (CSR), Curitiba (CUR), Santa Teresa (SAN), Floresta Nacional de Caxiuanã (CAX), Gaviões (GAV), Ibitipoca State Park (IBT), Manaus State (MAN), Manguinhos (MGN), Marechal Floriano (MFL), Miguel Pereira Municipality (MGP), Novo Progresso Municipality (NVP), Parati (PRT), Petrópoles (PTR), Porto Walter (POW), Reserva Biológica de Sooretama (SOO), Reserva Extrativista Beija-Flor Brilho-de-Fogo (BBF), Rio Ituxi (ITX), Salobra (SLB), Miranda Municipality, Salvador (SVD), Salvaterra Municipality (SVT), Santa Candida Municipal Biological Reserve (SCM), Santarém (SNT), São Luís do Paraitinga (SLP), São Paulo City (SPC), São Sebastião Island (SSI), Serra do Mar State Park (SDM), Tijuca (TIJ), Três Barras Municipality (TRB), Vila Dois Rios (VDR) Ilha Grande, Volta Redonda (VTR), Rondônia State (RO) and Pernambuco State (PE), Petrolina (PET).
Chile (CHL): Fundo San Martin (FSM), La Picada (LPI), Mehuín (MEH), Puyehue (PUY).
Costa Rica (CRI): Área de Conservación Guanacaste (ACG), Cerro de La Muerte (CLM), Paso Ancho (PSA), San José (SJE), Tilarán (TIL), Heredia Province (HER), Punta Arenas Province (PUN), San José Province (SJP).
Cuba (CUB): Isla de la Juventud (JVT), Botany Garden (BTG), Cayo Guin (CGY), La Mariposa (MRP), La Vuelta (VLT), Las Cañas (CNS), Santiago de Cuba (STC).
Dominica (DMA): Saint Andrew Parish (SAP), Saint Patrick Parish (SPP) and Saint George Parish (SGP).
Dominican Republic (DOM): Baharona Province (BAH).
Ecuador (ECU): Hacienda Primavera (HPR), Reserva de Producción de Fauna Cuyabeno (RCU), San Lorenzo (SLZ), San Pablo (SPB), Santa Cecilia (SCE) and Sucumbiós (SUC).
French Guiana: Cayenne (CAY), Crique Grégoire (CRQ) and Paramana (PAR).
Guadeloupe (GLP).
Guyana (GUY): Rupununi District (RPD).
Haiti (HTI): Sud Department (SUD).
Mexico (MEX): Acapulco (ACA), Armeria (ARM), Benito Juárez (BJZ), Cerro del Tepezcuintle (CDT), Chairel (CHR), Champayán (CHM), Coquimatlán (COQ), El Carrizal (ELC), Huixtla (HXT), Julio Carrillo Ranch in Ticuizitán (JCR), Laguna Escondida in Los Tuxtlas (LGE), Las Palmas Road in Escuintla (LPR), Los Mogotes Laguna de Coyuca (LMO), Parque Estatal Lagunas de Yalahau (YLH), Rodolfo Figueroa Road (RFR), San Antonio (SAT), Teapa (TEA), Tres Palos (TRP), Vallarta-Las Palmas (VLP), Xtoloc (XTL).
Nicaragua (NIC): Isla Diamante (ISD).
Panama (PAN): Cerro Mali (CRM), El Aguacate (EAG), Panama City (PNC), San Blas Territory (SBT), Chiriqui Province (CHI), Canal Zone (CNZ).
Paraguay (PRY): Asunción (ASU), Chacoí (CHA), Estancia Estrellas (EES) and Remanso Castillo (RCA).
Peru (PER): Ucayali Region (UCA), Panguana (PNG), Parque Nacional Manu (PNM), Reserva Cuzco Amazónico (CUZ), San Martin (SMT), Yulitunqui (YUL), La Libertad Department (LBT), Ancash Department (ANC).
Puerto Rico (PRI): Cupey (CPY), Luquillo Forest (LUQ), El Yunque Mountain (YNQ).
Saint Vincent and the Grenadines (VCT): Soufriere Volcano (SFV).
Trinidad and Tobago (TTO): Saint Joseph (STJ).
Uruguay (URY): Montevideo (MTV).
Venezuela (VEN): Mérida (MER), Santa Rita (STR).
Results
We recorded 53 species of molineid nematodes that parasitize amphibians and reptiles across the Panamanian, Caribbean and Neotropical regions. Among these, the genus Oswaldocruzia was the most represented, with 44 recorded species. This was followed by 4 species of Schulzia, 3 of Kentropyxia, 1 of Poekilostrongylus and 1 of Typhlopsia.
Regarding the hosts, we recorded molineids from 160 species across 31 families of amphibians and reptiles. Amphibians were the most numerous, comprising 146 species from 17 families. The anuran families recorded included Alsodidae (4 species), Batrachylidae (1 species), Brachycephalidae (1 species), Bufonidae (24 species), Ceratophryidae (1 species), Craugastoridae (5 species), Cycloramphidae (1 species), Dendrobatidae (1 species), Eleutherodactylidae (12 species), Hemiphractidae (1 species), Hylidae (17 species), Leptodactylidae (12 species), Microhylidae (1 species), Odontophrynidae (1 species), Ranidae (7 species) and Strabomantidae (8 species). In addition, the order Caudata was represented by a single family, Plethodontidae, which included 2 species (supplementary Table S1).
Reptiles were represented by 74 host species from 14 families. Among the lizards, we identified the following families and their respective number of species in each family: Alopoglossidae (3 species), Anolidae (28 species), Gekkonidae (1 species), Gymnophthalmidae (4 species), Iguanidae (2 species), Leiocephalidae (4 species), Leiosauridae (3 species), Phrynosomatidae (1 species), Scincidae (2 species), Teiidae (5 species), Tropidophiidae (1 species) and Tropiduridae (4 species). Additionally, we recorded 2 families of snakes: Colubridae (4 species) and Typhlopidae (1 species).
List of species
Family Molineidae Durette-Desset and Chabaud, 1977
Genus Kentropyxia Baker, 1982
Kentropyxia bakeri Willkens, Jesus, Borges, Ribeiro, Costa-Campos, Santos and Melo, 2023
Type hosts and type locality: Boana wavrini (Parker) (CAX)
Other hosts and locality records: Boana boans (Linnaeus) (BBF), Boana geographica (Spix) (BBF)
Host family: Hylidae.
Distribution: Neotropical (Brazil).
References: Willkens et al. (Reference Willkens, Jesus, Borges, Ribeiro, Costa-Campos, Santos and Melo2023).
Kentropyxia hylae Feitosa, Furtado, Santos and Melo, 2015
Type host and type locality: Osteocephalus taurinus Steindachner (CAX)
Host family: Hylidae.
Distribution: Neotropical (Brazil).
Available molecular data: MK492922 from O. taurinus (CAX), partial COI sequence.
References: Feitosa et al. (Reference Feitosa, Furtado, Santos and Melo2015); Willkens et al. (Reference Willkens, Furtado, Santos and Melo2021).
Kentropyxia sauria Baker, 1982
Type hosts and type locality: Kentropyx calcarata Spix (BEL)
Other hosts and locality records: Kentropyx calcarata (NVP), Kentropyx pelviceps (Cope) (UCA), Cnemidophorus gramivagus McCrystal and Dixon (ALT)
Host family: Teiidae
Distribution: Neotropical (Brazil, Peru)
References: Baker (Reference Baker1982); Goldberg et al. (Reference Goldberg, Bursey, Caldwell, Vitt and Costa2007b); McAllister et al. (Reference McAllister, Bursey and Freed2010a); Goldberg et al. (Reference Goldberg, Bursey, Vitt and Arreola2013).
Genus Oswaldocruzia Travassos, 1917
Oswaldocruzia albareti Ben Slimane and Durette-Desset, 1996
Type host and type locality: Rhinella marina (= Bufo marinus) (CAY)
Other hosts and locality records: Boana calcarata (Troschel) (=Hyla calcarata) (SPB), Boana fasciata (Günther) (=Hyla fasciata) (SPB), Boana geographica (=Hyla geographica) (SPB), Leptodactylus pentadactylus (Laurenti) (CAY), Rhinella margaritifera (= Bufo typhonius) (CAY).
Host families: Bufonidae, Hylidae (Hylinae), Leptodactylidae.
Distribution: Neotropical (Ecuador and French Guyana).
References: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1996a).
Remarks: For O. albareti, the authors incorrectly described this species with type II bursa. However, line drawings clearly show these nematodes with type III bursa. In the discussion section, Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1996a) also compare this species with congeners of type III bursa.
Oswaldocruzia anolisi Baruš and Coy Otero, 1968
Type hosts and type locality: Anolis equestris Merrem (CGY)
Other hosts and locality records: Anolis allisoni Barbour (CU), Anolis allogus Barbour and Ramsden (CU), Anolis baracoae Schwartz (CU), Anolis bartschi (Cochran) (CU), Anolis bremeri Barbour (CU), Anolis equestris (CU), Anolis homolechis (Cope) (CU), Anolis loysiana (Cocteau) (CU), Anolis lucius Duméril and Bibron (CU), Anolis luteogularis Noble and Hassler (CU), Anolis porcus (Cope) (=Chamaleolis porcus) (CU), Anolis quadriocellifer Barbour and Ramsden (CU), Anolis sagrei Duméril and Bibron (CU), Caraiba andreae (Reinhardt and Lütken) (=Antillophis andreae) (CU), Cubophis cantherigerus (Bibron) (=Alsophis cantherigerus) (CU), Cyclura nubila (Gray) (CU), Leiocephalus carinatus Gray (CU), Leiocephalus cubensis (Gray) (CU), Leiocephalus macropus (Cope) (CU), Leiocephalus stictigaster Schwartz (CU), Pholidoscelis auberi (Cocteau) (=Ameiva auberi) (CU), Tropidophis pardalis (Gundlach) (CU)
Host family: Anolidae.
Distribution: Caribbean (Cuba).
References: Baruš and Coy Otero (Reference Baruš and Coy Otero1968), Baruš and Coy Otero (Reference Baruš and Coy Otero1969), Coy Otero (Reference Coy Otero1970), Baruš and Coy Otero (Reference Baruš and Coy Otero1978), Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995b).
