Introduction
Catostomidae (suckers) is a diverse group of freshwater fishes within Cypriniformes, distributed across the Holarctic region, with a pronounced concentration of species in North America and limited representation in eastern Asia. The family comprises 86 species across 15 genera, with 85 species (14 genera) occurring in North America (Fricke et al. Reference Fricke, Eschmeyer and Fong2024a), making Catostomidae the third-largest group of freshwater fishes on the continent (Warren et al. Reference Warren, Burr, Walsh, Bart, Cashner, Etnier, Freeman, Kuhajda, Mayden, Robison, Ross and Starnes2000; Harris and Mayden Reference Harris and Mayden2001). Catostomids are traditionally classified into 4 subfamilies: Myxocyprininae, Cycleptinae, Ictiobinae and Catostominae, the latter of which includes several recognized tribes such as Catostomini, Moxostomatini, Thoburniini and Erimyzonini (Nelson Reference Nelson2006). Recent molecular phylogenetic analyses confirmed the monophyly of Catostomidae but revealed conflicting relationships among lineages depending on the type of dataset used (nuclear, mitochondrial) (e.g. Harris and Mayden Reference Harris and Mayden2001; Sun et al. Reference Sun, Xie, Wang, Liu, Treer and Chang2007; Doosey et al. Reference Doosey, Bart, Saitoh and Miya2010; Chen and Mayden Reference Chen and Mayden2012; Bagley et al. Reference Bagley, Mayden and Harris2018; Yang et al. Reference Yang, Mayden and Naylor2024). Although this family is almost exclusively native to North America, Catostomus catostomus is the only species with a disjunct distribution across the North Pacific region, occurring in both the Nearctic and the Siberian part of the Palearctic region (Harris et al. Reference Harris, Hubbard, Sandel, Warren and Burr2014). In contrast, Myxocyprinus asiaticus is the only catostomid species endemic to Eurasia, occurring in the Yangtze River basin in China (Smith Reference Smith and Mayden1992). The high species richness and distribution of Catostomidae across the Holarctic region, along with their well-studied phylogenetic background (e.g. Chen and Mayden Reference Chen and Mayden2012; Bagley et al. Reference Bagley, Mayden and Harris2018; Yang et al. Reference Yang, Mayden and Naylor2024), make this fish group a suitable model for exploring evolutionary and biogeographic patterns of their parasites and for studies of host–parasite diversification.
As exclusive hosts of Pseudomurraytrematidae (Platyhelminthes: Monopisthocotyla), catostomids provide a unique opportunity to investigate parasite diversification and host–parasite coevolution within a well-defined and geographically structured host lineages. Given the generally narrow host specificity observed in many monopisthocotylans and their frequently co-distributed evolutionary history with hosts, these ectoparasites represent powerful tools for exploring the biogeographic patterns of host lineages and serve as valuable phylogenetic markers in comparative and cophylogenetic studies of host–parasite systems (e.g. Benovics et al. Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018; Šimková et al. Reference Šimková, Řehulková, Choudhury and Seifertová2022; Šimková Reference Šimková2024).
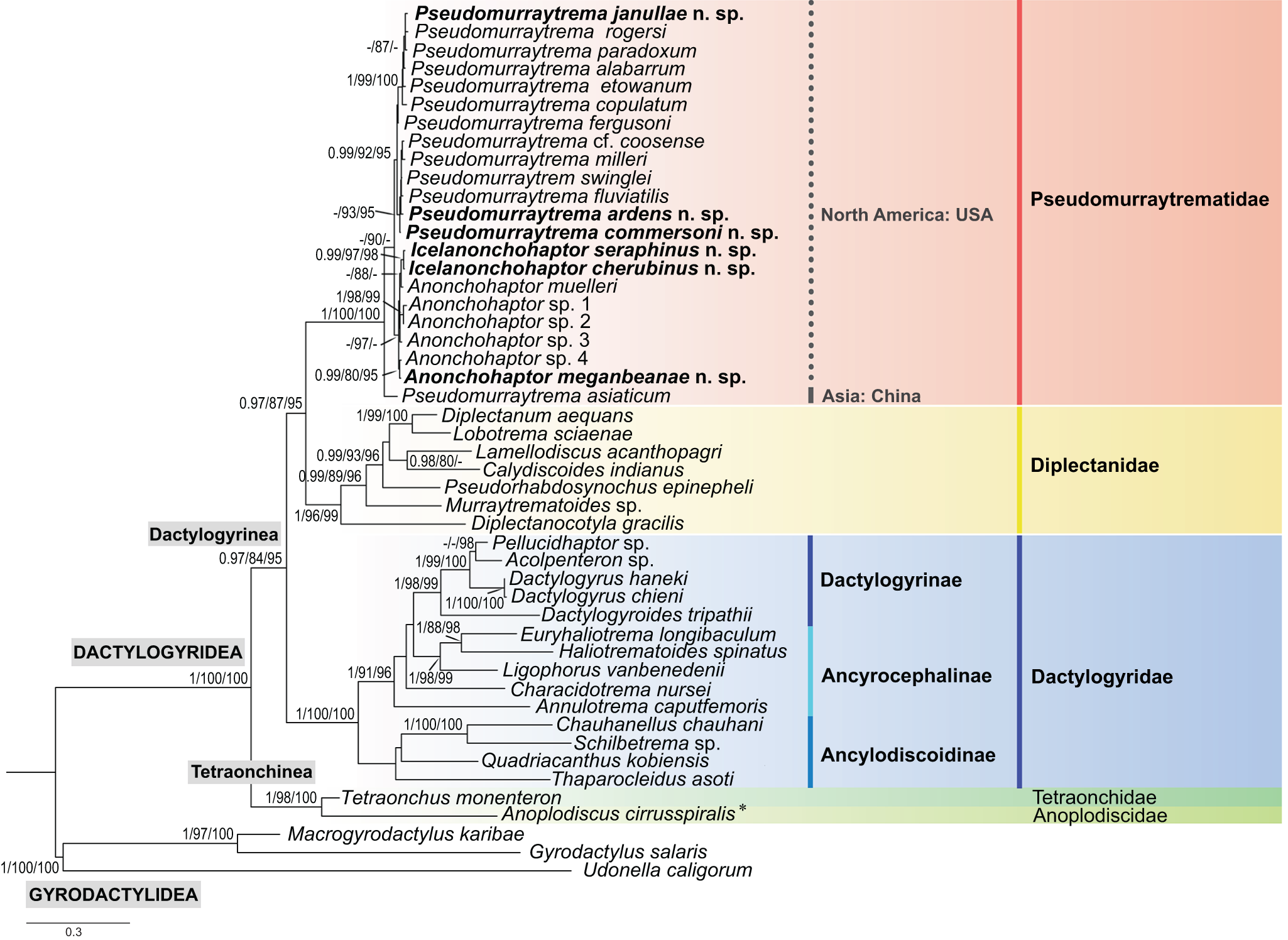
Pseudomurraytrematidae was initially established as a subfamily within Dactylogyridae by Kritsky et al. (Reference Kritsky, Mizelle and Bilqees1978) and was later elevated to family status within Dactylogyrinea by Beverley-Burton (Reference Beverley-Burton, Margolis and Kabata1984). This taxonomic rank was primarily based on the distinctive morphology of the reproductive system, first noted by Leiby et al. (Reference Leiby, Kritsky and Peterson1972) in 4 genera: Anonchohaptor Mueller, 1938, Icelanonchohaptor Leiby, Kritsky & Peterson, 1972, Myzotrema Rogers, 1967 and Pseudomurraytrema Bychowsky, 1957. The phylogenetic position of Pseudomurraytrematidae within Monopisthocotyla remains unresolved, as the family has been underrepresented in both morphological and molecular studies. Previous morphological analyses, which included only 4 species of Pseudomurraytrema (P. copulatum, P. etowanum, P. paradoxum and P. rogersi), suggested a close phylogenetic relationship between Pseudomurraytrematidae and Diplectanidae within Dactylogyrinea (Kritsky and Boeger Reference Kritsky and Boeger1989; Boeger and Kritsky Reference Boeger and Kritsky1993, Reference Boeger and Kritsky1997, Reference Boeger, Kritsky, Littlewood and Bray2001). Molecular data for this family are even scarcer. Only 1 unidentified species of Pseudomurraytrema (= P. ardens sensu Littlewood, Rohde & Clouth, Reference Littlewood, Rohde and Clough1998; treated as a nomen nudum by Boeger et al. Reference Boeger, Kritsky, Domingues and Bueno-Silva2014) has been included in molecular analyses. Several studies suggested that Pseudomurraytrematidae might be a sister group either to Diplectanidae alone or to both Diplectanidae and Dactylogyridae within Dactylogyrinea (Littlewood et al. Reference Littlewood, Rohde and Clough1998; Olson and Littlewood Reference Olson and Littlewood2002; Bentz et al. Reference Bentz, Combes, Euzet, Riutord and Verneau2003; Šimková et al. Reference Šimková, Plaisance, Matějusová, Morand and Verneau2003; Plaisance et al. Reference Plaisance, Littlewood, Olson and Morand2005; Boeger et al. Reference Boeger, Kritsky, Domingues and Bueno-Silva2014; Ogawa and Itoh Reference Ogawa and Itoh2017; Moreira et al. Reference Moreira, Luque and Šimková2019; Soares et al. Reference Soares, Domingues and Adriano2021). These findings highlight the need for more comprehensive and robust morphological and molecular analyses to resolve the phylogenetic relationships and evolutionary history of Pseudomurraytrematidae.
All known members of Pseudomurraytrematidae are parasitic on the gills and/or skin of catostomid fishes (Catostomoidei, Cypriniformes). The family currently comprises 20 nominal species distributed across 4 genera: Anonchohaptor, Icelanonchohaptor, Myzotrema and Pseudomurraytrema. The latter genus is the type genus and contains 12 species, of which 11 occur in North America and only 1 in China (P. asiaticum Chang & Ji, Reference Chang and Ji1978 on Myxocyprinus asiaticus) (Chang and Ji Reference Chang and Ji1978; Kuchta et al. Reference Kuchta, Řehulková, Francová, Scholz, Morand and Šimková2020; McAllister et al. Reference McAllister, Leis, Cloutman, Woodyard, Camus and Robison2022). Anonchohaptor and Icelanonchohaptor include 3 and 4 species, respectively, all reported from North America (Kuchta et al. Reference Kuchta, Řehulková, Francová, Scholz, Morand and Šimková2020; Mendoza-Franco et al. Reference Mendoza-Franco, Hernández-Gómez and Caspeta-Mandujano2023). Myzotrema is monotypic, with Myzotrema cyclepti described by Rogers (Reference Rogers1967) on Cycleptus elongatus (Cycleptinae) from Alabama. Host associations within Pseudomurraytrematidae differ among genera, with species of Anonchohaptor infecting multiple catostomid subfamilies, while species of Pseudomurraytrema and Icelanonchohaptor appear more host-restricted. However, the overall diversity and host specificity of these parasites remain poorly understood due to limited sampling across host taxa and regions (Mergo and White Reference Mergo and White1984; Kuchta et al. Reference Kuchta, Řehulková, Francová, Scholz, Morand and Šimková2020).
A survey aimed at investigating the diversity of monopisthocotylans parasitizing North American catostomid fishes was initiated in 2018. To date, 16 catostomid species have been examined across 8 US states, the Canadian province of Québec and 3 northern Mexican states. The first results of this effort reported 3 species of Dactylogyrus (Dactylogyridae) and 4 species of Gyrodactylus (Gyrodactylidae) infecting Hypentelium nigricans, Catostomus catostomus and Catostomus commersonii from Arkansas, New York and Québec (Šimková et al. Reference Šimková, Řehulková, Choudhury and Seifertová2022; Rahmouni et al. Reference Rahmouni, Seifertová and Šimková2023; Řehulková et al. Reference Řehulková, Seifertová, Francová and Šimková2023). As a continuation of this work, the present study focuses on members of Pseudomurraytrematidae collected from the catostomid hosts. The objectives are: (i) to identify and characterize newly collected pseudomurraytrematids, (ii) to compare their diversity with previous records and (iii) to reveal evolutionary relationships within Pseudomurraytrematidae and its placement within Dactylogyridea using an integrative approach.
Materials and methods
Collection and identification of fish hosts
A total of 16 catostomid species (Catostominae, Ictiobinae) were captured by electrofishing or seine nets from several localities in 8 US states (i.e. Arkansas, Illinois, Michigan, Mississippi, New York, Oregon, Texas and Wisconsin), the Canadian province of Québec and 3 northern Mexican states (Chihuahua, Coahuila and Durango). The fieldwork was carried out under permission from the relevant local authorities (provided by the US partners listed in the Acknowledgements). Fishes were initially identified by local experts well-versed in the regional ichthyofauna. This preliminary identification was later verified through cytochrome b (cyt b) mitochondrial gene sequencing. The taxonomic nomenclature and classification adopted in this study are in accordance with Eschmeyer’s Catalog of Fishes (Fricke et al. Reference Fricke, Eschmeyer and van der Laan2024b). Where original host names differ from the currently accepted ones, the original names are given in parentheses as synonyms. Live fishes were transported to the lab and housed in oxygenated containers. Within 3 days of capture, they were euthanized via spinal cord transection for immediate parasitological examination.