Remarks: In the studies conducted by Baruš and Coy Otero (Reference Baruš and Coy Otero1969) and Coy Otero (Reference Coy Otero1970), O. anolisi was reported in many Iguanidae and Teiidae lizards from Cuba. In Baruš and Coy Otero (Reference Baruš and Coy Otero1978) this species was synonymized with O. lenteixeirai. However, Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995c) re-examined the type-material and considered O. anolisi a valid species. Despite this, all posterior literature placed the reports from Baruš and Coy Otero (Reference Baruš and Coy Otero1969) and Coy Otero (Reference Coy Otero1970) within O. lenteixeirai, despite recognizing O. anolisi as a separate species.
Oswaldocruzia bainae Ben Slimane and Durette-Desset, 1996
Type host and type locality: Anolis chrysolepis Duméril and Bibron (SPB)
Other hosts and locality records: Anolis biporcatus (Wiegmann) (PNP), Anolis fuscoauratus D’orbigny (SPB), Plica umbra (Linnaeus) (SUC)
Host families: Anolidae, Tropiduridae
Distribution: Neotropical (Ecuador) and Panamanian (Panama)
Reference: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1996b), Goldberg et al. (Reference Goldberg, Bursey and Vitt2009a), Bursey et al. (Reference Bursey, Goldberg and Telford2003).
Oswaldocruzia barusi Ben Slimanei and Durette-Desset, 1995
Type hosts and type locality: Peltophryne empusa Cope (=Bufo empusus) (CGY)
Other hosts and locality records: Peltophryne dunni (Barbour) (=Bufo longinasus dunni) (BTG, MRP, CNS, JVT, VLT), Peltophryne empusa (=Bufo empusus) (BTG, MRP, CNS, JVT, VLT), Peltophryne fustiger (Schwartz) (=Bufo peltacephalus fustiger) (BTG, MRP, CNS, JVT, VLT), Peltophryne gundlachi (Ruibal) (CUB), Peltophryne taladai (Schwartz) (=Bufo taladai) (BTG, MRP, CNS, JVT, VLT).
Host family: Bufonidae.
Distribution: Caribbean (Cuba).
References: Baruš (Reference Baruš1973), Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995c).
Remarks: Baruš (Reference Baruš1973) identified parasites of various Bufonid host species from Cuba as O. lenteixeirai. However, these parasites were morphologically distinct, and Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995c) allocated them to O. barusi.
Oswaldocruzia belenensis Santos, Giese, Maldonado Jr. and Lanfredi, 2008
Type host and type locality: Rhinella marina (= Chaunus marinus) (BEL)
Other hosts and locality records: Rhinella margaritifera (CAX), Rhinella marina (SVT),
Host family: Bufonidae.
Distribution: Neotropical (Brazil).
Available molecular data: MK492915 from R. margaritifera (CAX), partial COI sequence; MK492916 from R. marina (BEL), partial COI sequence; MK492917 from R. marina (BEL), partial COI sequence; and MK492918 from R. margaritifera (CAX), partial COI sequence.
References: Santos et al. (Reference Santos, Giese, Maldonado and Lanfredi2008), Willkens et al. (Reference Willkens, Furtado, Santos and Melo2021).
Oswaldocruzia benslimanei Durette-Desset, Alves dos Anjos and Vrcibradic, 2006
Type hosts and type locality: Enyalius bilineatus (Duméril and Bibron) (MFL)
Host family: Leiosauridae
Distribution: Neotropical (Brazil)
References: Durette-Desset et al. (Reference Durette-Desset, Alves Dos Anjos and Vrcibradic2006).
Oswaldocruzia bonsi Ben Slimane and Durette-Desset, 1993
Type host and type locality: Bolitoglossa equatoriana Brame and Wake (SPB)
Other hosts and locality records: Oreobates quixensis Jiménez de la Espada (=Ischnocnema quixensis) (SPB)
Host families: Plethodontidae, Strabomantidae
Distribution: Neotropical (Ecuador)
References: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1993).
Oswaldocruzia brasiliensis Lent and Freitas, 1935
Type hosts and type locality: Palusophis bifossatus (Raddi) (= Drymobius bifossatus, Dryadophis bifossatus, Mastigodryas bifossatus) (MGN)
Other hosts and locality records: Copeoglossum nigropunctatum (Spix) (=Mabuya nigropunctata) (UCA), Erythrolamprus miliaris (Linnaeus) (=Liophis miliaris) (MGN), Hemidactylus mabouia (Moreau de Jonnès) (RJ)
Host family: Colubridae (Colubrinae, Dipsadinae)
Distribution: Neotropical (Brazil, Peru)
References: Lent and Freitas (Reference Lent and Freitas1935), Freitas (Reference Freitas1956), Rodrigues and Santos (Reference Rodrigues and Santos1974), Vicente et al. (Reference Vicente, Rodrigues, Gomes and Pinto1993) and McAllister et al. (Reference McAllister, Bursey and Freed2010a).
Oswaldocruzia brevispicula Moravec and Kaiser, 1995
Type hosts and type locality: Pristimantis shrevei (Schwartz) (= Eleutherodactylus shrevei) (SFV)
Host family: Strabomantidae
Distribution: Caribbean (Saint Vincent and the Grenadines)
References: Moravec and Kaiser (Reference Moravec and Kaiser1995)
Oswaldocruzia burseyi Durette-Desset, Alves dos Anjos and Vrcibradic, 2006
Type hosts and type locality: Enyalius perditus Jackson (SSI)
Other hosts and locality records: Enyalius perditus (SCM)
Host family: Leiosauridae
Distribution: Neotropical (Brazil)
References: Durette-Desset et al. (Reference Durette-Desset, Alves Dos Anjos and Vrcibradic2006), Vrcibradic et al. (Reference Vrcibradic, Alves Dos Anjos, Vicente and Bursey2008), Barreto-Lima et al. (Reference Barreto-Lima, Toledo and Alves Dos Anjos2012)
Oswaldocruzia cartagoensis Bursey and Goldberg, Reference Bursey and Goldberg2011
Type hosts and type locality: Bolitoglossa subpalmata (Boulenger) (CLM)
Host family: Plethodontidae
Distribution: Panamanian (Costa Rica)
References: Bursey and Goldberg (Reference Bursey and Goldberg2011)
Oswaldocruzia cassonei Ben Slimane and Durette-Desset, 1996
Type host and type locality: Pristimantis lanthanites (Lynch) (=Eleutherodactylus lanthanites) (SPB)
Other hosts and locality records: Pristimantis altamazonicus (Barbour and Dunn) (=Eleutherodactylus altamazonicus) (SPB), Pristimantis conspicillatus (Günther) (=Eleutherodactylus conspicillatus) (SPB), Pristimantis diadematus (Jiménez de la Espada) (=Eleutherodactylus diadematus) (SPB)
Host family: Strabomantidae
Distribution: Neotropical (Ecuador)
Reference: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1996b)
Oswaldocruzia chabaudi Ben Slimane and Durette-Desset, 1996
Type host and type locality: Boana boans (=Hyla boans) (SPB)
Other hosts and locality records: Boana fasciata (=Hyla fasciata) (SPB), Boana geographica (=Hyla geographica) (SPB, CAX), Boana wavrini (CAX), Itapotihyla langsdorffiii (Duméril & Bibron) (SAN). Osteocephalus cabrerai (BBF)
Host family: Hylidae (Hylinae)
Distribution: Neotropical (Ecuador, Brazil)
Available molecular data: MK492919 from Boana wavrini (CAX), partial COI sequence; and MK492920 from B. geographica (CAX), partial COI sequence.
References: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1996a), Willkens et al. (Reference Willkens, Furtado, Santos and Melo2021), Neves et al. (Reference Neves, Cardoso, Rebêlo, Silva Félix, Machado, Costa-Campos, Santos and Melo2024), Vrcibradic et al. (Reference Vrcibradic, Melo and Campião2025).
Oswaldocruzia chambrieri Ben Slimane and Durette-Desset, 1993
Type host and type locality: Rhinella margaritifera (=Bufo typhonius) (SPB)
Other hosts and locality records: Amazophrynella bokermanni (Izecksohn) (CAX), Rhinella margaritifera (CAX, HPR)
Host family: Bufonidae
Distribution: Neotropical (Ecuador, Brazil)
Available molecular data: MK492921 from A. bokermanni (CAX), partial COI sequence; and KU980934 from R. margaritifera (CAX), partial COI sequence.
References: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1993), Willkens et al. (Reference Willkens, Maldonado, Santos, Maschio and Melo2016), Willkens et al. (Reference Willkens, Furtado, Santos and Melo2021)
Oswaldocruzia costaricensis Bursey and Goldberg, 2005
Type hosts and type locality: Lithobates cf. forreri (Boulenger) (=Rana cf. forreri) (GUA)
Other hosts and locality records: Agalychnis callidryas (Cope) (CR), Anolis aquaticus (Taylor) (ACG), Anolis lionotus Cope (ACG), Craugastor fitzingeri (Schmidt) (CR, ACG), Craugastor gollmeri (Peters) (CR), Craugastor ranoides (Cope) (CR), Craugastor taurus (Taylor) (CR), Ctenosaura quinquecarinata (Gray) (ACG), Incilius coccifer (Cope) (ACG), Incilius luetkenii (Boulenger) (ACG), Lithobates taylori (Smith) (CR), Lithobates warszewitschii (Schmidt) (GUA, PUN, SJP), Rhaebo haematiticus (Cope) (ACG), Rhinella marina (ACG), Sceloporus variabilis Wiegmann (ACG), Scinax elaeochroa (Cope) (CR), Smilisca phaeota (Cope) (CR).
Host family: Anolidae, Bufonidae, Craugastoridae, Hylidae, Iguanidae, Phrynosomatidae, Ranidae.
Distribution: Panamanian (Costa Rica)
References: Bursey and Goldberg (Reference Bursey and Goldberg2005), Bursey and Goldberg, (Reference Bursey and Goldberg2007), Bursey et al. (Reference Bursey, Goldberg and Telford2007a), Goldberg and Bursey (Reference Bursey, Goldberg and Telford2007a), Goldberg and Bursey (Reference Goldberg and Bursey2008a), Goldberg and Bursey (Reference Goldberg and Bursey2008b), Bursey (Reference Bursey2010), Bursey and Brooks (Reference Bursey and Brooks2010)
Oswaldocruzia dlouhyi Ben Slimane and Durette-Desset 1995
Type hosts and type locality: Rhinella sp. (=Bufo sp.) (GAV)
Host family: Bufonidae
Distribution: Neotropical (Brazil)
References: Ben Slimane and Durette-Desset 1995b
Oswaldocruzia dorsarmata Ben Slimane, Durette-Desset and Chabaud, 1995
Type hosts and type locality: Anolis marmoratus Duméril and Bibron (GLP)
Host family: Anolidae
Distribution: Caribbean (Guadeloupe)
References: Ben Slimane et al. (Reference Ben Slimane, Durette-Desset and Chabaud1995a)
Oswaldocruzia franciscoensis Vieira, Pereira, Ribeiro, Oliveira, Silva, Muniz-Pereira and Felix-Nascimento, 2023
Type hosts and type locality: Leptodactylus macrosternum Miranda-Ribeiro (PET)
Host family: Leptodactylidae
Distribution: Neotropical (Brazil)
References: Vieira et al. (Reference Vieira, Pereira, Ribeiro, Oliveira, Silva, Muniz-Pereira and Felix-Nascimento2023)
Oswaldocruzia fredi Durette-Desset, Alves dos Anjos and Vrcibradic, 2006
Type hosts and type locality: Enyalius iheringii Boulenger (SSI)
Host family: Leiosauridae
Distribution: Neotropical (Brazil)
References: Durette-Desset et al. (Reference Durette-Desset, Alves Dos Anjos and Vrcibradic2006); Vrcibradic et al. (Reference Vrcibradic, Alves Dos Anjos, Vicente and Bursey2008),
Oswaldocruzia jeanbarti Ben Slimane, Durette-Desset and Chabaud, 1995
Type hosts and type locality: Anolis marmoratus (GLP)
Host family: Anolidae
Distribution: Caribbean (Guadeloupe)
References: Ben Slimane et al. (Reference Ben Slimane, Durette-Desset and Chabaud1995a)
Oswaldocruzia lamotheargumedoi Ruiz-Torres, García-Prieto, Osorio-Sarabia and Violante-González, 2013
Type hosts and type locality: Rhinella marina (LMO)
Host family: Bufonidae
Distribution: Panamanian (Mexico)
References: Ruiz-Torres et al. (Reference Ruiz-Torres, García-Prieto, Osorio-Sarabia and Violante-González2013)
Oswaldocruzia lanfrediae Larrat, Melo, Gomes, Willkens and Santos, 2018
Type hosts and type locality: Leptodactylus paraensis Heyer (CAX)
Host family: Leptodactylidae
Distribution: Neotropical (Brazil)
References: Larrat et al. (Reference Larrat, Melo, Gomes, Willkens and Santos2018)
Oswaldocruzia lenteixeirai Vigueras, 1938
Type hosts and type locality: Osteopilus septentrionalis (Duméril and Bibron) (=Hyla insulsa, Hyla septentrionalis) (CUB)
Other hosts and locality records: Anolis armouri (Cochran) (SUD), Anolis bahorucoensis Noble and Hassler (BAH), Anolis bonairensis Ruthven (BES), Aquarana catesbeiana Shaw (=Rana catesbeiana) (CUB), Eleutherodactylus atkinsi Dunn (CUB), Eleutherodactylus coqui Thomas (LUQ), Eleutherodactylus cuneatus (Cope) (= Eleutherodactylus sierramaestrae) (CUB), Eleutherodactylus dimidiatus (Cope) (CUB), Eleutherodactylus goini Schwartz (CUB), Eleutherodactylus greyi Dunn (CUB), Eleutherodactylus klinikowskii Schwartz (CUB), Eleutherodactylus pinarensis Dunn (CUB), Eleutherodactylus planirostris (Cope) (CUB), Eleutherodactylus portoricensis Schmidt (CPY), Eleutherodactylus zeus Schwartz (CUB), Eleutherodactylus zugi Schwartz (CUB), Osteopilus septentrionalis (Duméril and Bibron, 1841) (BFS)
Host families: Hylidae (Hylinae), Anolidae, Ranidae, Eleutherodactylidae,
Distribution: Caribbean (Bahamas, Bonaire, Cuba, Dominican Republic, Haiti, Puerto Rico)
References: Vigueras (Reference Vigueras, Silva and Travassos1938), Baruš and Moravec (Reference Baruš and Moravec1967), Baruš and Coy Otero (Reference Baruš and Coy Otero1968), Baruš (Reference Baruš1972), Baruš (Reference Baruš1973), Schmidt and Whittaker (Reference Schmidt and Whittaker1975), Baruš and Coy Otero (Reference Baruš and Coy Otero1978), Coy Otero and Baruš (Reference Coy Otero and Baruš1979), Martinez et al. (Reference Martinez, Coy Otero and Ventosa1982), Coy Otero and Ventosa (Reference Coy Otero and Ventosa1984), Goldberg et al. (Reference Goldberg, Bursey and Tawil1994), Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995c), Moravec and Kaiser (Reference Moravec and Kaiser1995), Goldberg et al. (Reference Goldberg, Bursey and Cheam1996), Goldberg et al. (Reference Goldberg, Bursey and Cheam1998), Goldberg and Bursey (Reference Goldberg and Bursey2019)
Remarks: Prokopic (Reference Prokopic1957) reported O. lenteixeirai in Rana dalmatina Fitzinger from the former Czechoslovakia; however, Moravec and Vojtková (Reference Moravec and Vojtková1975) later identified this as misidentification. Baruš and Moravec (Reference Baruš and Moravec1967) redescribed O. lenteixeirai using specimens from the type host and type locality, but these were subsequently transferred to O. moraveci by Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995c). Additionally, O. lenteixeirai identified by Baruš (Reference Baruš1973) in various bufonid host species from Cuba were assigned by Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995c) to O. barusi. Similarly, O. lenteixeirai identified by Goldberg and Bursey (Reference Goldberg and Bursey1996) were redefined to O. marechali by Goldberg et al. (Reference Goldberg, Bursey and Cheam1997).
Baruš and Coy Otero (Reference Baruš and Coy Otero1978) synonymized O. anolisi with O. lenteixeirai, a classification later considered inaccurate by Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995c). However, all subsequent literature continued to consider the reports of O. anolisi from Baruš and Coy Otero (Reference Baruš and Coy Otero1969) and Coy Otero (Reference Coy Otero1970) in numerous Iguanidae and Teiidae lizards in Cuba as belonging to O. lenteixeirai, even while they treated as separate species with a distinct diagnosis. Thus, we have decided to categorize all reports of nematodes previously identified as O. anolisi separately from this species.
Oswaldocruzia lescurei Ben Slimane and Durette-Desset, 1996
Type host and type locality: Rhinella margaritifera (= Bufo typhonius) (PAR)
Other hosts and locality records: Incilius marmoreus (Wiegmann) (CDT), Rhinella margaritifera (= Bufo typhonius) (PAR, CRQ)
Host family: Bufonidae
Distribution: Neotropical (French Guyana) and Panamanian (Mexico)
References: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1996a), Trejo-Meléndez et al. (Reference Trejo-Meléndez, Osorio-Sarabia, García-Prieto and Mata-López2019).
Remarks: For several years, the definition of Rana typhonia was unclear, leading to confusion with other species names, such as Bufo typhonius, which has been associated with different amphibians from the families Bufonidae and Hylidae. Trejo-Meléndez et al. (Reference Trejo-Meléndez, Osorio-Sarabia, García-Prieto and Mata-López2019) consider Bufo typhonius from Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1996a) as a synonym of Trachycephalus typhonius (Hylidae). However, Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1996a) treated these hosts as bufonids, not hylids. Therefore, we have decided to update this classification to R. margaritifera.
Oswaldocruzia lopesi Freitas and Lent, 1938
Type hosts and type locality: Leptodactylus latrans (Steffen) (=Leptodactylus ocellatus) (MGN)
Other hosts and locality records: Ameerega picta (Tschudi) (=Epipedobates pictus) (CUZ), Boana fasciata (=Hyla fasciata) (CUZ), Hamptophryne boliviana (Parker) (CUZ), Leptodactylus bolivianus Boulenger (CUZ), Leptodactylus latrans (=Leptodactylus ocellatus) (CAM, SLB, SOO, MTV), Pristimantis fenestratus (Steindachner) (CUZ), Rhaebo glaberrimus (Günther) (CUZ), Rhinella icterica (Spix) (=Bufo ictericus) (MGP), Rhinella margaritifera (CUZ), Rhinella marina (=Bufo marinus) (MAN), Trachycephalus coriaceus (Peters) (=Phrynohyas coriacea) (CUZ)
Host family: Bufonidae
Distribution: Neotropical (Brazil, Peru, Uruguay)
References: Freitas and Lent (Reference Freitas and Lent1938), Travassos et al. (Reference Travassos, Freitas and Lent1939), Freitas (Reference Freitas1956), Bursey et al. (Reference Bursey, Goldberg and Parmelee2001), Gonçalves et al. (Reference Gonçalves, Vicente and Pinto2002), Luque et al. (Reference Luque, Martins and Tavares2005)
Oswaldocruzia manuensis Guerrero, 2013
Type hosts and type locality: Rhinella marina (PNM)
Host family: Bufonidae
Distribution: Neotropical (Peru)
References: Guerrero (Reference Guerrero2013)
Oswaldocruzia marechali Ben Slimane, Durette-Desset and Chabaud, 1995
Type hosts and type locality: Anolis marmoratus (GLP)
Other hosts and locality records: Anolis oculatus (Cope) (SAP, SPP, SGP)
Host family: Anolidae
Distribution: Caribbean (Dominica and Guadeloupe)
References: Ben Slimane et al. (Reference Ben Slimane, Durette-Desset and Chabaud1995a), Goldberg and Bursey (Reference Goldberg and Bursey1996), Goldberg et al. (Reference Goldberg, Bursey and Cheam1997)
Remarks: This was previously identified as O. lenteixeirai by Goldberg and Bursey (Reference Goldberg and Bursey1996) as redefined by Goldberg et al. (Reference Goldberg, Bursey and Cheam1997).