Collection and preparation of parasites
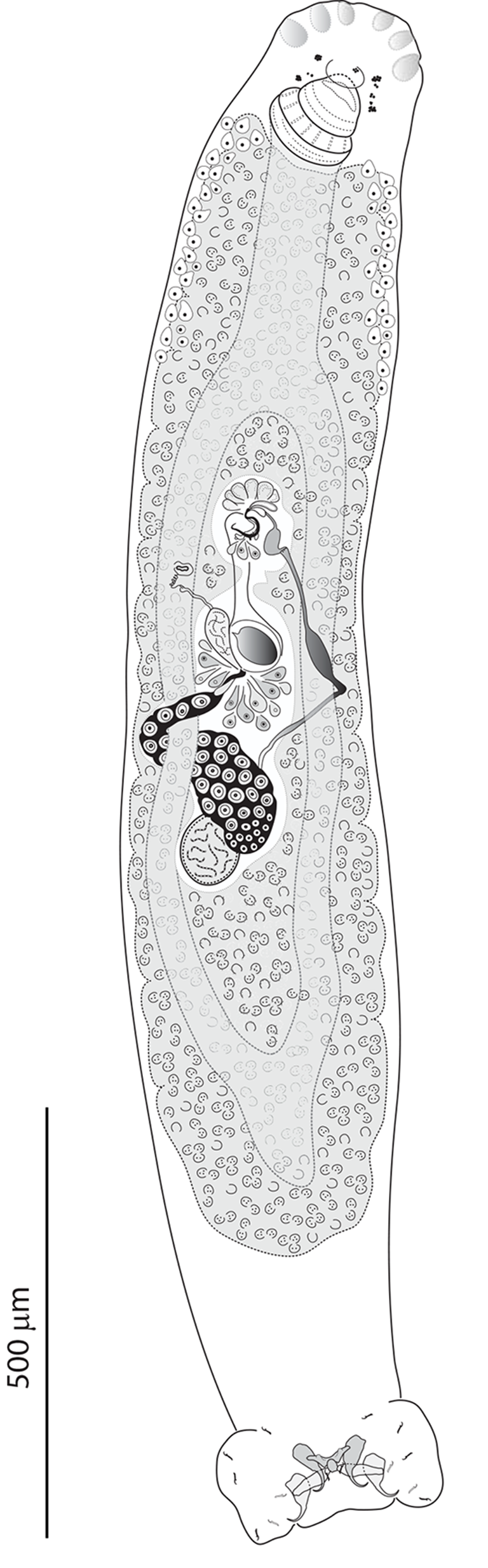
Skin scrapings, fins and gills were removed from the host, placed in Petri dishes with tap water and examined using a stereomicroscope (Olympus SZX7, Tokyo, Japan) to detect parasites. Live pseudomurraytrematids were individually removed from host tissues using fine needles and immediately processed for morphological (see Řehulková and Gelnar Reference Řehulková and Gelnar2005) and molecular analyses. To draw and measure their hard structures (i.e. haptoral attachment structures, male copulatory organ and vagina), parasites were thoroughly pressed and preserved in a solution of glycerine and ammonium picrate (GAP; Malmberg Reference Malmberg1957). After morphometric examination, these GAP-fixed specimens were re-mounted in Canada balsam to ensure their long-term preservation (Ergens Reference Ergens1969). Some worms were fixed in 70% ethanol prior to staining with iron acetocarmine or Gomori’s trichrome, dehydration and mounting to allow examination of their internal structures. For DNA analysis, specimens were bisected with fine needles and then one half of the body (either the posterior part containing haptoral sclerites or the anterior part with the male copulatory organ and vagina) was fixed in 96% ethanol to facilitate future DNA extraction; the other half was mounted on a slide, fixed with GAP for species identification, and kept as a hologenophore (sensu Pleijel et al. Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008). The mounted parasites were studied using an Olympus BX61 microscope (Tokyo, Japan) equipped with phase contrast optics. Illustrations were created using a drawing attachment, scanned and redrawn using a graphics tablet (Wacom Intuos5 Touch) compatible with Adobe Illustrator software (Adobe Systems Inc., San Jose, CA, USA). Measurements (given in micrometres) were taken using an Olympus digital camera coupled with Stream Motion 1.9.2 image analysis software (Olympus) and are reported as the mean, with the range and number (n) of specimens measured in parentheses. The numbering of hook pairs follows the method recommended by Mizelle (Reference Mizelle1936). The male copulatory organ is henceforth abbreviated to MCO. Type and voucher specimens of parasites examined during the present study were deposited in the Smithsonian Institution, National Museum of Natural History (USNM), Washington, DC, USA, as indicated in the respective species accounts.
DNA extraction, amplification and sequencing
Bisected parasite specimens preserved in 96% ethanol were dried using a centrifugal vacuum concentrator (Eppendorf, Hamburg, Germany). DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the protocol for the purification of total DNA from animal tissues. A fragment spanning partial 18S rDNA and the internal transcribed spacer (18S–ITS1) and a fragment of partial 28S rDNA (28S) were amplified and sequenced. A list of primers and PCR conditions are provided in Table 1. Amplifications were carried out in a 20 μL reaction mixture containing 13 μL nuclease-free water, 4 μL FIREPol Master Mix Ready to Load (Solis BioDyne, Tartu, Estonia), 0.5 μL of each primer (10 μM concentration), and 2 μL of eluted DNA. PCR products were detected by electrophoresis in 1.5% agarose gels stained with GoodView (SBS Genetech, Bratislava, Slovakia). Amplified products were purified using EPPiC Fast (Amplia, Bratislava, Slovakia), following the manufacturer’s protocol. Sequencing was performed in both directions using PCR primers with the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Thermo Fisher Scientific, Prague, Czech Republic) on an ABI Prism 3130 Genetic Analyzer (Applied Biosystems). Raw sequencing data were analysed using Sequencing Analysis software v.5.2 (Applied Biosystems) and processed with Sequencer software (Gene Codes Corporation, Ann Arbor, MI, USA) to assemble contigs. Newly obtained sequences have been deposited in GenBank (accession numbers are listed in Table 3).
Table 1. List of primers used for PCR amplification of nuclear rDNA markers in the present study

Table 2. Monopisthocotylan species included in the phylogenetic analyses, with their family assignment, corresponding host taxa (species, family, order), country of origin and GenBank accession numbers (28S rDNA)

Table 3. Pseudomurraytrematid species from catostomid hosts in North America, with site of infection, host taxa (species, subfamily/tribe), locality and GenBank accession numbers (28S rDNA, 18S–ITS1). N – number of specimens molecularly analysed

Phylogenetic analyses
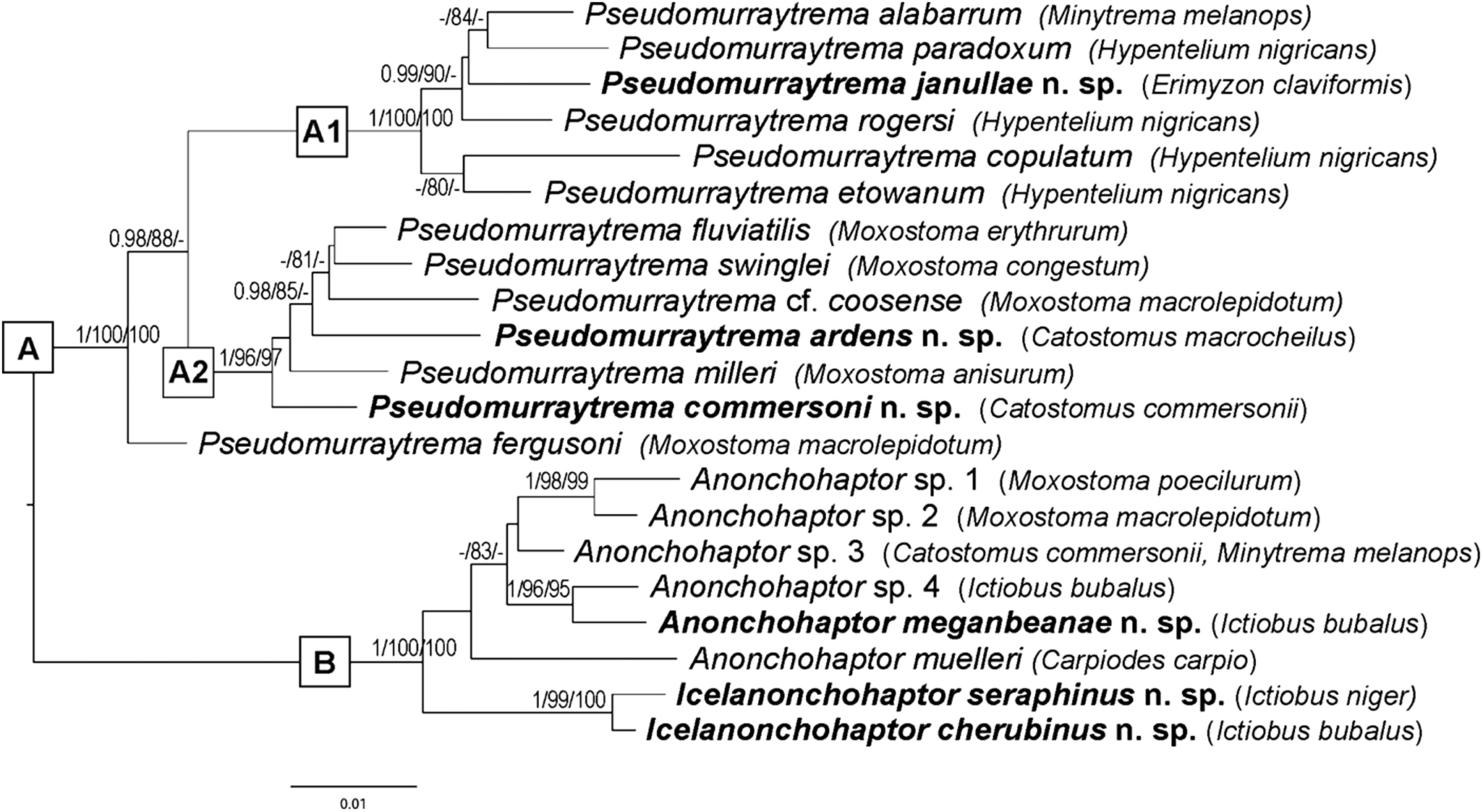
To infer the phylogenetic relationships among species of Pseudomurraytrematidae, 2 datasets were compiled: a single-gene dataset (28S rDNA) and a concatenated dataset comprising 18S rDNA, ITS1 and 28S rDNA sequences. The 28S dataset included 13 sequences of Pseudomurraytrema species, 2 species of Icelanonchohaptor and 6 species of Anonchohaptor newly sequenced in the present study, as well as 1 sequence of P. asiaticum from Myxocyprinus asiaticus (China). In addition, 21 sequences of selected representatives from Diplectanidae, Dactylogyridae, Tetraonchus monenteron and Anoplodiscus cirrusspiralis were retrieved from GenBank. Representatives of Gyrodactylidea were used as outgroups. Accession numbers, host species and locality data for the 28S dataset are listed in Table 2. The concatenated dataset (18S, ITS1 and 28S) was restricted to Nearctic Pseudomurraytrematidae and included 21 sequences representing the species examined in the present study. Accession numbers, host species and locality data are provided in Table 3.
The Maximum Likelihood (ML) and Bayesian inference (BI) algorithms were used to build the phylogenetic trees. Sequence alignments were performed on the MAFFT v.7 server (https://mafft.cbrc.jp/alignment/server/) (Katoh et al. Reference Katoh, Rozewicki and Yamada2019) using the G-INS-i algorithm. The best-fit sequence substitution models were determined based on the Bayesian information criterion using ModelFinder (Kalyaanamoorthy et al. Reference Kalyaanamoorthy, Minh, Wong, von Haeseler and Jermiin2017) implemented in IQ-TREE (Nguyen et al. Reference Nguyen, Schmidt, von Haeseler and Minh2015). The TIM3 + F + I + G4 model was chosen for the 28S dataset, while the K2P + I (18S), TIM2 + F + G4 (ITS1) and TPM2 + F + I (28S) models were selected for the concatenated dataset. The ML analyses were conducted using IQ-TREE (Nguyen et al. Reference Nguyen, Schmidt, von Haeseler and Minh2015) on the IQ-TREE web server (Trifinopoulos et al. Reference Trifinopoulos, Nguyen, von Haeseler and Minh2016). Branch supports were calculated using both the ultrafast bootstrap approximation (UFBoot; Hoang et al. Reference Hoang, Chernomor, von Haeseler, Minh and Vinh2018) and the Shimodaira–Hasegawa-like approximate likelihood ratio test (SH-aLRT; Guindon et al. Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010) with 1000 replicates. UFBoot values ≥95% can be considered strong support, while values <80% should be treated with caution (Hoang et al. Reference Hoang, Chernomor, von Haeseler, Minh and Vinh2018). UFBoot provides nearly unbiased estimates of branch support, while SH-aLRT is more conservative. Each branch is assigned with SH-aLRT and UFBoot supports (Minh et al. Reference Minh, Lanfear, Wong, Ly-Trong, Trifinopoulos, Schrempf and Schmidt2025). Combining both tests (e.g. UFBoot ≥ 95 and SH-aLRT ≥ 80) provides reliable support for branches, while a discrepancy between them may indicate model errors or polytomies.
The BI analysis was conducted using MrBayes v.3.2 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012) with the following Markov chain Monte Carlo (MCMC) settings: 4 simultaneously running chains (1 cold and 3 heated), 5 million generations with a sampling frequency of every 100 generations, and a burn-in fraction of 25%. Posterior probabilities (PP) higher than 0.95 are generally considered to be strong support for a given clade, while values between 0.80 and 0.95 indicate weaker or moderate support, and values below 0.80 are usually considered unsupported. Convergence of the MCMC chains was assessed by examining the Potential Scale Reduction Factor (PSRF), which was close to 1.0 for all parameters, and by calculating Effective Sample Sizes (ESS) using Tracer v1.7 (Rambaut et al. Reference Rambaut, Drummond, Xie, Baele and Suchard2018), all of which exceeded 200. The obtained trees for BL and ML were visualized in FigTree v.1.4.3 (Rambaut, Reference Rambaut2017) and edited in Adobe Photoshop. The uncorrected pairwise genetic distances (p-distance) between the taxa were estimated using the software MEGA11 (Tamura et al. Reference Tamura, Stecher and Kumar2021) separately for each genetic marker.
Results
Fourteen of the 16 examined catostomid species were found to host pseudomurraytrematid parasites. Only Carpiodes cyprinus from Wisconsin (1 specimen examined) and Pantosteus nebuliferus from Mexico (20 specimens examined) were uninfected. Representatives of Pseudomurraytrematidae were recorded in all surveyed regions except Mexico, New York and Québec. In total, 22 monopisthocotylan species across 3 genera – Anonchohaptor, Icelanonchohaptor and Pseudomurraytrema – were collected from the gills or fins of the catostomid hosts. Pseudomurraytrema was the most species-rich genus (14 species), followed by Anonchohaptor (6 species) and Icelanonchohaptor (2 species). The highest parasite diversity was observed on Hypentelium nigricans (Arkansas), which harboured 4 species of Pseudomurraytrema (P. copulatum, P. etowanum, P. paradoxum, P. rogersi). Ictiobus bubalus hosted 2 Anonchohaptor species (Illinois, Texas) and 1 Icelanonchohaptor species (Mississippi). Most pseudomurraytrematid species were recovered from gills; only Icelanonchohaptor cherubinus n. sp. and Icelanonchohaptor seraphinus n. sp. were found on the fins of I. bubalus and I. niger, respectively (Mississippi).
Pseudomurraytrematidae Kritsky, Mizelle & Bilqees, 1978
Dactylogyridea Bychowsky, 1937
Diagnosis: Body divisible into cephalic region, trunk, peduncle and haptor. Tegument thin, smooth. Cephalic region developed into cephalic lappets or lobes; cephalic glands unicellular, arranged in 2 bilateral groups in the anterolateral trunk. Two pairs of irregular eyespots. Mouth subterminal and midventral. Pharynx muscular, glandular. Oesophagus short or elongated. Intestinal caeca 2, terminating blindly or confluent, lacking diverticula. Gonads in tandem, slightly overlapping; testis postovarian. Vas deferens looping around the left intestinal caecum; seminal vesicle an expansion of vas deferens. One or 2 prostatic reservoirs. MCO consisting of a U-shaped copulatory tube, basally articulated with a 3-ramous accessory piece (exceptionally modified as a whole in Pseudomurraytrema fergusoni). Ovary elongate, looping around the right intestinal caecum. Vagina dextroventral, consisting of a distal lightly sclerotized funnel and a proximal coiled portion (exceptionally modified as a whole in P. fergusoni). Seminal receptacle preovarian. Peduncle short or elongated. Haptor disc-shaped, cup-shaped, or polygonal. Haptoral structures comprising 14 hooks and, when present, 2 anchor-bar complexes (1 ventral, 1 dorsal). Each complex consisting of a pair of anchors supported by a bar. Anchors without defined roots. Ventral bar single; dorsal bar either single or paired. Parasites of catostomid fishes (Catostomoidei).