Oswaldocruzia mauleoni Ben Slimane, Durette-Desset and Chabaud, 1995
Type hosts and type locality: Anolis marmoratus (GLP)
Host family: Anolidae
Distribution: Caribbean (Guadeloupe)
References: Ben Slimane et al. (Reference Ben Slimane, Durette-Desset and Chabaud1995a)
Oswaldocruzia mazzai Travassos, 1935
Type host and type locality: Rhinella diptycha (= Bufo marinus) (JUY)
Other hosts and locality records: Anolis brasiliensis Vanzolini and Williams (=Anolis nitens) (RPD), Boana raniceps (Cope) (=Hypsiboas raniceps, Hyla spegazzini), Leptodactylus bufonius Boulenger (SLB, BOD), Leptodactylus fuscus (Schneider) (CSR), Leptodactylus latrans (= Leptodactylus ocellatus, Cystignathus ocellatus) (CSR), Leptodactylus mystaceus (Spix) (SCE), Leptodactylus pentadactylus (SCE), Leptodactylus pustulatus (Peters) (CSR), Pristimantis altamazonicus (=Eleutherodactylus altamazonicus) (SCE), Rhinella diptycha (Cope) (=Bufo marinus, Rhinella schneideri, Bufo paracnemis) (SLB, BOD), Rhinella icterica (=Bufo ictericus) (MGP), Rhinella margaritifera (=Bufo typhonius) (SCE, MAN, RPD), Rhinella marina (=Bufo marinus) (SPB, MAN, RPD), Rhinella major (TAC), Tropidurus torquatus (Wied-Neuwied) (CHB, BOD).
Host families: Anolidae, Bufonidae, Hylidae (Hylinae), Leptodactylidae, Strabomantidae, Tropiduridae.
Distribution: Neotropical (Argentina, Brazil, Ecuador, Guyana)
Reference: Travassos (Reference Travassos1935); Travassos (Reference Travassos1937); Travassos et al. (Reference Travassos, Freitas and Lent1939); Lent et al. (Reference Lent, Teixeira de Freitas and Proença1946); Freitas (Reference Freitas1956); Masi-Pallares and Maciel (Reference Masi-Pallares and Maciel1974); Dyer and Altig (Reference Dyer and Altig1977); Vicente et al. (Reference Vicente, Rodrigues, Gomes and Pinto1991); Vicente et al. (Reference Vicente, Rodrigues, Gomes and Pinto1993); Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995a), Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995b); Gonçalves et al. (Reference Gonçalves, Vicente and Pinto2002); Luque et al. (Reference Luque, Martins and Tavares2005); Goldberg et al. (Reference Goldberg, Bursey, Caldwell and Shepard2009b); McAllister et al. (Reference McAllister, Bursey and Freed2010b), Hamann and González (Reference Hamann and González2015)
Remarks: This species was first described by Travassos (Reference Travassos1935) in Bufo sp., which was later updated to R. marina (=Bufo marinus) in Travassos et al. (Reference Travassos, Freitas and Lent1939). However, since this species does not occur in Argentina, we recognize R. diptycha, a member of the R. marina group that has known distribution in Argentina, as the type host.
The specimens reported by Lent et al. (Reference Lent, Teixeira de Freitas and Proença1946) in amphibians from Paraguay were later identified as O. proencai Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995b) and were not considered here. Gonçalves et al. (Reference Gonçalves, Vicente and Pinto2002) argued that Freitas (Reference Freitas1956) incorrectly referred to B. parachinemis as a synonym of B. marinus. However, it did not seem to us that Freitas (Reference Freitas1956) considers both names as synonyms; instead, he highlights the correction given by Travassos and Freitas (Reference Travassos and Freitas1942) of the host’s name presented in Travassos (Reference Travassos1937). Freitas (Reference Freitas1956) also synonymized O. subauricularis sensu Travassos et al. (Reference Travassos, Freitas and Lent1939) to O. mazzai.
Oswaldocruzia moraveci Ben Slimanei and Durette-Desset, 1995
Type hosts and type locality: Osteopilus septentrionalis (=Hyla insula) (CGY)
Host family: Bufonidae
Distribution: Caribbean (Cuba)
References: Baruš and Coy Otero (Reference Baruš and Coy Otero1968), Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995c)
Remarks: Baruš and Moravec (1968) reported specimens of O. lenteixeirai from the same host and locality of the type material. Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995c) re-examined these specimens and pointed out several characters that justified the proposal of O. moraveci (= O. lenteixeirai sensu Baruš and Moravec, Reference Baruš and Moravec1967, nec; Vigueras, Reference Vigueras, Silva and Travassos1938).
Oswaldocruzia neghmei Puga, 1981
Type hosts and type locality: Hylorina sylvatica Bell (PUY)
Other hosts and locality records: Alsodes vittatus (Philippi) (=Eupsophus vittatus) (FSM, MEH, LPI), Eupsophus migueli Formas (MEH), Eupsophus roseus (Duméril and Bibron) (FSM), Eupsophus vertebralis Grandison (FSM, MEH)
Host family: Batrachylidae, Alsodidae
Distribution: Neotropical (Chile)
References: Puga (Reference Puga1980), Puga (Reference Puga1981), Puga (Reference Puga1994)
Oswaldocruzia nicaraguensis Bursey, Goldberg and Vitt, 2006
Type hosts and type locality: Holcosus festivus (Lichtenstein and Martens) (=Ameiva festiva) (ISD)
Other hosts and locality records: Anolis biporcatus (PUN, EAG), Anolis capito Peters (=Norops capito) (RSJ, PNP), Anolis humilis Peters (ISD, HER, CHI), Anolis limifrons Cope (ISD, HER, CNZ, SBT), Anolis lionotus (ISD, PNP), Scincella cherriei (Cope) (=Sphenomorphus cherriei) (CR)
Host family: Teiidae, Scincidae, Anolidae
Distribution: Panamanian (Nicaragua, Costa Rica, Panama)
References: Bursey et al. (Reference Bursey, Goldberg and Vitt2006b), Bursey et al. (Reference Bursey, Goldberg and Vitt2007b), Bursey et al. (Reference Bursey, Goldberg and Telford2007a), Goldberg et al. (Reference Goldberg, Bursey and Vitt2010), Bursey et al. (Reference Bursey, Goldberg, Telford and Vitt2012)
Oswaldocruzia panamaensis Bursey, Goldberg and Telford Jr. 2007
Type hosts and type locality: Loxopholis rugiceps Cope (=Leposoma rugiceps) (PNC)
Host family: Gymnophthalmidae
Distribution: Panamanian (Panama)
References: Bursey et al. (Reference Bursey, Goldberg and Telford2007a)
Oswaldocruzia peruensis Ben Slimane, Verhaagh and Durette-Desset, 1995
Type hosts and type locality: Stenocercus roseiventris D’orbigny In Duméril and Bibron (PNG)
Other hosts and locality records: Anolis punctatus Daudin (CUZ), Stenocercus roseiventris (CUZ)
Host families: Tropiduridae, Anolidae
Distribution: Neotropical (Peru)
References: Ben Slimane et al. (Reference Ben Slimane, Verhaagh and Durette-Desset1995b), Bursey et al. (Reference Bursey, Goldberg and Parmelee2005)
Oswaldocruzia petterae Ben Slimane and Durette-Desset, 1996
Type host and type locality: Leptodactylus pentadactylus (SPB)
Host family: Leptodactylidae
Distribution: Neotropical (Ecuador)
Reference: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1996b)
Oswaldocruzia proencai Ben Slimane and Durette-Desset, 1995
Type host and type locality: Rhinella diptycha (=Bufo paracnemis, Rhinella schneideri) (PRY)
Other hosts and locality records: Gastrotheca peruana (Boulenger) (LBT, ANC), Leptodactylus bufonius (ASU, CHA, RCA), Leptodactylus fuscus (CSR), Leptodactylus latrans (= Leptodactylus ocellatus) (ASU, CHA, RCA), Leptodactylus pustulatus (CSR), Rhinella arenarum (Hensel) (= Chaunus arenarum) (AST, ITR), Rhinella diptycha (=Rhinella schneideri) (ASU, CHA, RCA, ITR, COR), Rhinella margaritifera (=Bufo typhonius) (UCA)
Host families: Bufonidae, Hemiphractidae, Leptodactylidae
Distribution: Neotropical (Argentina, Brazil, Paraguay, Peru)
References: Lent et al. (Reference Lent, Teixeira de Freitas and Proença1946); Vicente et al. (Reference Vicente, Rodrigues, Gomes and Pinto1991), Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995b), Ramallo et al. (Reference Ramallo, Bursey and Goldberg2007a), Ramallo et al. (Reference Ramallo, Bursey and Goldberg2007b), González and Hamann (Reference González and Hamann2008), Goldberg et al. (Reference Goldberg, Bursey, Caldwell and Shepard2009b) and McAllister et al. (Reference McAllister, Bursey and Freed2010a), Gómez et al. (Reference Gómez, Sánchez, Ñacari and Espínola-Novelo2020)
Remarks: Lent et al. (Reference Lent, Teixeira de Freitas and Proença1946) reported specimens of O. mazzai in Rhinella diptycha (=Bufo paracnemis), Leptodactylus latrans (= Leptodactylus ocellatus) and L. bufonius from Paraguay. However, Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995b), after a further examination of those specimens, proposed a new species, reassigning those nematodes to O. proencai.
Oswaldocruzia subauricularis (Rudolphi, Reference Rudolphi1819) Travassos, 1917 (=Strongylus subauricularis)
Type host and type locality: Rhinella ornata (Spix) (= Rana musica, Bufo musicus, Bufo lentiginosus, Bufo ornatus) (BR)
Other hosts and locality records: Boana faber (Wied-Neuwied) (=Hyla faber) (ANG), Ceratophrys cornuta (Linnaeus) (PTP), Enyalius perditus (IBT), Incilius marmoreus (CDT), Leptodactylus latrans (= Leptodactylus ocellatus, Cystignathus ocellatus) (VTR, SVD, SLP), Leptodactylus melanonotus (Hallowell) (COQ, ARM, TRP, ACA, ELC, VLP, SAT, BJZ, TEA, CHM, CHR), Leptodactylus pentadactylus (SVD), Lithobates brownorum (Sanders) (YLH), Lithobates cf. forreri (=Rana cf. forreri) (ACA, RFR, LPR), Lithobates sp. (JCR), Lithobates vaillanti (Brocchi) (=Rana vaillanti) (LGE), Phyllomedusa burmeisteri Boulenger (ANG), Physalaemus olfersii (Lichtenstein and Martens) (SDM), Rhinella crucifer (Wied-Neuwied) (=Bufo crucifer) (ANG), Rhinella diptycha (=Bufo paracnemis) (SVD, INJ), Rhinella dorbignyi (Duméril and Bibron) (=Rhinella fernadezae) (COR), Rhinella horribilis (Wiegmann) (=Bufo horribilis) (HXT), Rhinella icterica (=Bufo marinus bimaculatus) (CUR, MGP, BOT), Rhinella marina (=Bufo agua, Bufo marinus) (PRT, BOC, SPC, SLB, PSA, SJE, TIL, XTL), Rhinella ornata (=Bufo ornatus) (BR), Rhinella sp. (=Bufo sp.) (GAV), Trachycephalus mesophaeus (Hensel) (= Hyla mesophaea, Phrynohias mesophaea) (ANG)
Host families: Bufonidae, Ceratophryidae, Hylidae (Hylinae, Phylomedusinae), Leiosauridae, Leptodactylidae, Ranidae and Strabomantidae
Distribution: Neotropical (Argentina, Brazil) and Panamanian (Costa Rica, Mexico)
Remarks: Rudolphi (Reference Rudolphi1819) first described this species from the host ‘Rana musica’ in Brazil. This host name was later updated to Bufo musicus by Travassos (Reference Travassos1917). Travassos (Reference Travassos1921) states that B. musicus corresponded to B. lentiginosus americanus collected by Natterer from Brazil.
Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1993) recommend following the redescription provided by Travassos (Reference Travassos1937), although they noted that the host could be either Bufo aguae (a name previously used for R. marina, R. icterica and R. crucifer) or Ceratophrys cornuta. According to Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995b) the original host determination was likely incorrect, as the name was synonymous with Bufo americanus and Bufo terrestris (both of which are currently assigned to genus Anaxyrus). These species are native to North America and do not occur in Brazil.
The name B. lentiginosus has also been used for several species of Bufonidae across North America and South America, but only Rhinella ornata is found in Brazil and was documented with the original material. Thus, we opted to update this name to R. ornata.
Freitas (1955) reported O. subauricularis (Rudolphi, Reference Rudolphi1819) in Enyalius perditus (=Enyalius catenatus) from Tijuca, Rio de Janeiro, Brazil. These nematode specimens were considered species inquirenda by Durette-Desset et al. (Reference Durette-Desset, Alves Dos Anjos and Vrcibradic2006).
References: Rudolphi (Reference Rudolphi1819), Travassos (Reference Travassos1917, Reference Travassos1921), Pearse (Reference Pearse1936), Travassos (Reference Travassos1937), Caballero (Reference Caballero1949), Fahel (Reference Fahel1952), Freitas (Reference Freitas1956), Brenes-Madrigal and Bravo-Hollis (Reference Brenes-Madrigal and Bravo-Hollis1959), Tantalean (Reference Tantalean1976), Vicente and Santos (Reference Vicente and Santos1976), Rodrigues et al. (Reference Rodrigues, Rodrigues and Cristófaro1982), Baker (Reference Baker1987), Vicente et al. (Reference Vicente, Rodrigues, Gomes and Pinto1991), Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995a), Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1995b), Paredes-Calderón et al. (Reference Paredes-Calderón, León-Règagnon and García-Prieto2004), Luque et al. (Reference Luque, Martins and Tavares2005), Cabrera-Guzmán et al. (Reference Cabrera-Guzmán, León-Règagnon and García-Prieto2007), Espinoza-Jiménez et al. (Reference Espinoza-Jiménez, García-Prieto, Osorio-Sarabia and León-Règagnon2007), Sousa et al. (Reference Sousa, Lima and Oliveira2007), Pinhão et al. (Reference Pinhão, Wunderlich, Alves Dos Anjos and Silva2009), Cabrera-Guzmán et al. (Reference Cabrera-Guzmán, Garrido-Olvera and León-Règagnon2010), Yáñez-Arenas and Guillén-Hernández (Reference Yáñez-Arenas and Guillén-Hernández2010), Mata-López et al. (Reference Mata-López, León-Règagnon and García-Prieto2013), Toledo et al. (Reference Toledo, Aguiar, Silva and Alves Dos Anjos2013), Toledo et al. (Reference Toledo, Morais, Silva and Alves Dos Anjos2015), Hamann et al. (Reference Hamann, Kehr and González2013), Velázquez-Urrieta and León-Règagnon (Reference Velázquez-Urrieta and León-Règagnon2018), Trejo-Meléndez et al. (Reference Trejo-Meléndez, Osorio-Sarabia, García-Prieto and Mata-López2019), González et al. (Reference González, Duré, Palomas, Schaefer, Etchepare and Acosta2021).
Oswaldocruzia taranchoni Ben Slimane and Durette-Desset 1995
Type hosts and type locality: Rhinella marina (=Bufo marinus) (PE)
Host family: Bufonidae
Distribution: Neotropical (Brazil)
References: Ben Slimane and Durette-Desset 1995b
Oswaldocruzia tcheprakovae Ben Slimane and Durette-Desset, 1996
Type host and type locality: Pristimantis altamazonicus (=Eleutherodactylus altamazonicus) (SPB)
Host family: Strabomantidae
Distribution: Neotropical (Ecuador)
Reference: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1996b)
Oswaldocruzia touzeti Ben Slimane and Durette-Desset, 1993
Type host and type locality: Pristimantis variabilis (Lynch) (=Eleutherodactylus variabilis) (SPB)
Host family: Strabomantidae
Distribution: Neotropical (Ecuador)
References: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1993)
Oswaldocruzia urubambaensis Guerrero, 2013
Type hosts and type locality: Rhinella marina (SMT)
Host family: Bufonidae
Distribution: Neotropical (Peru)
References: Guerrero (Reference Guerrero2013)
Oswaldocruzia vaucheri Ben Slimane and Durette-Desset, 1993
Type host and type locality: Oreobates quixensis (=Ischnocnema quixensis) (SPB)
Other hosts and locality records: Leptodactylus fuscus (NVP)
Host families: Strabomantidae, Leptodactylidae
Distribution: Neotropical (Ecuador, Brazil)
References: Ben Slimane and Durette-Desset (Reference Ben Slimane and Durette-Desset1993); Goldberg et al. (Reference Goldberg, Bursey, Caldwell, Vitt and Costa2007b)
Oswaldocruzia venezuelensis Ben Slimane, Guerrero and Durette-Desset, 1996
Type hosts and type locality: Rhinella marina (=Bufo marinus) (STR)
Other hosts and locality records: Rhinella marina (=Bufo marinus) (STJ)
Host family: Bufonidae
Distribution: Neotropical (Venezuela, Trinidad and Tobago)
References: Ben Slimane et al. (Reference Ben Slimane, Guerrero and Durette-Desset1996b), Ragoo and Omah-Maharaj (Reference Ragoo and Omah-Maharaj2003)
Oswaldocruzia vitti Bursey and Goldberg, 2004
Type-host and type-locality: Cercosaura eigenmanni (Griffin) (=Prionodactylus eigenmanni) (AM)
Other hosts and locality records: Alopoglossus angulatus (Linnaeus) (AC, RO, SUC), Alopoglossus atriventris Duellman (AC), Anolis fuscoauratus (SNT, POW, ITX, CCA, SUC, RCU), Anolis punctatus (AM, RO, SUC, RCU), Cercosaura eigenmanni (=Prionodactylus eigenmanni) (RO, CUZ), Cercosaura ocellata Wagler (GUA), Cercosaura oshaughnessyi (Boulenger) (=Prionodactylus oshaughnessyi) (AC, SUC, RCU), Cnemidophorus gramivagus (ALT), Plica plica (Linnaeus) (AC, PA, RO), Plica umbra (PA, RO)
Host families: Alopoglossidae, Anolidae, Gymnophthalmidae, Teiidae, Tropiduridae
Distribution: Neotropical (Brazil, Ecuador, Peru)
References: Bursey and Goldberg (Reference Bursey and Goldberg2004); Bursey et al. (Reference Bursey, Goldberg and Parmelee2005); Goldberg et al. (Reference Goldberg, Bursey and Vitt2006a); Goldberg et al. (Reference Goldberg, Bursey and Vitt2006b); Goldberg et al. (Reference Goldberg, Bursey and Vitt2007a), Goldberg et al. (Reference Goldberg, Bursey and Vitt2009a), Ávila and Silva (Reference Ávila and Silva2011), Goldberg et al. (Reference Goldberg, Bursey, Vitt and Arreola2013).
Genus Poekilostrongylus Schmidt and Whittaker, 1975
Poekilostrongylus puertoricensis Schmidt and Whittaker, 1975
Type hosts and type locality: Eleutherodactylus coqui (YNQ)
Host family: Eleutherodactylidae
Distribution: Caribbean (Puerto Rico)
References: Schmidt and Whittaker (Reference Schmidt and Whittaker1975) and Ben Slimane et al. (Reference Ben Slimane, Chabaud and Durette-Desset1996a)
Genus Schulzia Travassos, 1937
Schulzia chiribita Durette-Desset, Florindez and Morales, 2000
Type hosts and type locality: Leptodactylus rhodonotus (Günther) (YUL)
Host family: Leptodactylidae
Distribution: Neotropical (Peru)
References: Durette-Desset et al. (Reference Durette-Desset, Florindez and Morales2000)
Schulzia ptychoglossi Bursey, Goldberg and Telford Jr., 2006
Type hosts and type locality: Alopoglossus festae (Peracca) (=Ptychoglossus festae) (CRM)
Host family: Alopoglossidae
Distribution: Panamanian (Panama)
References: Bursey et al. (Reference Bursey, Goldberg and Telford2006a)
Schulzia travassosi Durette-Desset, Baker and Vaucher, 1985
Type hosts and type locality: Rhinella crucifer (= Bufo crucifer) (ANG)
Hosts and locality records: Haddadus binotatus (Spix) (ANG), Ischnocnema guentheri (Steindachner) (=Hylodes guntheri, Eleutherodactylus guentheri) (ANG), Leptodactylus bufonius (EES, INJ, LOM, TAC), Leptodactylus latrans (ANG), Proceratophrys appendiculata (Günther) (VDR), Rhinella granulosa (Spix) (= Bufo granulosus) (EES), Rhinella icterica (= Chaunus ictericus) (TRB), Rhinella major (CON, LOM), Thoropa miliaris (Spix) (=Hylodes miliaris) (CVD) and Xenodon merremii (Wagler) (= Rhadinea merremii).
Host family: Bufonidae, Leptodactylidae and Odontophrynidae
Distribution: Neotropical (Brazil, Paraguay, Argentina)
References: Travassos (Reference Travassos1925), Travassos (Reference Travassos1937), Durette-Desset et al. (Reference Durette-Desset, Baker and Vaucher1985), Boquimpani-Freitas et al. (Reference Boquimpani-Freitas, Vrcibradic, Vicente, Bursey, Rocha and Van Sluys2001), Bursey et al. (Reference Bursey, Goldberg and Telford2006a), Lux Hoppe et al. (Reference Lux Hoppe, Pedrassani, Hoffmann-Inocente, Tebaldi, Storti, Zanuzzo, Avancini and Do Nascimento2008), González and Hamann (Reference González and Hamann2015b), Hamann and González (Reference Hamann and González2015), González et al. (Reference González, Duré, Palomas, Schaefer, Etchepare and Acosta2021).
Remarks: In 1866, Schneider described Strongylus subventricosus. This species was later transferred to the genus Oswaldocruzia by Travassos (Reference Travassos1917) and mentioned again in Travassos (Reference Travassos1921), though without clear justification. Travassos (Reference Travassos1925) redescribed the species based on new material he collected. In 1937, Travassos established the genus Schulzia, designating O. subventricosa as its type species.
Durette-Desset et al. (Reference Durette-Desset, Baker and Vaucher1985) observed that materials from Schneider (Reference Schneider1866) and Travassos (Reference Travassos1925) allocated to Schulzia subventricosa represent distinct taxa. Since the species description provided by Travassos corresponded to his material only, the authors reclassified Schneider‘s specimens into the genus Macielia and introduced the name Schulzia travassosi for the material described 1925.
Consequently, Macielia subventricosa (Schneider) corresponds to Strongylus subventricosus Schneider and Oswaldocruzia subventricosa as defined by Travassos (Reference Travassos1917, Reference Travassos1921). While Schulzia travassosi is equivalent to O. subventricosa, as defined by Travassos (Reference Travassos1925).
Schulzia usu Lent and Portes Santos, 1989
Type hosts and type locality: Atelopus oxyrhynchus Boulenger (MER)
Host family: Bufonidae
Distribution: Neotropical (Venezuela)
References: Lent and Portes Santos (Reference Lent and Portes Santos1989)
Genus Typhlopsia Baruš and Otero, 1978
Typhlopsia kratochvilli Baruš and Coy Otero, 1978
Type hosts and type locality: Typhlops lumbricalis (Linnaeus) (STC)
Host family: Typhlopidae
Distribution: Caribbean (Cuba)
References: Baruš and Coy Otero (Reference Baruš and Coy Otero1978) and Ben Slimane et al. (Reference Ben Slimane, Chabaud and Durette-Desset1996a)
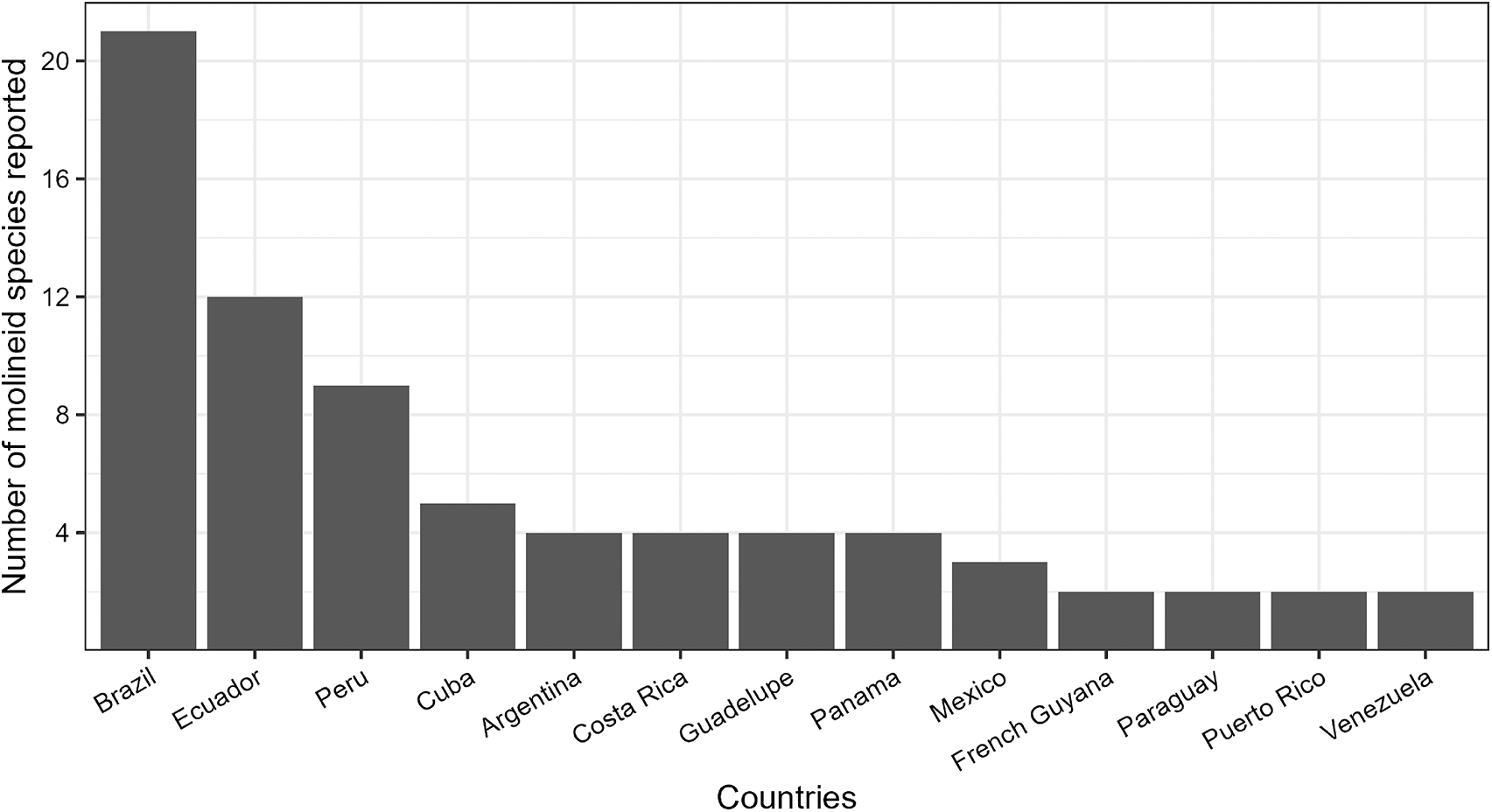
In the Neotropical region, 36 molineid species have been recorded, of which 33 are endemic. In comparison, the Caribbean region has 11 species (all endemic), while Panamanian region has 9 species, with 6 being endemic. Among the 24 countries recorded, Brazil has the highest number of molineid species, totalling 21 species. It is followed by Ecuador with 12 species, Peru with 9 species and Cuba with 5 species. Other countries have fewer species registered: Argentina, Costa Rica, Guadeloupe and Panama have 4 species each; Mexico have 3 species, French Guiana, Paraguay, Puerto Rico and Venezuela have 2 species each. Finally, Bahamas, Bonaire, Chile, Dominica, Dominican Republic, Guyana, Haiti, Nicaragua, Saint Vincent and the Grenadines, Trinidad and Tobago and Uruguay all have 1 species each (see Figure 1).

Figure 1. Countries with the highest number of molineid species recorded in this study. Countries are listed in descending order based on species count. Countries with only one recorded species are not shown.
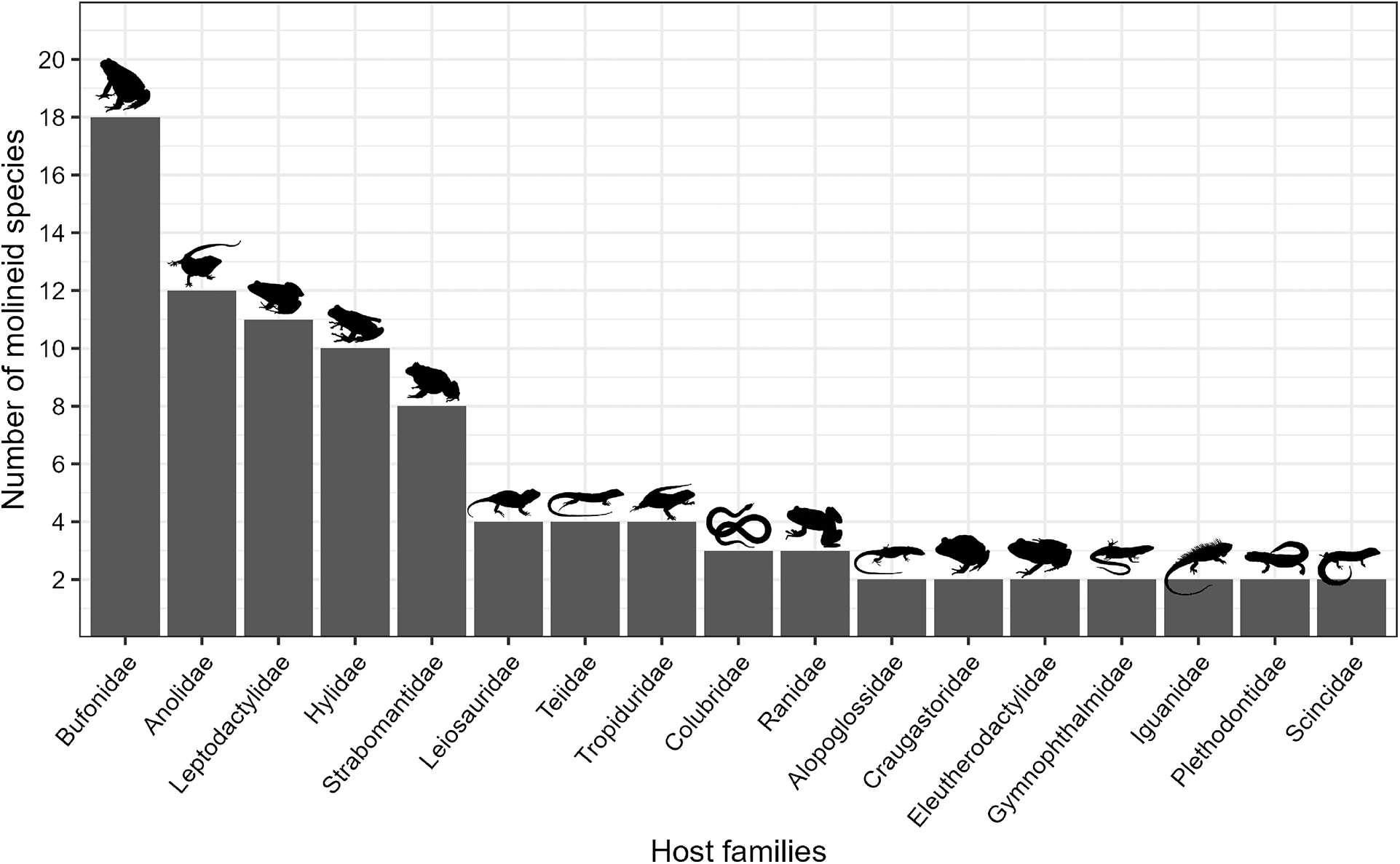
Among the 31 host families reported, 17 were parasitized by more than 1 species of molineid parasites. The families with higher molineid species identified were as follows: Bufonidae with 18 species, Anolidae with 12 species, Leptodactylidae with 11 species, Hylidae with 10 species and Strabomantidae with 8 species. The families Leiosauridae (Squamata), Teiidae (Squamata), Tropiduridae (Squamata) each had 4 species, while Colubridae (Squamata) and Ranidae with 3 species each. Additionally, the families Alopoglossidae, Craugastoridae, Eleutherodactulidae, Gymnophthalmidae, Iguanidae, Plethodontidae and Scincidae were represented by 2 species each. The remaining 14 host families had only 1 helminth species each, including Alsodidae, Batrachylidae, Brachycephalidae, Ceratophryidae, Cycloramphidae, Dendrobatidae, Gekkonidae, Hemiphractidae, Leiocephalidae, Microhylidae, Odontophrynidae, Phrynosomatidae, Tropidophiidae and Typhlopidae (Figure 2).