Genera included: Anonchohaptor Mueller, 1938; Icelanonchohaptor Leiby, Kritsky & Peterson, 1972; Myzotrema Rogers, 1967; Pseudomurraytrema Bychowsky, 1957 (type genus).
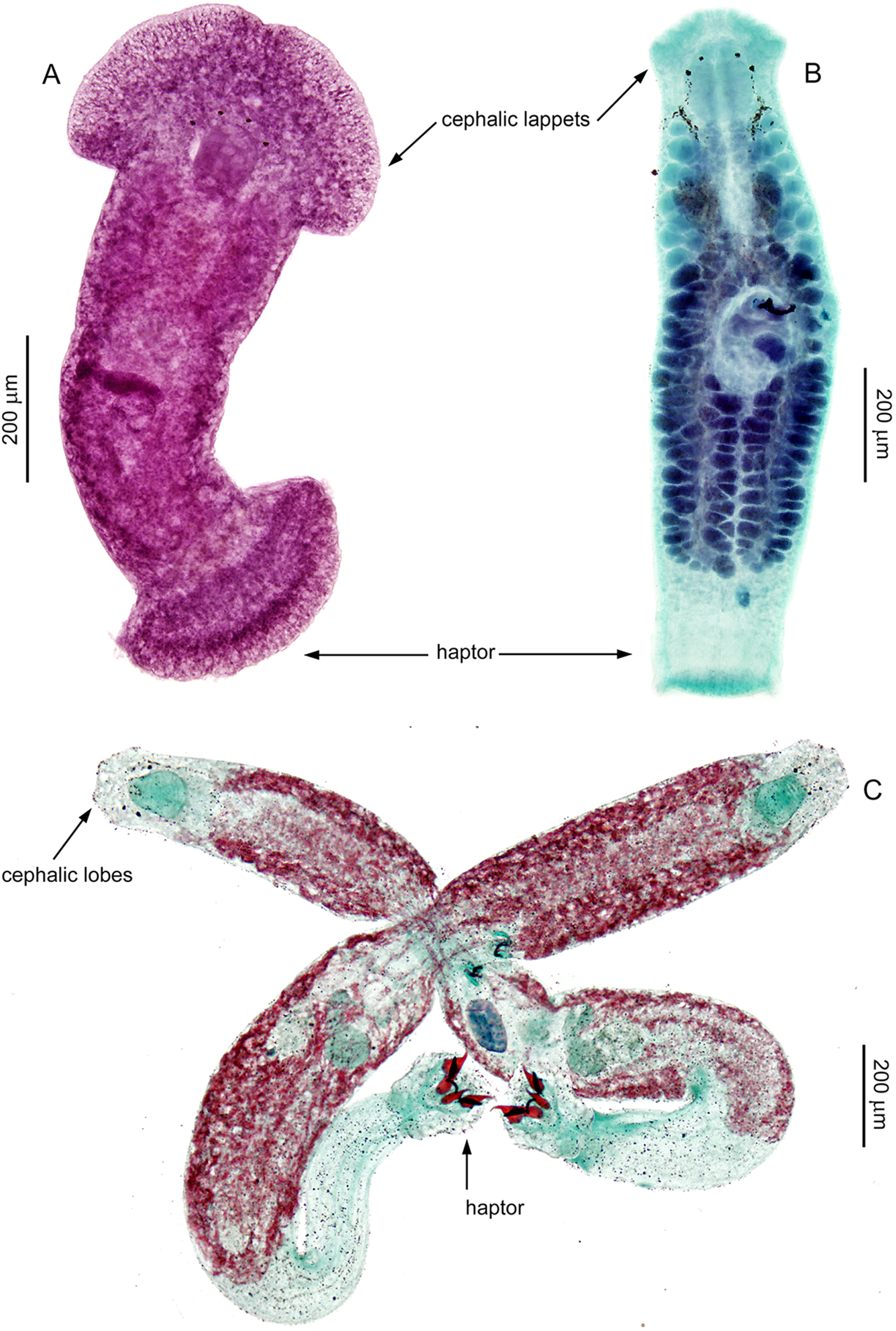
Remarks: The main taxonomic criterion for distinguishing between genera of this family is the shape and structure of the anterior (prohaptor) and posterior (haptor) attachment organs (Leiby et al. Reference Leiby, Kritsky and Peterson1972; Kritsky et al. Reference Kritsky, Mizelle and Bilqees1978; Beverley-Burton Reference Beverley-Burton, Margolis and Kabata1984). In species of Anonchohaptor and Icelanonchohaptor, the prohaptor resembles the triangular head of free-living Platyhelminthes (e.g. Dugesia) and bears 2 bilateral lappets, which appear less developed in species of Icelanonchohaptor compared to those of Anonchohaptor (Beverley-Burton Reference Beverley-Burton, Margolis and Kabata1984; as confirmed by the present study; see Figure 1A, B). In contrast, the prohaptor in Myzotrema and Pseudomurraytrema consists of cephalic lobes (Rogers Reference Rogers1967; Beverley-Burton Reference Beverley-Burton, Margolis and Kabata1984; present study, Figure 1C), similar to those observed in diplectanids and dactylogyrids. While the prohaptor is largely similar in Anonchohaptor and Icelanonchohaptor, the overall morphology and size of the haptor provide more reliable features for distinguishing these genera. Our study confirms the diagnostic characteristics outlined by Beverley-Burton (Reference Beverley-Burton, Margolis and Kabata1984), indicating that the haptor in Anonchohaptor is disc-shaped, notably wider than the body diameter, and slightly muscular, whereas in Icelanonchohaptor, it is cup-shaped, approximately the same diameter as the body, and strongly muscular (Figure 1A, B). With regard to the configuration of the haptoral sclerotized structures, species of all genera within Pseudomurraytrematidae possess 7 pairs of hooks. However, a fully developed ventral and dorsal anchor–bar complex is present only in species of Pseudomurraytrema (Figure 1C) and in the monotypic Myzotrema. In Pseudomurraytrema species, the dorsal bar is a paired structure, in contrast to a single bar observed in Myzotrema cyclepti (Rogers Reference Rogers1967). The presence or absence of the anchor–bar complexes appears to be a crucial trait for differentiating species of the 2 aforementioned genera from those of Anonchohaptor and Icelanonchohaptor. Species within Pseudomurraytrematidae generally share a conserved internal anatomy and, in particular, a basic structure of the MCO and vagina, with the exception of P. fergusoni (see Remarks to the species). The MCO consists of a copulatory tube supported by a 3-armed accessory piece (Figure 2). The copulatory tube is U-shaped (resembling a lyre). Its proximal portion is S-shaped, formed by 2 opposing curvatures, while the distal portion is narrowed. The 2 parts are separated by a submedial spine (Figure 2A). Among species, variation is observed in the thickness of the tube, the degree and position of the curves, and the form of the distal end. In P. fergusoni, the distal end of the copulatory tube is truncated, and the base fuses with the accessory piece to form a bulbous structure. Moreover, the vagina of P. fergusoni lacks the coiled proximal portion that characterizes all other known species of the family (typical morphology shown in Figure 2E, F).

Figure 1. Photomicrographs of stained specimens representing 3 genera of Pseudomurraytrematidae. A – Anonchohaptor meganbeanae n. sp. ex Ictiobus bubalus (Texas); B – Icelanonchohaptor cherubinus n. sp. ex Ictiobus bubalus (Mississippi); C – Pseudomurraytrema rogersi – mating pair ex Hypentelium nigricans (Arkansas). Diagnostic features distinguishing these genera are labelled in the image and correspond to characters used in the identification key.

Figure 2. Photomicrographs of the MCO (Pseudomurraytrema alabarrum) and vagina (Icelanonchohaptor seraphinus n. sp.). A: submedial spine demarcating the proximal and distal parts of the copulatory tube; B–D: arms of the accessory piece (proximal, medial and distal, respectively); E: multiply coiled proximal portion of the vagina; F: pouch-like distal portion of the vagina. These photomicrographs illustrate the typical morphology of the MCO and vagina in members of Pseudomurraytrematidae.
Species identification of pseudomurraytrematids is based primarily on the morphology and size of the haptoral hard structures and the distal parts of the reproductive system (i.e. MCO and vagina). However, existing taxonomic works on these parasites often lack consistency in representing all these structures, frequently omitting comprehensive details of the hook(s) and/or vagina. This lack of detail, combined with oversimplified depictions of the MCO, complicates accurate species delimitation – particularly within Anonchohaptor and Icelanonchohaptor, where the haptor bears only hooks.
Key to genera of Pseudomurraytrematidae
1. (2) Haptor with 7 pairs of hooks ………………………………………………………………………………………………. 3
2. (1) Haptor with 7 pairs of hooks, dorsal and ventral anchor-bar complexes — each with a pair of anchors without differentiated roots, and a single bar ……………………………………….... 5
3. (4) Haptor wider than the peduncle, disc-shaped, slightly muscular ……………………………………………………………….…………………………........... Anonchohaptor (Figure 1A)
4. (3) Haptor as wide as the peduncle, cup-shaped, highly muscular ………………………… ……………………………………………………….…………….... Icelanonchohaptor (Figure 1B)
5. (6) Ventral bar single; dorsal bar single …………………….. ……………………………………….......... Myzotrema cyclepti
6. (5) Ventral bar single; dorsal bar paired …………… ……………………................ Pseudomurraytrema (Figure 1C)
The following section provides formal descriptions of 7 new species and redescriptions of 11 known species of Pseudomurraytrematidae. Several additional specimens were identified as Anonchohaptor sp. based on general morphology but lacked sufficient diagnostic features for species-level identification; these specimens are included in the phylogenetic analyses pending further taxonomic resolution.
Anonchohaptor meganbeanae n. sp. (Figure 3)
Type host and locality: Ictiobus bubalus (Rafinesque) – Guadalupe River, San Marcos (29°53′24″N, 97°56′03″W), Texas (4 June 2023).

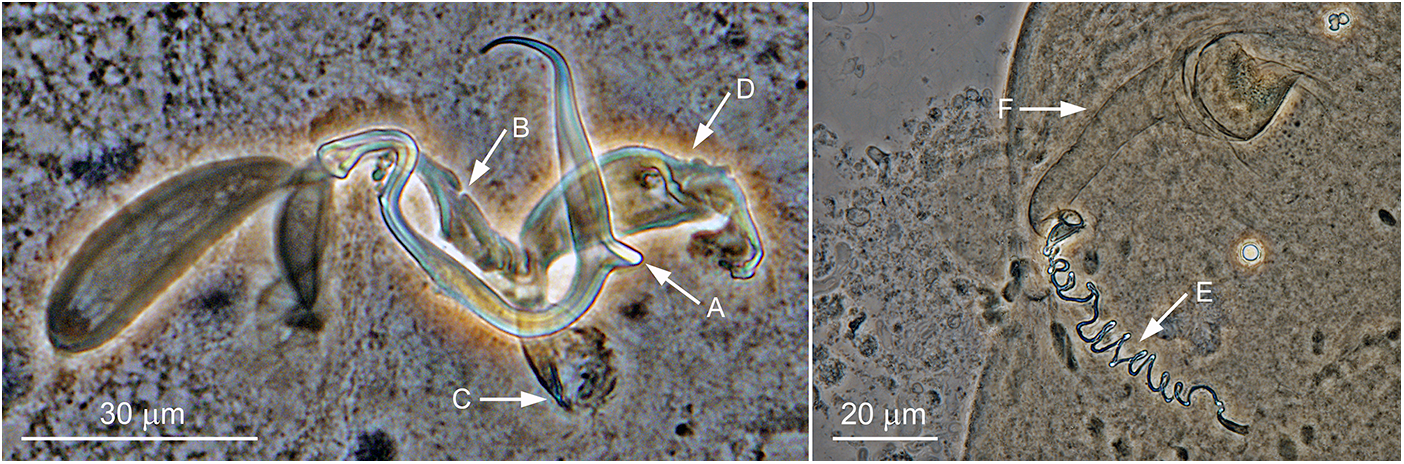
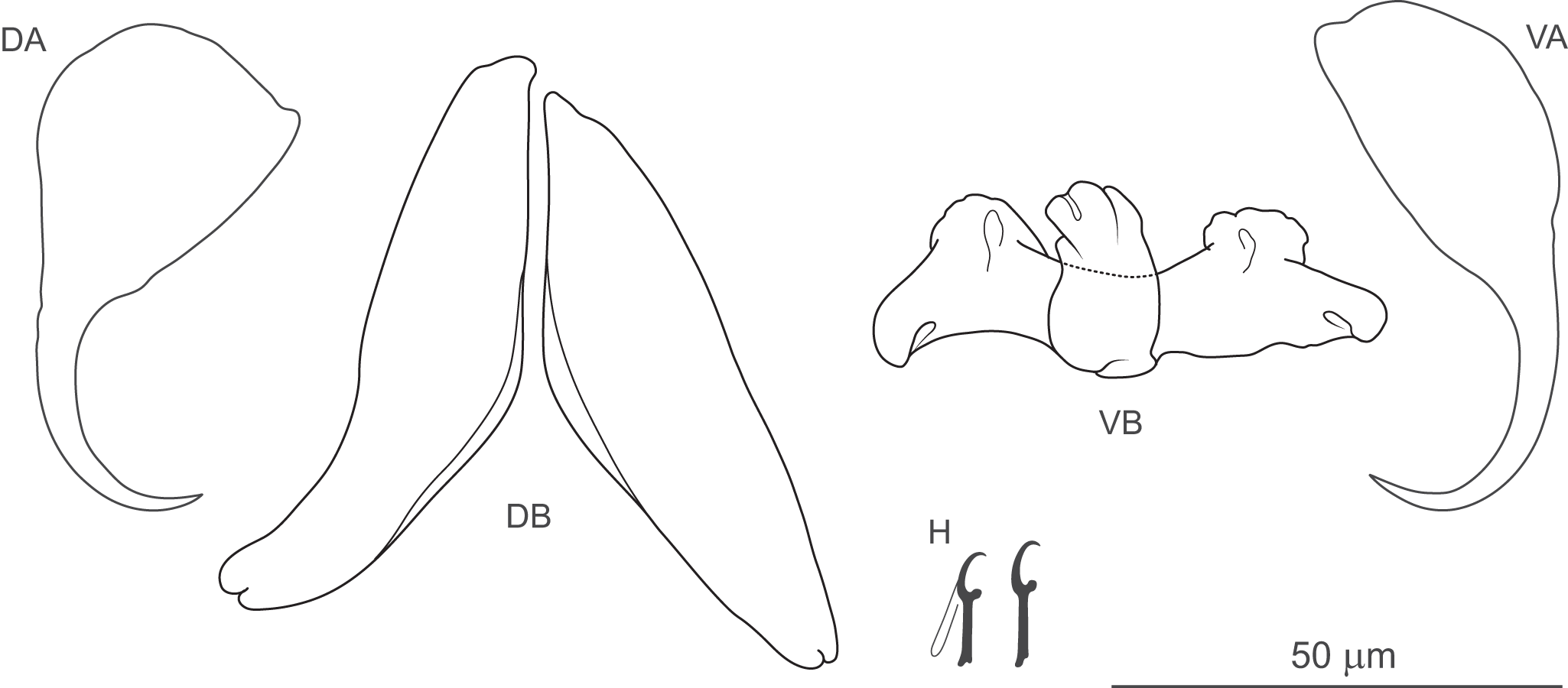
Figure 3. Anonchohaptor meganbeanae n. sp. ex Ictiobus bubalus (Texas): Hc – central hook; Hm – marginal hook; MCO – male copulatory organ; VG – vagina.
Other locality: Mississippi River, Illinois (12 July 2022).
Site of infection: Gills.
Etymology: The species is named in honour of Megan Bean (Texas Parks and Wildlife Department, USA) for her valuable contributions to the research and conservation of North American freshwater fishes, and for her support during fish sampling in Texas in 2023.
ZooBank registration (LSID): urn:lsid:zoobank.org:act:169FD241-5A6B-4EA2-8BE5-91576CE8C422.
Prevalence and intensity of infection: Texas – 67% (2 fish infected/3 fish examined); 3 parasites per infected host. Illinois – 25% (1 fish infected/4 fish examined); 4 parasites per infected host (juveniles).
Type and voucher specimens: Holotype (USNM 1762124); 1 paratype (USNM 1762125); 1 hologenophore (USNM 1762126).
Description: Body dorsoventrally flattened, tapering posteriorly, with maximum width in anterior trunk region, just posterior to cephalic lobes. Cephalic region hammer-shaped, well defined, markedly wider than anterior trunk. Peduncle short to absent; haptor clearly delimited, distinctly wider than peduncle. Head organs indistinct. Eyes 4; members of posterior pair farther apart than those of anterior pair. Accessory eye granules absent. Approximately 8–10 large cephalic glands per bilateral group, extending from just posterior to pharynx to level of oesophageal bifurcation. Mouth subterminal, located just anterior to pharynx; pharynx subspherical. Oesophagus elongate, bifurcating into 2 intestinal caeca immediately anterior to MCO; caeca apparently united posteriorly.
Ovary looping around right intestinal caecum. Vagina dextroventral, consisting of lightly sclerotized distal funnel-shaped portion and a multiply coiled proximal tube (approximately 17 coils). Vitellarium absent in region of reproductive organs. Testis postovarian, ovoid, small relative to ovary; vas deferens looping around left intestinal caecum. MCO comprising copulatory tube and articulated accessory piece. Copulatory tube broadly U-shaped, with submedial spine separating proximal S-shaped portion from narrowed distal portion. S-shaped portion with distinct bends; distal portion curved inward terminally, forming a blunt hook-shaped end; submedial spine moderately developed. Accessory piece 3-armed: proximal arm composed of 2 parts situated bilaterally to the proximal region of the copulatory tube – 1 grooved, the other wing-like; medial arm paddle-shaped; distal arm longest, robust and groove-like.
Haptor clearly delimited, disc-shaped, armed with 2 central and 12 marginal hooks; anchor-bar complexes absent. Hooks of similar shape and size; each with an elongate thumb projecting perpendicularly from shaft; shank uniform or slightly tapered proximally, with a rounded and slightly recurved base; filamentous hooklet (FH) loop about three-quarters of shank length.
Measurements: Body length 723 (461–985; n = 2); greatest width 192 (125–258; n = 2). Pharynx 68 (54–81; n = 2) in diameter. Haptor 149 (81–217; n = 2) long, 240 (120–360; n = 2) wide. Central hook 13 (n = 3) long, marginal hook 13 (12–13; n = 3) long. MCO – tube curved length 76 (n = 1).
Remarks: Anonchohaptor currently comprises 3 valid species: A. anomalum Mueller, 1938; A. muelleri Kritsky, Leiby & Shelton, 1972; and A. olseni Leiby, Kritsky & Bauman, 1973 (Mueller Reference Mueller1938; Kritsky et al. Reference Kritsky, Leiby and Shelton1972; Leiby et al. Reference Leiby, Kritsky and Bauman1973). Anonchohaptor meganbeanae n. sp. clearly differs from A. muelleri by having hooks of uniform size (vs central hooks distinctly larger than marginal hooks in A. muelleri). It can be distinguished from A. anomalum by its smaller hooks (13 vs 17 in A. anomalum) and a vagina that appears to be less coiled (17 vs 11 coils in A. anomalum), based on the original illustration (see Figure 4 in Mueller Reference Mueller1938). Compared to A. olseni, the new species has slightly larger hooks (12–13 vs 9–12).
Icelanonchohaptor cherubinus n. sp. (Figure 4)
Type host and locality: Ictiobus bubalus (Rafinesque) – Oxbow south of Cumbest Bridge landing, Pascagoula River (30°30′23″N, 88°32′25″W), Mississippi (19 June 2019).

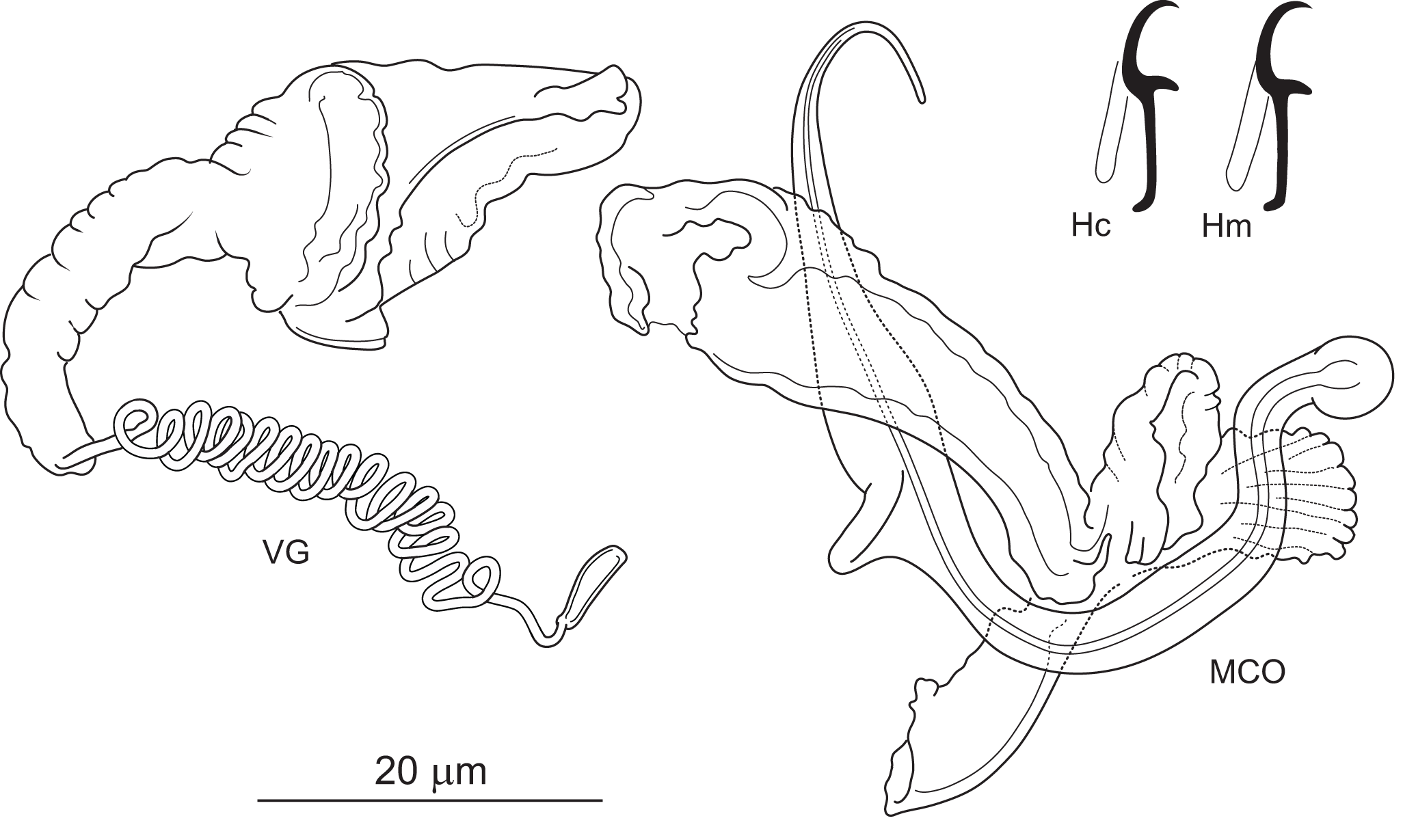
Figure 4. Icelanonchohaptor cherubinus n. Sp. ex Ictiobus bubalus (Mississippi): Hc – central hook; Hm – marginal hook; MCO – male copulatory organ; VG – vagina.
Site of infection: Fins.
Etymology: The specific name cherubinus is derived from kerūv (Hebrew, Latinized), referring to the Cherubim – a class of celestial beings traditionally associated with strength and guardianship. The name refers to wing-like extensions flanking the proximal region of the MCO.
ZooBank registration (LSID): urn:lsid:zoobank.org:act:CF1B713C-D437-42C0-8E05-FD2EBDB10A6A.
Prevalence and intensity of infection: 50 % (1 fish infected/2 fish examined); 4 parasites per infected host.
Type and voucher specimens: Holotype (USNM 1762127); 2 paratypes (USNM 1762128, 1762129); 2 hologenophores (USNM 1762130, 1762131).
Description: Body elongate, with nearly uniform width throughout its length, dorsoventrally flattened. Cephalic region trapezoidal to bluntly arrow-shaped. Peduncle merging into haptor; boundary indistinct, peduncle and haptor of equal width. Head organs 4 bilateral pairs. Two large cells, function unknown, present just anterior to eyes. Eyes 4; members of posterior pair farther apart than those of anterior pair. Fine line of pigment granules extending posteriorly from each posterior eye, branching radially at level of posterior part of pharynx. Accessory eye granules absent. Approximately 11 large cephalic glands in each bilateral group, extending from just posterior to pharynx to level of oesophageal bifurcation. Mouth subterminal, just anterior to large pharynx. Pharynx subspherical. Oesophagus elongate, bifurcating into 2 intestinal caeca immediately anterior to MCO; caeca apparently united posteriorly.
Ovary looping right intestinal caecum. Vagina dextroventral, comprising lightly sclerotized distal funnel-shaped portion and multiply coiled proximal tube. Vitellarium absent in region of reproductive organs; 2 vitelline bulbs lateral to oesophagus, narrowing at level of bifurcation, continuing posteriorly as 2 lateral bands along intestinal caeca. Testis postovarian, ovoid, small relative to ovary; vas deferens loops left intestinal caecum. MCO comprising copulatory tube and articulated accessory piece. Copulatory tube broadly U-shaped, with submedial spine separating proximal S-shaped portion from narrowed distal portion. S-shaped portion with shallow and indistinct bends; distal portion curved inward terminally, forming a blunt sickle-shaped end; submedial spine well-developed. Accessory piece 3-armed; proximal arm formed by wing-like, finely striated extensions, situated bilaterally to proximal part of copulatory tube; medial arm elongate, leaf-shaped; distal arm longest, robust, groove-like.
Haptor poorly demarcated from a short peduncle, cup-shaped, armed with 2 central and 12 marginal hooks; anchor-bar complexes absent. Hooks of similar shape and size; each with an elongate thumb projecting obliquely downward from shaft; shank slightly curved and expanded proximally; FH loop about one-half of shank length.
Measurements: Body length 1906 (1250–2980; n = 3); greatest width 225 (320–430; n = 3). Pharynx 135 (126–148; n = 3) in diameter. Haptor 282 (244–320; n = 3) wide. Central hook 19 (n = 4) long, marginal hook 19 (19–20; n = 4) long. MCO – tube curved length 107 (104–111; n = 3).
Remarks: To date, 4 species of Icelanonchohaptor have been described: I. icelanonchohaptor Leiby, Kritsky & Peterson, 1972 from Ictiobus cyprinellus (Missouri River, North and South Dakota), I. microcotyle Kritsky, Leiby & Shelton, 1972 from Carpioides carpio (Missouri River, North and South Dakota), I. fyviei Dechtiar & Dillon, 1974 from Carpiodes cyprinus (Lake Erie, Canada) (Kritsky et al. Reference Kritsky, Leiby and Shelton1972, Leiby et al. Reference Leiby, Kritsky and Peterson1972, Dechtiar and Dillon Reference Dechtiar and Dillon1974) and recently I. tropicalis Mendoza-Franco, Hernández-Gómez & Caspeta-Mandujano, 2023 from Ictiobus meridionalis (Recreo River, Tabasco, Mexico) (Mendoza-Franco et al. Reference Mendoza-Franco, Hernández-Gómez and Caspeta-Mandujano2023).
Icelanonchohaptor cherubinus n. sp. is similar to I. microcotyle and I. seraphinus n. sp. (see below) in possessing a MCO with conspicuous wing-like expansions of the accessory piece situated bilaterally to the proximal part of the copulatory tube. Icelanonchohaptor cherubinus n. sp. differs clearly from both congeners in the morphology of the hooks: the thumb is obliquely truncated (vs beak-shaped in I. microcotyle and I. seraphinus n. sp.), and they are slightly longer (19–20 vs 16–19 and 16–17 in I. microcotyle and I. seraphinus n. sp., respectively). In addition, I. cherubinus n. sp. differs from I. microcotyle in having a vagina with a longer vaginal tube comprising approximately 18 coils (vs 10 coils in I. microcotyle).
Icelanonchohaptor seraphinus n. sp. (Figure 5)
Type host and locality: Ictiobus niger (Rafinesque) – Hutson Lake, Pascagoula River (30°52′21″N, 88°45′47″W), Mississippi (17 June 2019).

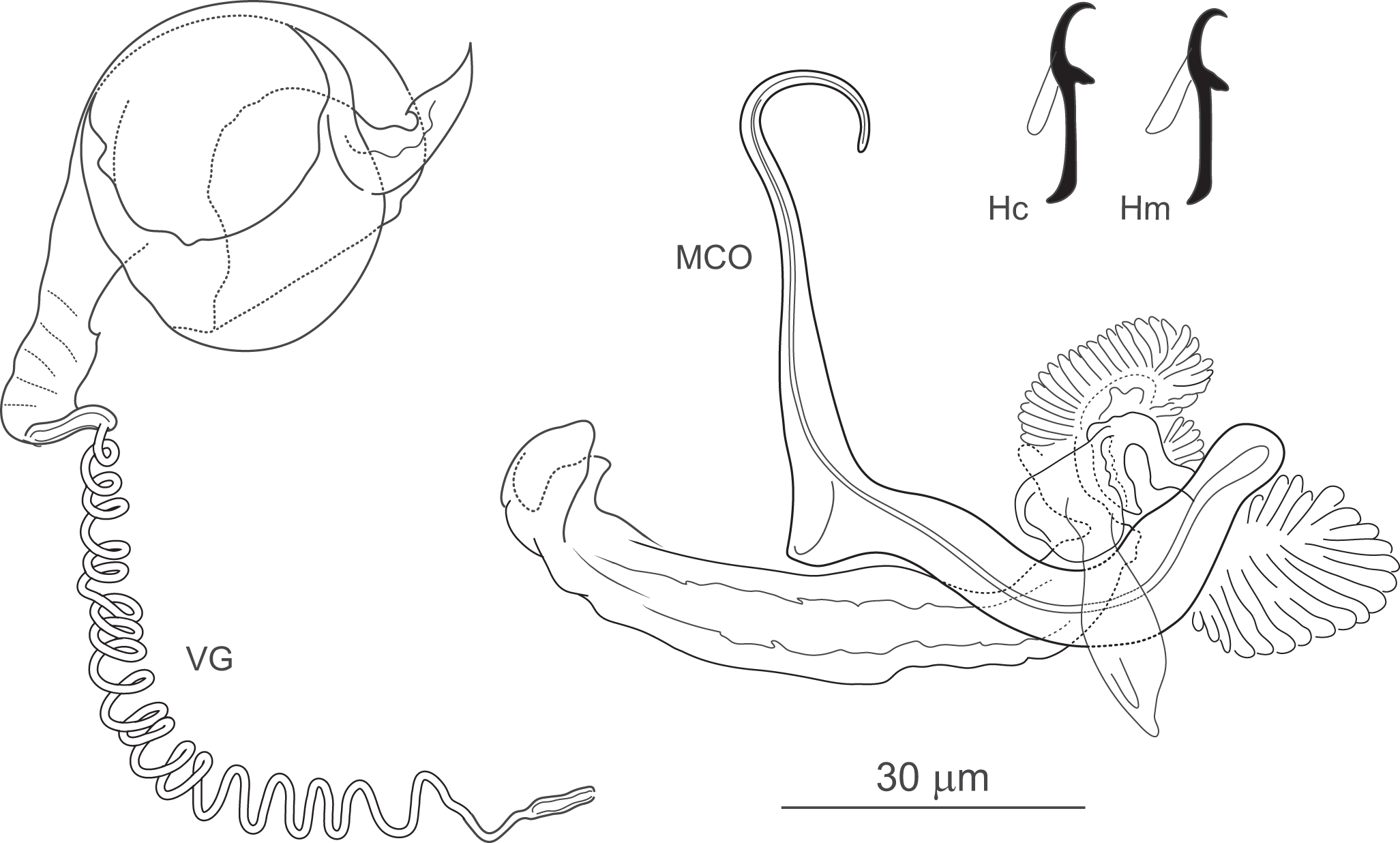
Figure 5. Icelanonchohaptor seraphinus n. sp. ex Ictiobus Niger (Mississippi): Hc – central hook; Hm – marginal hook; MCO – male copulatory organ; VG – vagina.
Site of infection: Fins.
Etymology: The specific name seraphinus is derived from śərāfîm (Hebrew, Latinized), referring to the Seraphim – a class of high-ranking angels traditionally associated with wings. The name alludes to wing-like extensions flanking the proximal region of the copulatory tube.
ZooBank registration (LSID): urn:lsid:zoobank.org:act:4EEEF393-E855-41C3-9BDA-1DAF7A93C294.
Prevalence and intensity of infection: 67% (2 fish infected/3 fish examined); 3 parasites per infected host (mostly juveniles).
Type and voucher specimens: Holotype (USNM 1762132); 1 hologenophore (USNM 1762133).
Description: Due to limited material, internal anatomy could not be described. Four of 5 specimens were juveniles; the only adult (hologenophore) was incomplete, with part of the body processed for DNA extraction. Description is based on morphometry of sclerotized structures, illustrated by line drawings. MCO comprising copulatory tube and articulated accessory piece. Copulatory tube broadly U-shaped, with submedial spine separating proximal S-shaped portion from narrowed distal portion. S-shaped portion with shallow and indistinct bends; distal portion curved inward terminally, forming a blunt sickle-shaped end; submedial spine massive.
Haptor poorly demarcated from a short peduncle, cup-shaped; armed with 2 central and 12 marginal hooks; anchor-bar complexes absent. Hooks of similar shape and size; each with beak-like thumb projecting perpendicularly from shaft; shank slightly expanded proximally; FH loop about one-half of shank length.
Measurements: Central hook 17 (n = 1) long, marginal hook 17 (16–17; n = 1) long. MCO – tube curved length 119 (117–119; n = 3).
Remarks: Icelanonchohaptor seraphinus n. sp. is recovered as sister species to I. cherubinus n. sp. in our phylogenetic analyses (see Figures 23 and 24). Icelanonchohaptor seraphinus n. sp. differs from the latter species in the shape and size of the hooks (see Remarks for I. cherubinus n. sp.) and in having an MCO that appears more robust. Icelanonchohaptor seraphinus n. sp. also resembles I. microcotyle in the general shape of the MCO and hooks, both bearing hooks with a beak-like thumb. It differs from I. microcotyle by possessing a vagina with a longer vaginal tube comprising approximately 18 coils (vs 10 coils in I. microcotyle).
Pseudomurraytrema species parasitizing species of Catostomus (Catostomini)
Pseudomurraytrema ardens n. sp. (Figure 6)
Type host and locality: Catostomus macrocheilus Girard – Siuslaw River, Oregon (27 June 2024).

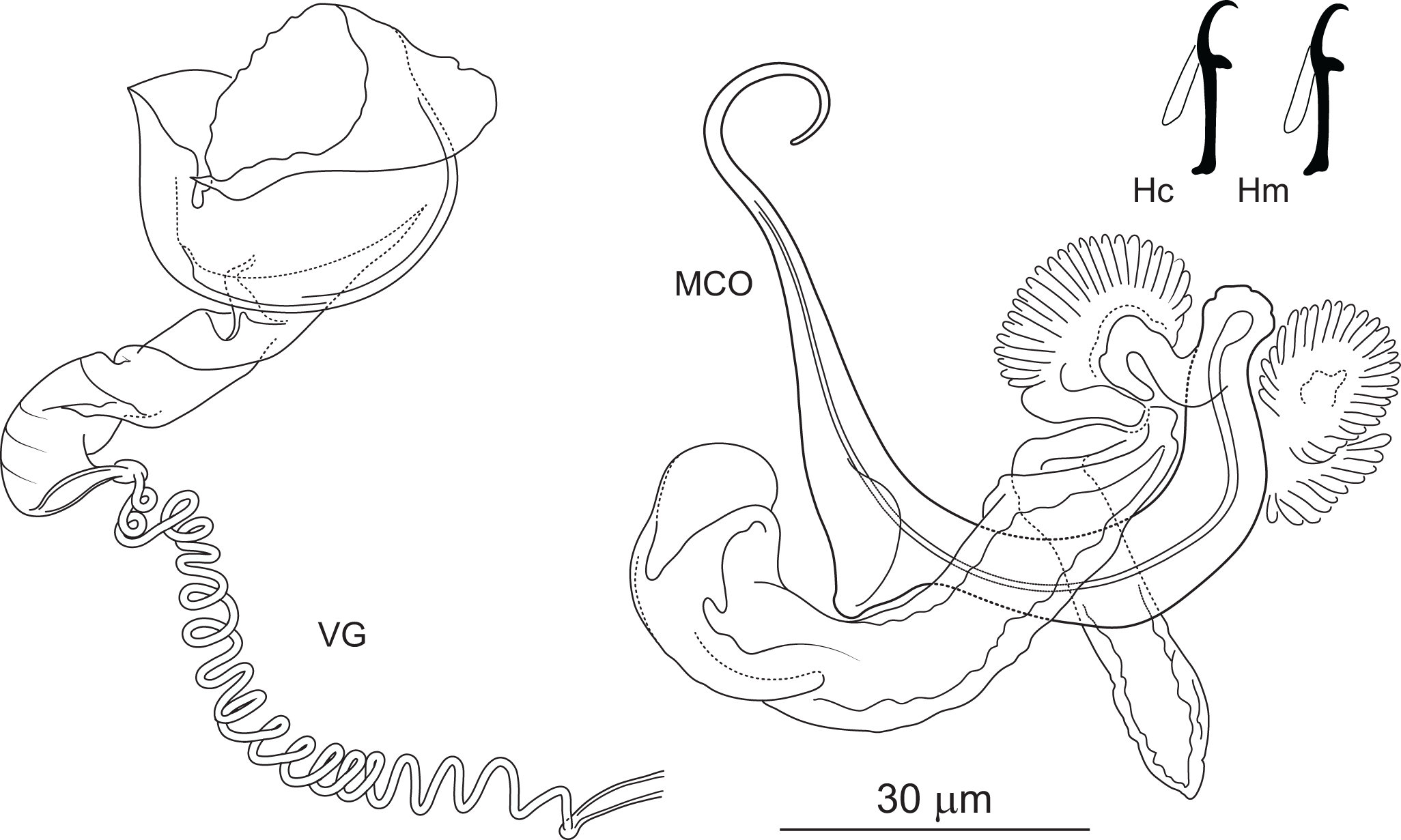
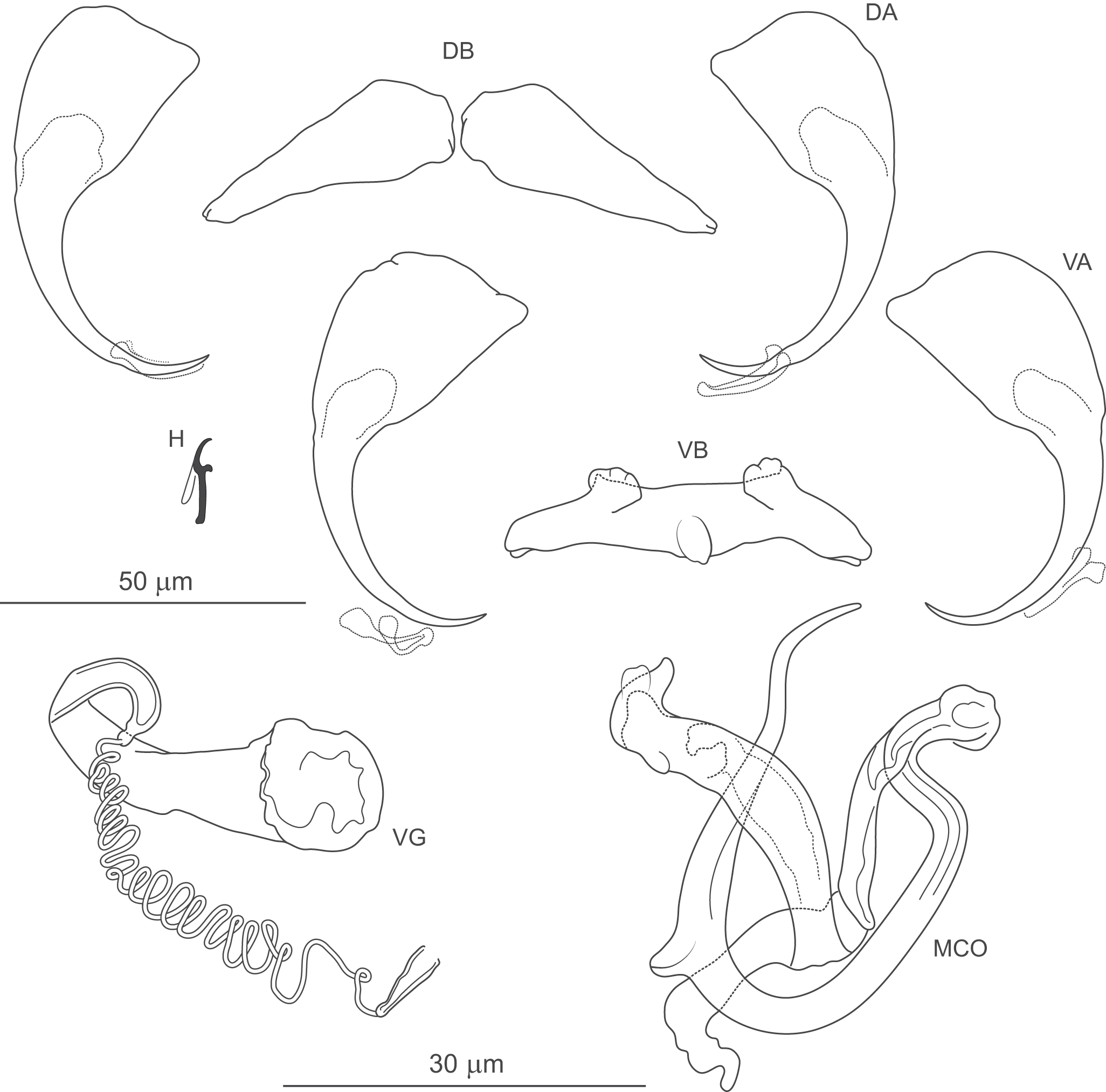
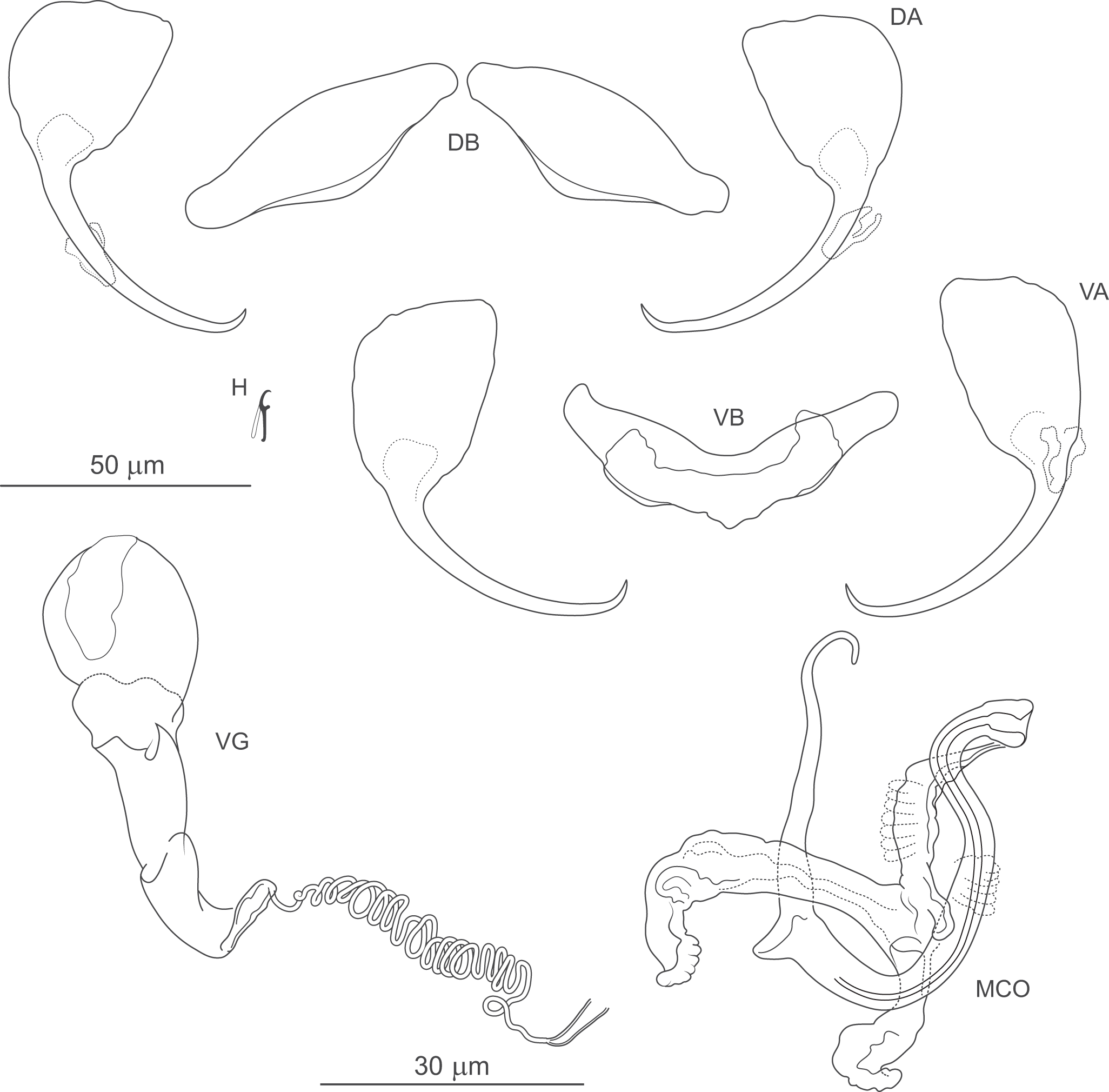
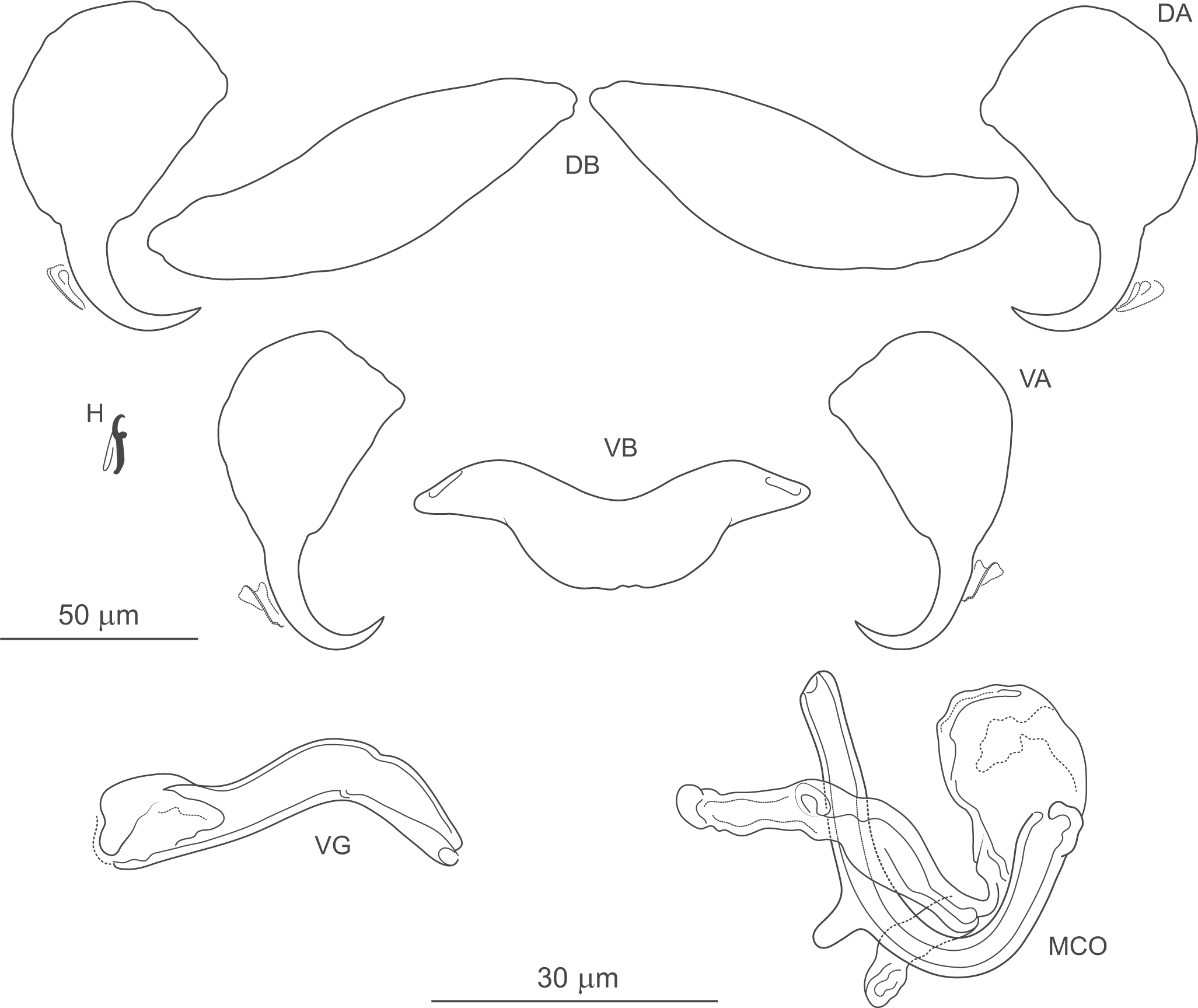
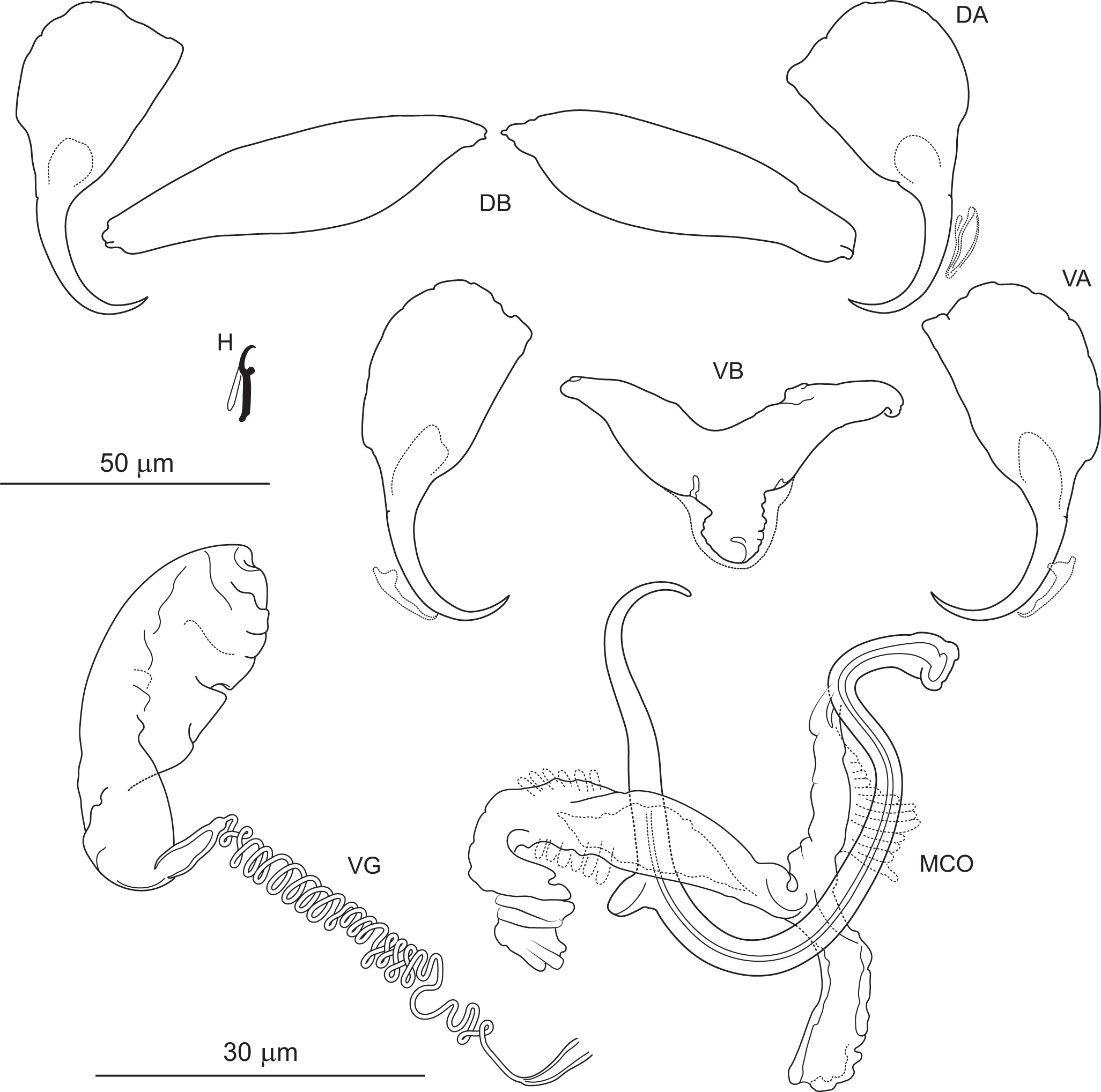
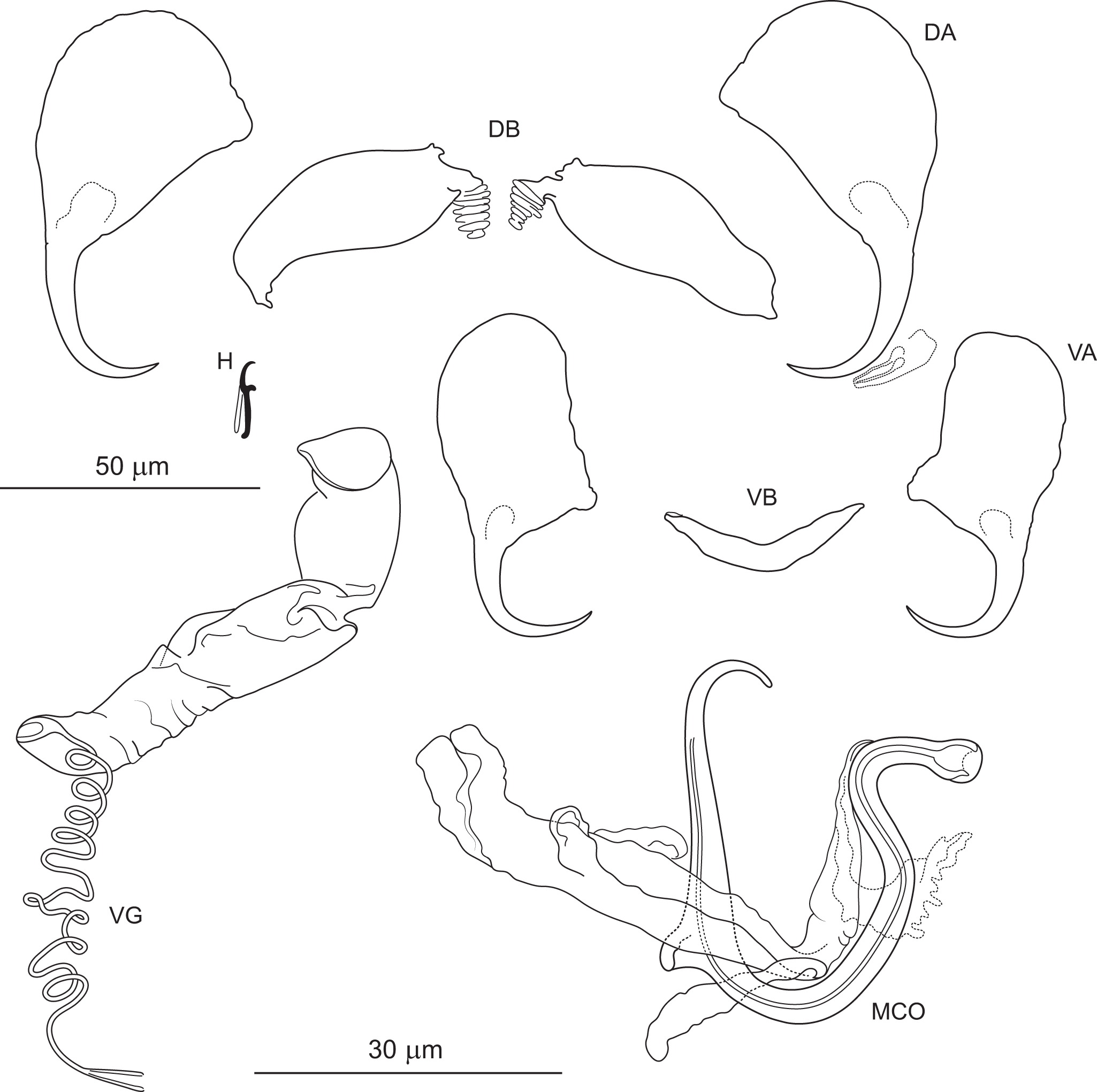
Figure 6. Pseudomurraytrema ardens n. sp. ex Catostomus macrocheilus (Oregon): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Previous records: Catostomus ardens Jordan & Gilbert – Snake River, Idaho (as Pseudomurraytrema sp. ‘ardens’; Littlewood et al. Reference Littlewood, Rohde and Clough1998).
Site of infection: Gills.
Etymology: The specific name ardens is after the host on which the species was first found.
ZooBank registration (LSID): urn:lsid:zoobank.org:act:6EC27A7D-15FB-4B73-A7D2-2CBB1A6754F1.
Prevalence and intensity of infection: 50% (4 fish infected/8 fish examined); 2–5 parasites per infected host (mostly juveniles).
Type and voucher specimens: Holotype (USNM 1762081); 1 paratype (USNM 1762082); 3 hologenophores (USNM 1762083, 1762084, 1762085).
Description: Body elongate to fusiform, dorsoventrally flattened. Cephalic region relatively wide; trunk width uniform; peduncle short or absent. Haptor subhexagonal in dorsoventral view, wider than long, medium to large relative to trunk size. Head organs poorly defined. Two pairs of eyespots with poorly associated chromatic granules; members of both pairs similar in size and spacing. Accessory chromatic granules sparse, scattered in cephalic and anterior trunk region. Ventral mouth located between anterior eyespots. Pharynx subovate to spherical; oesophagus and intestinal caeca indistinct.
Gonads tandem or with testis slightly overlapping ovary dorsally. Ovary looping dorsoventrally around right intestinal caecum. Ootype and uterus not observed. Vagina dextroventral, located at about half of body length, composed of trumpet-shaped distal part and multiple-coiled vaginal tube with proximal fork. Vaginal canal and seminal receptacle not observed. Vitellarium follicular, extending from level of oesophagus to near haptor; absent in region of other reproductive organs. Transverse vitelline ducts not observed. Testis postovarian. Vas deferens and seminal vesicle not observed. Two prostatic reservoirs unequal in size. MCO composed of articulated copulatory tube and accessory piece. Copulatory tube U-shaped, with sharply S-shaped proximal portion (2 distinct bends in proximal third), separated by submedial spine from narrowed, curved distal part. Accessory piece with 3 rami: proximal ramus articulated to base; distal ramus largest, forming V-shaped structure with proximal 1; medial ramus arising externally to their junction.
Haptor armed with dorsal and ventral anchor–bar complexes and 7 pairs of hooks. Ventral and dorsal anchors similar in shape and size; each with narrow, terminally flattened base, elongate curved shaft and short point. Distal shaft undulation more evident in dorsal anchor. Ventral bar saddle-shaped, with 2 submedial protuberances on anterior margin and posteromedial knob-like process; ends slightly recurved posteriorly. Paired dorsal bar subtriangular, with broad, angularly rounded medial end tapering smoothly to pointed lateral ends. Hooks similar in shape and size; each with hooked thumb projecting perpendicularly from shank; shank uniform or slightly tapered proximally; base slightly enlarged, flattened; FH loop about three-quarters of shank length.
Measurements: Body 786 (n = 1) long; greatest width 174 (n = 1). Pharynx 69 (n = 1) long, 63 (n = 1) wide. Haptor 96 (n = 1) long, 209 (n = 1) wide. Ventral anchor 57 (54–60; n = 4) long; base width 21 (19–23; n = 4). Dorsal anchor 57 (55–59; n = 4) long; base width 21 (19–22; n = 4).Ventral bar 56 (53–59; n = 2) long. Paired dorsal bar 40 (37–43; n = 2) long. Hooks 14 (13–15; n = 4) long. MCO – tube curved length 87 (84–92 n = 3); tube height 34 (31–37; n = 3).
Remarks: This species was first reported by Littlewood et al. (Reference Littlewood, Rohde and Clough1998) as Pseudomurraytrema ardens, based on specimens collected by Delane Kritsky from Catostomus ardens in Idaho. Although Littlewood et al. (Reference Littlewood, Rohde and Clough1998) included genetic sequences of this species in their phylogenetic analysis, they did not formally describe it. Consequently, Boeger et al. (Reference Boeger, Kritsky, Domingues and Bueno-Silva2014) considered P. ardens a nomen nudum. Since then, Pseudomurraytrema sp. ‘ardens’ has been used in several other phylogenetic analyses as the only representative of Pseudomurraytrematidae with sequences deposited in GenBank (Olson and Littlewood Reference Olson and Littlewood2002; Bentz et al. Reference Bentz, Combes, Euzet, Riutord and Verneau2003; Šimková et al. Reference Šimková, Plaisance, Matějusová, Morand and Verneau2003; Plaisance et al. Reference Plaisance, Littlewood, Olson and Morand2005; Boeger et al. Reference Boeger, Kritsky, Domingues and Bueno-Silva2014; Ogawa and Itoh Reference Ogawa and Itoh2017; Moreira et al. Reference Moreira, Luque and Šimková2019; Soares et al. Reference Soares, Domingues and Adriano2021). This species is formally described here based on 3 specimens recovered from Catostomus macrocheilus in Oregon, whose DNA sequences match those of Pseudomurraytrema sp. ‘ardens’ in BLAST analyses. Pseudomurraytrema ardens n. sp. exhibits morphological similarities with P. commersoni n. sp. and P. kritsdelani n. sp., particularly in having a trumpet-shaped distal portion of the vagina and a copulatory tube with an undulating distal section that typically curves outward at the tip. Among these, P. ardens n. sp. most closely resembles P. kritsdelani n. sp. (see below) in the morphology of the ventral anchors and the vagina. In both species, the ventral anchors possess a moderately broad base with a flattened proximal margin and an elongate, curved shaft exhibiting slight undulation near its junction with a short point. The vaginas of both species consist of a trumpet-shaped distal portion and a multiply coiled vaginal tube. Pseudomurraytrema ardens n. sp. clearly differs from P. kritsdelani n. sp. by having: (1) morphologically similar ventral and dorsal anchors (vs a dorsal anchor with a significantly wider fan-shaped base compared to the ventral anchor in P. kritsdelani n. sp.), (2) ventral and dorsal anchors with a smooth outer margin between shaft and base (vs an abrupt junction between shaft and base, especially evident in the dorsal anchors of P. kritsdelani n. sp.), (3) a hook with a narrower shank of nearly uniform diameter (vs thicker and slightly inflated shank on its inner side, except for the curved terminal part, in P. kritsdelani n. sp.; see Figure 7 for comparison) and (4) narrower paired dorsal bar with a less bulgy medial end. In addition, P. ardens n. sp. ranks among the smallest representatives of Pseudomurraytrema, differing in body size from P. kritsdelani n. sp. (786 vs 1040 in P. kritsdelani n. sp.).

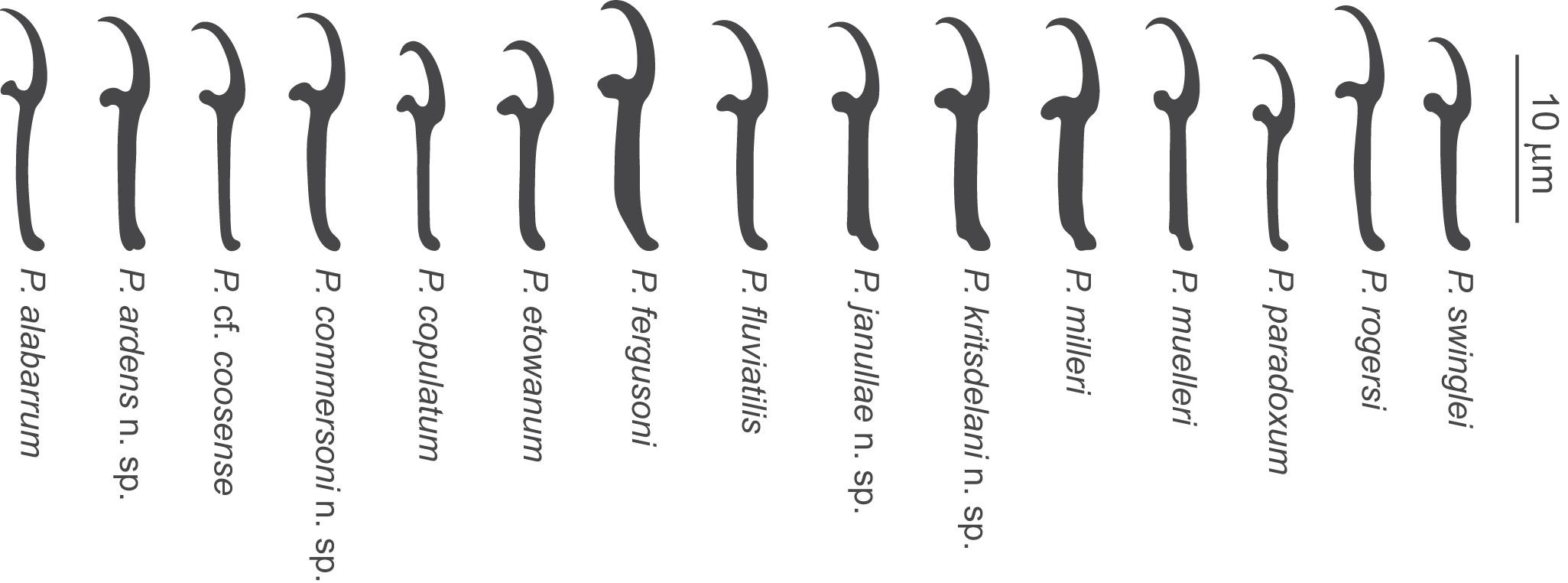
Figure 7. Comparative morphology of hooks of Pseudomurraytrema species: all 11 previously described Nearctic species and 4 new species. Pseudomurraytrema coosense tentatively identified (cf.); P. Muelleri illustrated from the holotype, not among present material.
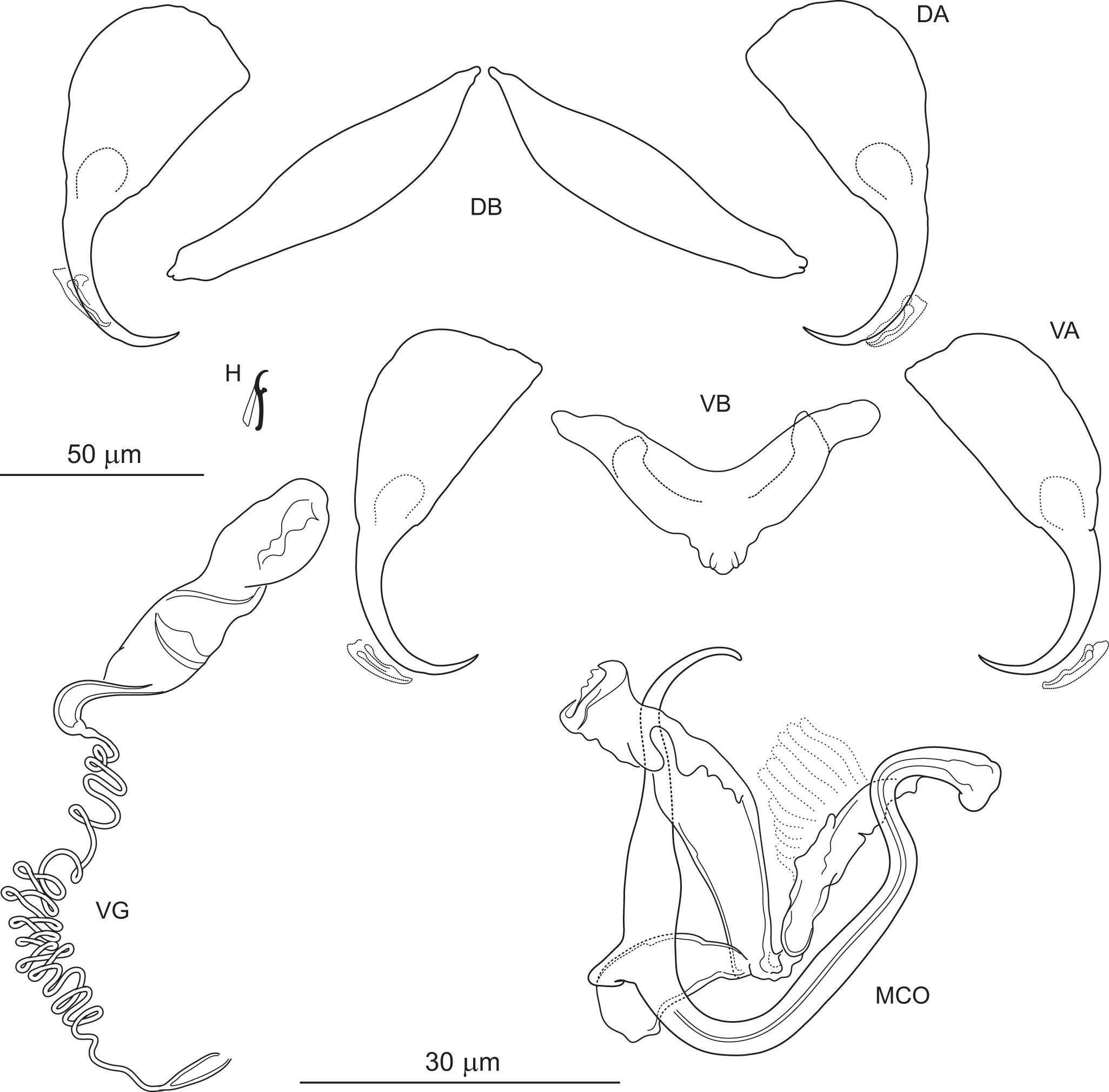
Pseudomurraytrema commersoni n. sp. (Figure 8)
Synonym: Murraytrema copulatum Mueller, Reference Mueller1938 (in part).

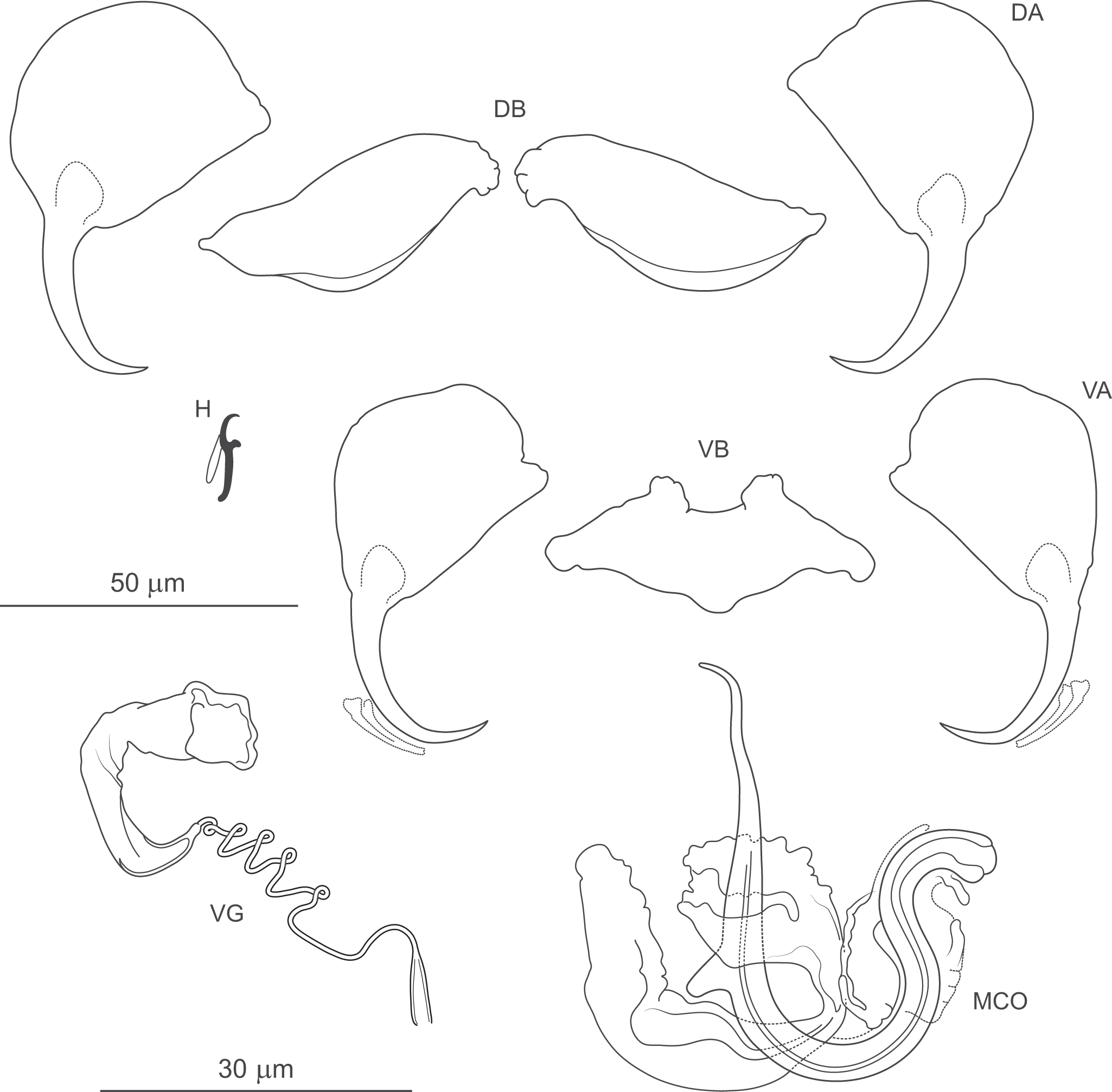
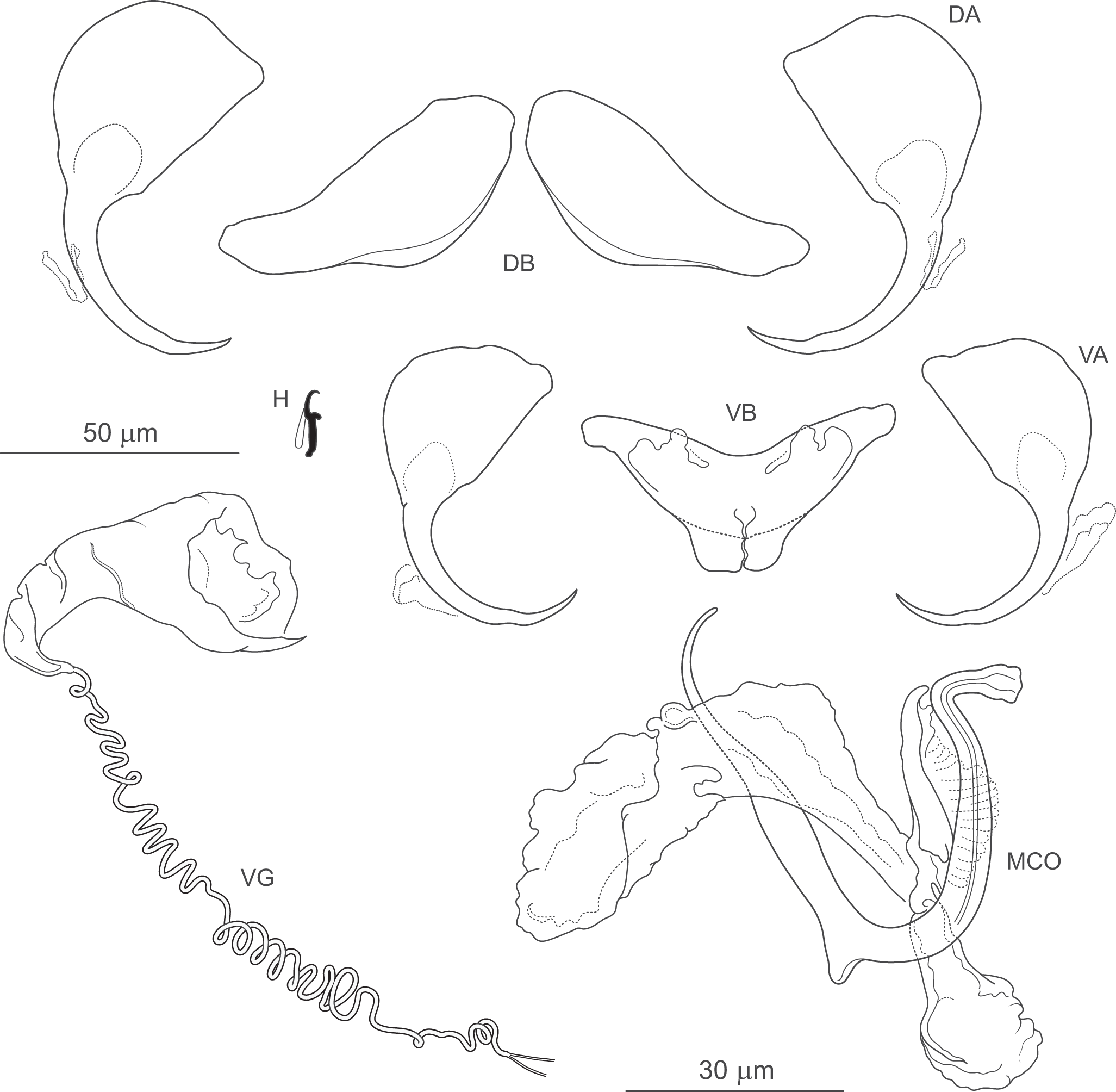
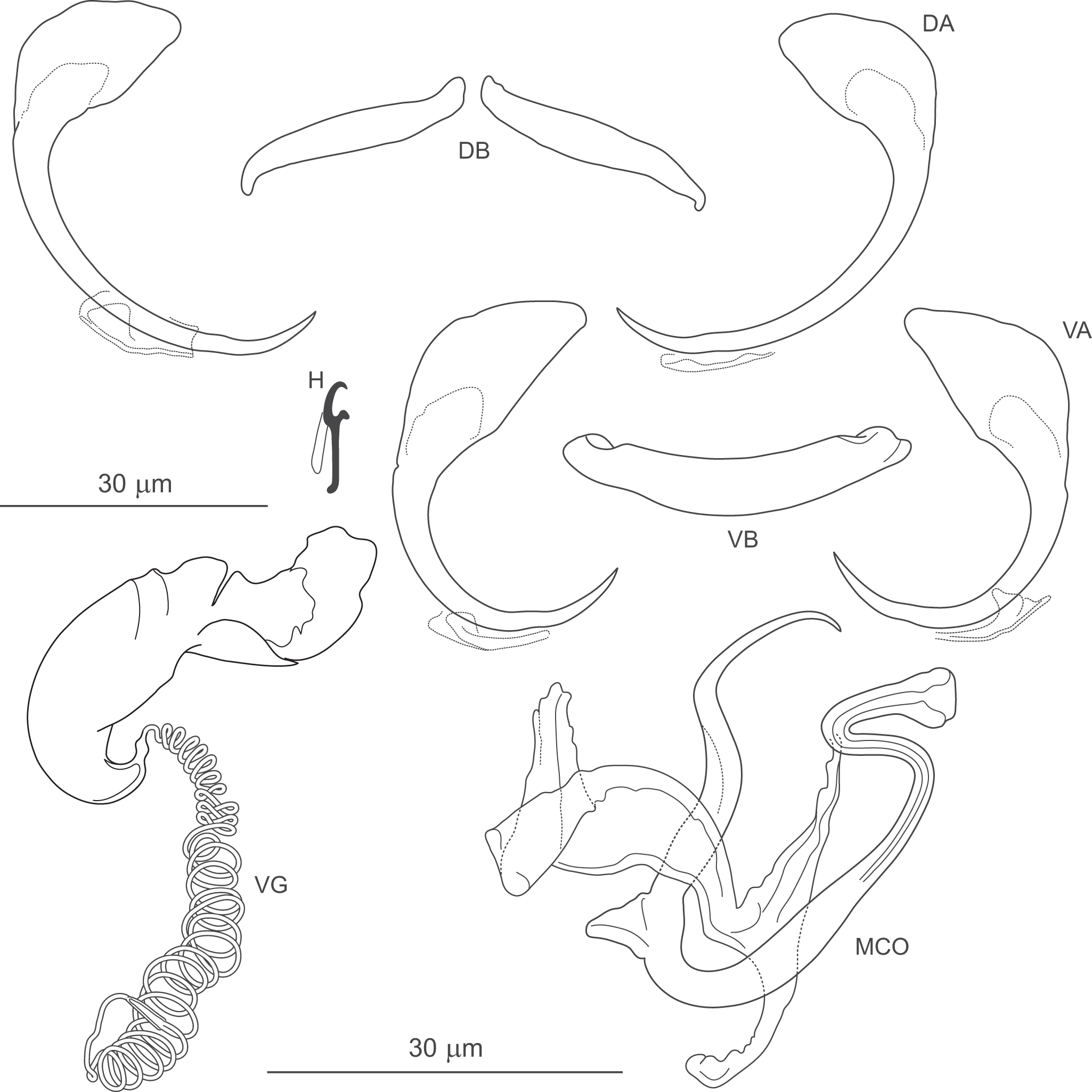
Figure 8. Pseudomurraytrema commersoni n. sp. ex Catostomus commersonii (Wisconsin): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Type host and locality: Catostomus commersonii (Lacepède) – Hickory Oak Pond, Wisconsin (23 September 2018).
Other records: Catostomus commersonii – Moore’s Creek (43°54′15″N, 91°13′31″W), Wisconsin (9 July 2022).
Previous records: Catostomus commersonii – Chautauqua Lake and French Creek, near Panama, New York (as Murraytrema copulatum; Mueller Reference Mueller1938).
Unconfirmed host and locality records: Catostomus commersonii and Moxostoma pisolabrum Trautman & Martin (syn. M. aureolum) – Silver Creek (Fond du Lac Co.), St. Croix River (Burnett Co.), Wisconsin (Mizelle and Klucka Reference Mizelle and Klucka1953).
Site of infection: Gills.
Etymology: The specific name commersoni is, like the host, in honour of the French naturalist Philibert Commerson.
ZooBank registration (LSID): urn:lsid:zoobank.org:act:62CD8EFD-97F3-4496-8776-57E0C721C5A7.
Prevalence and intensity of infection: Wisconsin (2018) – 8% (1 fish infected/13 fish examined); 2 parasites per infected host. Wisconsin (2022) – 25% (2 fish infected/8 fish examined); 1–2 parasites per infected host.
Type and voucher specimens: Holotype (USNM 1762087); 1 hologenophore (USNM 1762088).
Description (based on 1 whole-mounted specimen and 1 hologenophore; both specimens were incomplete and did not allow assessment of body morphology or internal anatomy). MCO composed of articulated copulatory tube and accessory piece. Copulatory tube U-shaped, with thick, loosely S-shaped proximal portion (2 shallow, equally spaced bends), separated by submedial spine from narrowed distal part, usually curved outwards at tip. Accessory piece with 3 rami: proximal ramus articulated to base, bearing finger-like processes; distal ramus largest, forming V-shaped structure with proximal one; medial ramus massive, with frilled margin. Vagina dextroventral, with trumpet-shaped distal part and short vaginal tube with approximately 5 coils.
Haptor armed with dorsal and ventral anchor–bar complexes and 7 pairs of hooks. Ventral and dorsal anchors similar in shape and size; each with markedly broad, fan-shaped base, slightly bent shaft well delimited from short recurved point. Ventral bar saddle-shaped, with 2 submedial protuberances along anterior margin and posteromedial expansion; ends recurved posteriorly. Paired dorsal bar robust, with convex posterior margin (maximum convexity just beyond mid-length) and narrowed lateral ends. Hooks similar in shape and size; each with slightly erect, hooked thumb; shank slightly tapered and proximally curved; FH loop about three-quarters of shank length.
Measurements: Ventral anchor 59 (n = 1) long; base width 27 (n = 1). Dorsal anchor 62 (n = 1) long; base width 33 (n = 1). Ventral bar 59 (n = 1) long. Paired dorsal bar 51 (n = 1) long. Hooks 15 (14–15; n = 1) long. MCO – tube curved length 80 (80–81; n = 2); tube height 37 (35–39; n = 2).
Remarks: Mueller (Reference Mueller1938) described Pseudomurraytrema copulatum from the gills of C. commersonii, Hypentelium nigricans, Moxostoma anisurum and Moxostoma erythrurum, but noted that specimens collected from the former host exhibited morphological differences compared to those from the other 3 fish species. He further suggested that these specimens could represent 2 distinct species. Price (Reference Price1967) described Pseudomurraytrema muelleri from C. commersonii in Georgia and stated that his specimens matched those originally described by Mueller (Reference Mueller1938) from the same host. Subsequently, Rogers (Reference Rogers1969) considered P. muelleri to be morphologically identical to Pseudomurraytrema alabarrum, previously described from Minytrema melanops (Rogers Reference Rogers1966), and later that year, Chien (Reference Chien1969) proposed that P. muelleri should be regarded as a synonym of P. alabarrum. Finally, Kritsky and Leiby (Reference Kritsky and Leiby1973) indicated that the specimens identified as P. copulatum from C. commersonii in Wisconsin by Mizelle and Klucka (Reference Mizelle and Klucka1953) likely represent P. alabarrum, and therefore the earlier records of P. copulatum on C. commersonii from New York (Mueller Reference Mueller1938) and Wisconsin (Mizelle and Klucka Reference Mizelle and Klucka1953) should be reassigned to P. alabarrum.
Illustrations by Mueller (Reference Mueller1938; Figures 9, 12 and 13) clearly show that the specimens he identified as P. copulatum from C. commersonii are conspecific with those examined in the present study from the same host species. The morphology of the ventral and dorsal anchor–bar complexes depicted by Mueller (Reference Mueller1938) closely corresponds to that of the present material. Likewise, the MCOs are of highly similar appearance, and the vaginas are clearly distinct from those of all other known Pseudomurraytrema species due to their significantly shorter vaginal tubes. Taken together, this evidence suggests that neither Mueller’s specimens nor those examined herein belong to any of the 3 previously described species (P. alabarrum, P. copulatum, P. muelleri), but instead represent a distinct species, which is described here as P. commersoni n. sp. The most important characters distinguishing P. commersoni n. sp. from the 3 aforementioned species (P. alabarrum, P. copulatum and P. muelleri) are as follows: (1) dorsal and ventral anchors with a broad, fan-shaped base (vs both anchors with comparatively narrower bases in P. alabarrum, P. copulatum and P. muelleri); (2) a robust paired dorsal bar with a markedly widened posterior margin and a medial end distinctly broader than the lateral one (vs paired dorsal bar elongated, slightly widened medially, with both ends similarly narrowed in P. alabarrum, P. copulatum and P. muelleri); (3) a saddle-shaped ventral bar with 2 closely positioned anteromedial protuberances (vs rod-shaped in P. copulatum; saddle-shaped with more widely spaced anteromedial protuberances in P. alabarrum and P. muelleri); (4) a copulatory tube with a thicker, loosely S-shaped proximal portion and a wavy distal portion with the tip usually recurved outwards (vs proximal portion sharply S-shaped and thinner in diameter; distal portion angularly recurved inwards in P. alabarrum, P. copulatum and P. muelleri); and (5) a markedly shorter, and only slightly coiled, vaginal tube (vs long and multiply coiled vaginal tube in all 3 species).
As noted above, although Mueller (Reference Mueller1938) pointed out the differences between monopisthocotylans from C. commersonii and H. nigricans (= type host species for P. copulatum), Mizelle and Klucka (Reference Mizelle and Klucka1953) continued to identify their specimens from C. commersonii and M. pisolabrum (syn. M. aureolum) as P. copulatum. Unfortunately, their illustrations are insufficient for reliable species identification, as figures of the haptoral bars and vagina are lacking, and no voucher specimens were deposited. Although comparison of their drawings of the anchors (Figures 21 and 22) with those of the present material suggests that they were probably dealing with P. commersoni n. sp., the authors did not specify the host species from which the illustrated specimens were obtained. Therefore, the occurrence of P. commersoni n. sp. on M. pisolabrum remains unconfirmed. Apart from its shorter vaginal tube, P. commersoni n. sp. can be readily distinguished from the 3 other congeneric species parasitizing Catostomus species, namely P. ardens n. sp., Pseudomurraytrema kritsdelani n. sp. and P. muelleri, by having ventral and dorsal anchors that are morphologically almost identical, each characterized by a broad, fan-shaped base and a relatively straight shaft clearly delimited from the short point. In contrast, P. kritsdelani n. sp. and P. muelleri possess morphologically dissimilar anchors, whereas P. ardens n. sp. exhibits morphologically similar anchors that are narrower, with a terminally flattened base.
Pseudomurraytrema kritsdelani n. sp. (Figure 9)
Type host and locality: Catostomus ardens – Riverton Road, Blackfoot River (43°09ʹ00ʹʹN, 112