Figure 2. Number of molineid species per host family. Host families are ordered in descending order based on their number of species recorded. Families that have only one helminth species are not shown.
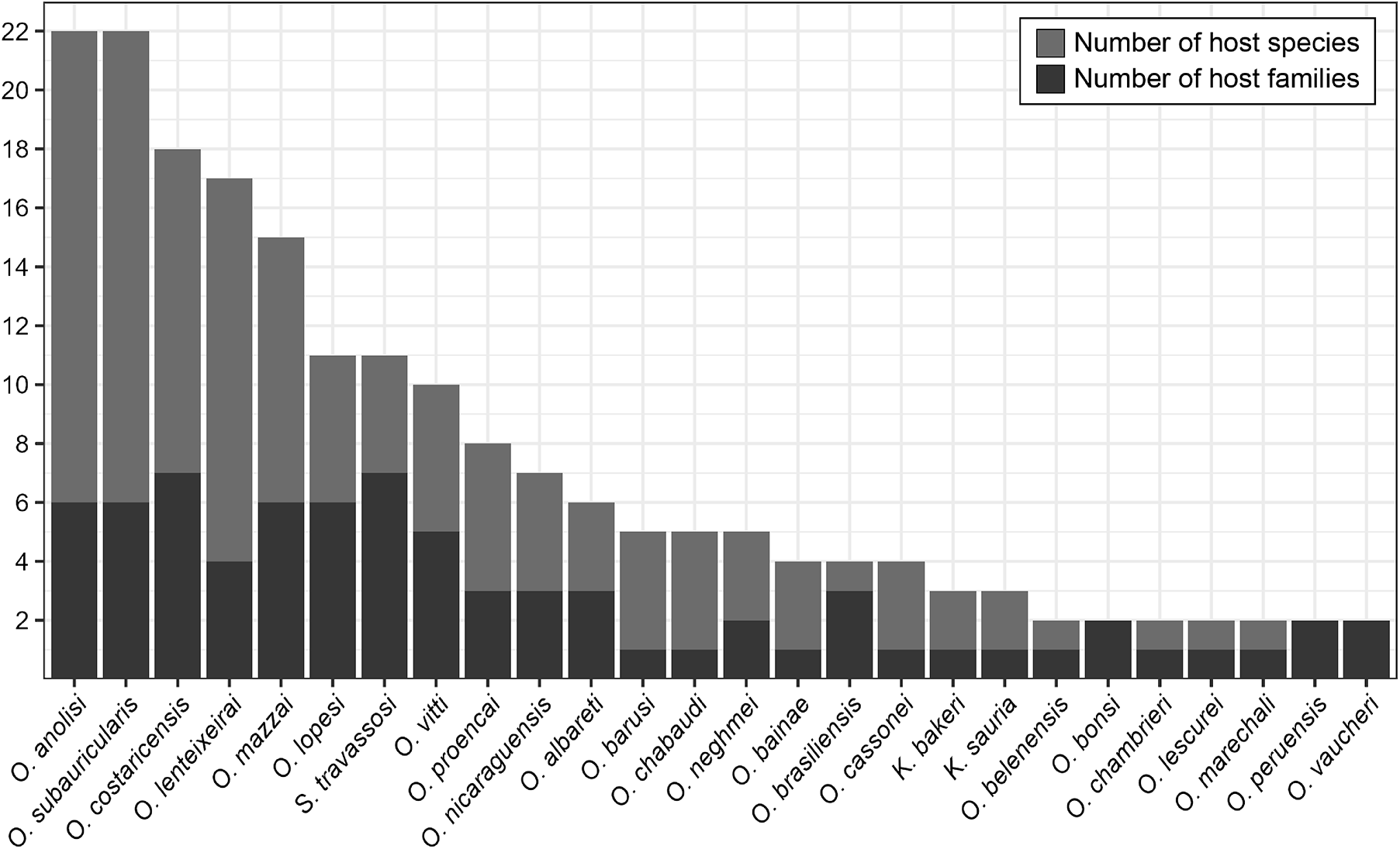
Oswaldocruzia anolisi and O. subauricularis were the most common molineid species overall. Oswaldocruzia anolisi was reported in 22 species from 6 families: Anolidae, Colubridae, Iguanidae, Leiocephalidae, Teiidae and Tropidophiidae). Similarly, O. subauricularis also was reported in 22 species of hosts from 6 families (Bufonidae, Ceratophryidae, Hylidae, Leiosauridae, Leptodactylidae and Ranidae) from Argentina, Brazil and Costa Rica.
Following these 2 species, O. costaricensis was reported in 18 species from 7 host families, O. lenteixeirai in 17 species from 4 families. Other species included O. mazzai (15 species from 6 families), S. travassosi (11 species from 7 families), O. lopesi (11 species from 6 families), O. vitti (10 species from 5 families), O. proencai (8 species from 3 families). Additionally, O. nicaraguensis was reported in 7 species from 3 families, O. albareti in 6 species from 3 families, O. barusi in 5 species from 1 family, O. chabaudi in 5 species from 1 family and O. neghmei in 5 species from 2 families.
We should also highlight that O. bainae was found in 4 host species from 1 family, O. brasiliensis in 4 species from 3 families, O. cassonei in 4 species from 1 family. Lastly, K. bakeri and K. sauria each appeared in 3 species from 1 family. Oswaldocruzia belenensis, O. chambrieri, O. marechali and O. lescurei were found in 2 species from 1 family each, while O. bonsi, O. peruensis and O. vaucheri in 2 species from 2 families each (Figure 3). All 27 remaining taxa were reported for a single host species.

Figure 3. The most common species of molineid nematodes and their range of recorded hosts. Species are organized in descending order based on the number of host species (light grey), compared to the number of host families in which they occur (dark grey).
Discussion
Biogeographical division and species distribution
Ben Slimane et al. (Reference Ben Slimane, Chabaud and Durette-Desset1996a) classified the species of the genus Oswaldocruzia into 5 biogeographical groups based on differences in the spicular morphology, it aligns with the biogeographic division proposed by Sclater (Reference Sclater1858) and adopted by Wallace (Reference Wallace1876). In this division the Neotropical region encompasses the tropical and subtropical areas of the Americas, extending from southern Mexico to the southern tip of South America, including Central America and the Caribbean islands.
However, currently, 2 biogeographical realms proposed by Holt et al. (Reference Holt, Lessard, Borregaard, Fritz, Araújo, Dimitrov, Fabre, Graham, Graves, Jønsson, Nogués-Bravo, Wang, Whittaker, Fjeldså and Rahbek2013) that are equivalent to the former Neotropical realm, have been accepted. The Panamanian realm extends from Mexico to Panama, while the Neotropical realm, as it stands today, includes only the countries of South America. Thus, in the present study, we adopt the biogeographic distribution proposed by Holt et al. (Reference Holt, Lessard, Borregaard, Fritz, Araújo, Dimitrov, Fabre, Graham, Graves, Jønsson, Nogués-Bravo, Wang, Whittaker, Fjeldså and Rahbek2013).
Our results indicate that all 11 Caribbean species are endemic to that region. Only O. bainae, O. lescurei and O. subauricularis were found in more than 1 geographical region, specifically shared distribution among Panamanian and Neotropical regions. These findings provide evidence that molineid species from these regions represent distinct groups, particularly for the Caribbean species with a very particular morphology of spicules, as also observed by Ben Slimane et al. (Reference Ben Slimane, Chabaud and Durette-Desset1996a).
Brazil has the highest number of molineid species among the countries studied. This can be attributed to several factors, one of which is the fact that Brazil has the largest territorial area. This area encompasses diverse biomes and a rich diversity of hosts, which likely contributes to its rich parasite diversity, as noted by Campião et al. (Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Da Silva2014). Also, other fact is the long-standing tradition of helminthology in South America, particularly in Brazil. Research on nematodes in Brazilian species dates back to 1648, with significant contributions from 19th-century expeditions of European researchers such as Rudolphi, Diesing and Molin, as well as the 20th-century contributions of Travassos and other prominent helminthologists (Vicente et al. Reference Vicente, Rodrigues, Gomes and Pinto1993). In last decades, new research groups have been contributing to our knowledge on nematode diversity, regarding its herpetofauna (Ávila and Silva, Reference Ávila and Silva2010; Campião et al. Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Da Silva2014; Müller et al. Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018; Morais et al. Reference Morais, Müller, Melo, Aguiar, Willkens, Sousa Silva, Giese, Ávila and da Silva2020; Araujo Filho et al. Reference Araujo Filho, Teixeira, Teles, Rocha, Almeida, Mesquita and Lacerda2020; Benício et al. Reference Benício, Dos Santos, Freire, Ávila, Da Silva and Fonseca2022; Macedo et al. Reference Macedo, Willkens, Silva, Gardner, Melo and Santos2023, Vieira et al. (Reference Vieira, Pereira, Ribeiro, Oliveira, Silva, Muniz-Pereira and Felix-Nascimento2023)).
Other South American countries also have a distinguished history in helminthology, with numerous authors and research groups dedicating significant efforts to the study of nematode diversity in Argentina (González and Hamann, Reference González and Hamann2015a; Draghi et al. Reference Draghi, Drago and Lunaschi2020), Cuba (Baruš and Coy Otero, Reference Baruš and Coy Otero1978), Ecuador (Ben Slimane and Durette-Desset, Reference Ben Slimane and Durette-Desset1996b), Venezuela (Lent and Portes Santos, Reference Lent and Portes Santos1989; Ben Slimane et al. Reference Ben Slimane, Guerrero and Durette-Desset1996b) and Peru (Bursey et al. Reference Bursey, Goldberg and Parmelee2005; Guerrero, Reference Guerrero2013; Gómez et al. Reference Gómez, Sánchez, Ñacari and Espínola-Novelo2020; Cuellar et al. Reference Cuellar, Sáez, Cantú, Sánchez, Mendoza, Conga, Cruces, Luque and Chero2022; Luque and Chero, Reference Luque and Chero2022), among several others.
Unconfirmed registers and invalid names
There are 2 unconfirmed reports of molineid species from South American amphibians. Walton (Reference Walton1935) reported O. filiformis in Ceratophrys aurita (= Ceratophrys dorsata) and in Leptodactylus latrans (= Leptodactylus ocellatus) from Brazil and O. pipiens in Leptodactylus sp. from South America. Those reports are the only reference for these molineid species for South American amphibians. Travassos (Reference Travassos1937) discussed that these identifications were inaccurate, and Freitas and Lent (Reference Freitas and Lent1938) also commented that the identifications cannot be confirmed since O. filiformis and O. pipiens have been reported exclusively across the North America in a wide range of amphibians and reptiles. Thus, this report should be analysed carefully during species comparisons, and we did not consider valid in this study.
Travassos (Reference Travassos1925) reported O. subauricularis from Leptodactylus latrans (= Leptodactylus ocellatus) in Brazil. However, Travassos (Reference Travassos1937) later stated that the record of O. subauricularis from L. latrans was incorrect. Thus, we did not consider this report here.
The taxonomic history of Schulzia is complex. Travassos (Reference Travassos1937) proposed this genus to allocate Strongylus subventricosus from Schneider (Reference Schneider1866), designating Schulzia subventricosa as a type-species of the genus. These nematodes were previously placed within Oswaldocruzia by Travassos (Reference Travassos1917) and described by Travassos (Reference Travassos1925) with material from different hosts that he named O. subventricosus. However, the author provided no reasons for separating it into a new genus.
In 1985, Durette-Desset et al., identified nematodes similar to those described by Travassos (Reference Travassos1925) and proposed the name Schulzia travassosi for specimens found in Paraguay. However, they noticed that the description given by Travassos (Reference Travassos1937) corresponded only to the material he had described in 1925 and not from Schneider (Reference Schneider1866). Consequently, Durette-Desset et al. (Reference Durette-Desset, Baker and Vaucher1985) reallocated Schneider‘s specimens to the genus Macielia Travassos (Nematoda, Trichostrongylidae). They redefined Schulzia by proposing the name Schulzia travassosi for Paraguayan specimens and material from Travassos (Reference Travassos1925) and designating them as a type-species of the genus and Macielia subventricosa for Schneider‘s specimens. Thus, the name Schulzia subventricosa is currently not considered valid and not presented in this work.
Host–parasite associations and specificity
We observed that, until now, molineid nematodes have been mainly found in amphibian hosts (146 species), while in reptiles, a lower number have been reported (74 species). The most common strategies used by nematodes to infect their hosts are by oral route or by skin penetration (Anderson, Reference Anderson2000). Studies on development and life cycle were conducted only for O. filiformis (Hendrikx Reference Hendrikx1981; Hendrikx Reference Hendrikx1983, Hendrikx and Van Moppes, Reference Hendrikx and Van Moppes1983; Griffin, Reference Griffin1988) and for O. pipiens (Baker, Reference Baker1978) which demonstrated that their cycles are direct. The infection route in these molineid nematodes is unclear, but one possible explanation of our results could be related to the direct cycle of these nematodes, particularly for skin penetration. Reptiles are covered in overlapping epidermal scales, reducing the area exposed to infection, whereas amphibians have smooth skins covered with mucus secretions that are more accessible to infective larvae.
Bufonids had more molineid species richness than all other amphibians’ host families. Also, for R. marina we observed the highest number of molineid species (11 species), followed by R. margaritifera (6 species). Willkens et al. (Reference Willkens, Furtado, Santos and Melo2021) proposed that the habitat use of the host (primarily terrestrial or predominantly arboreal) could account for the observed species diversity in certain amphibian families like Bufonidae. These terrestrial amphibians and their increased mobility compared to arboreal species makes contact with a higher diversity of parasites more likely (Poulin, Reference Poulin2007). Furthermore, several studies on helminth diversity have consistently demonstrated a higher functional diversity of parasite communities in terrestrial hosts (Campião et al. Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Da Silva2014; Hamann and González, Reference Hamann and González2015; Gómez et al. Reference Gómez, Sánchez, Ñacari and Espínola-Novelo2020; Euclydes et al. Reference Euclydes, Dudczak and Campião2021). In contrast, considering that Bufonids are relatively common in these regions, and comparatively easier to collect and study, the apparent richness may partly reflect an artefact of rather different sampling effort.
Poulin (Reference Poulin1997) suggested that host body size is a good predictor of parasite richness, as larger hosts may offer more habitats, ecological niches and resources than smaller ones. Similarly, Poulin (Reference Poulin2007) proposed that larger hosts might harbour more parasites due to their greater surface area, higher food intake and the fact that they are typically older, providing more time for parasite accumulation. Hamann et al. (Reference Hamann, González and Kehr2006) and Campião et al. (Reference Campião, Ribas and Tavares2015) reported positive correlations between host body size and parasite richness in Neotropical amphibians, which likely explains why Rhinella marina exhibited the highest number of parasite species in the present study. However, Cardoso et al. (Reference Cardoso, Jesus, Silva-Filho, Willkens, Santana, Santos, Santos and Melo2021) found no significant influence of host body size on the composition of helminth taxa, suggesting that this pattern may not be consistent across all amphibian hosts.
The family Anolidae, represented by hosts of the genus Anolis, showed the second higher molineid species richness among all host families and the highest among reptiles, of which Anolis marmoratus presented the highest number of molineid species (4 species). This is the most abundant genus of lizards, and most species of this genus are arboreal or semi-arboreal (Uetz et al. Reference Uetz, Freed, Aguilar, Reyes and Hošek2025). Still, there are also terrestrial and semiaquatic species, of which the Caribbean species present particular ectomorphs that inhabit different niches (Losos, Reference Losos1992) and might explain the richness observed for this family of reptiles.
Disregarding species reported only once and from a single host species, some nematodes occurred exclusively in a particular host family. For instance, K. bakeri was reported from 3 species of Hylidae (all from the genus Boana), K. sauria from 3 species of Teiidae (from the genera Cnemidophorus and Kentropyx), O. chabaudi from 6 different species of Hylidae (from the genera Boana and Osteocephalus), O. lescurei from 3 species of Bufonidae and O. barusi from 5 species of Bufonidae (all from the genus Peltophryne). These nematodes occurred in hosts with overlapping distributions and similar niches. Thus, this result might also reflect an association of these species with different habitats of their hosts, as Willkens et al. (Reference Willkens, Furtado, Santos and Melo2021) hypothesized. However, the authors also discussed a host-parasite cophylogeny hypothesis, which considers potential cospeciation events among hosts and their parasites and could partially explain our results.
Notably, other molineid species did not exhibit a specific association with a single host species or family. For instance, the most common species, O. subauricularis was found in 22 species of amphibian and reptilian hosts (Bufonidae, Ceratophryidae, Hylidae, Leiosauridae, Leptodactylidae, Ranidae and Strabomantidae) from Argentina, Brazil and Costa Rica. Similarly, O. anolisi, O. costaricensis, O. lenteixeirai, O. mazzai, O. lopesi, O. vitti, O. proencai and O. nicaraguensis also showed a broad host range. The occurrence of molineids infecting hosts from different families with different habitat uses has been reported previously (Bursey and Goldberg, Reference Bursey and Goldberg2011; Willkens et al. Reference Willkens, Maldonado, Santos, Maschio and Melo2016). Also, we highlight that most species are poorly studied, and details regarding their distribution and host range are yet unknown.
Compiling and maintaining a comprehensive species list within the field of helminthology are indispensable for scientific research and advancements in understanding parasitic diversity. These species lists provide a systematic and organized record of all known helminth species, categorizing them based on taxonomic classifications, geographical distributions and host associations. Such information is crucial for researchers investigating helminths’ ecology, epidemiology and evolution.
In conclusion, our study and the checklist provided here, offers a comprehensive overview of nematodes of the family Molineidae, summarize details regarding their distribution and host range, and provide basis for further studies on this group. The taxonomic history of these Molineid genera and their species is complex, studies integrating morphological and molecular data are still required to achieve a broader understanding of their biology and to confirm their phylogenetic relationships and historical records. We also endorse that their diversity might still be underestimated, and new species and records from different localities and hosts are awaiting discovery.
Supplementary material
The Supplementary Material for this paper can be found at https://doi.org/10.1017/S0031182025101364.
Acknowledgements
The authors acknowledge the doctors Arnaldo Maldonado Jr., Drausio H. Morais, Gleomar F. Maschio and Cythya E. González for their support and assistance in this work during the elaboration of the manuscript.
Author contributions
Conceptualization and study design: F.T.V.M. and Y.W.; methodology: Y.W.; investigation: Y.W.; taxonomy and nomenclature: Y.W. and F.T.V.M.; writing – original draft preparation: Y.W.; writing – review and editing: Y.W., F.T.V.M. and J.N.S.; supervision: F.T.V.M.; funding acquisition: F.T.V.M. and J.N.S.
Financial support
This study was part of the Ph.D. thesis of Yuri Willkens in the Graduate Program in Biology of Infectious and Parasitic Agents, UFPA. It was supported by CAPES/PPGBAIP/UFPA the National Council for Scientific and Technological Development (CNPq); CNPq Research productivity grant to MELO, F. T. V. (Process no. 304955/2018-3).
Competing interests
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable.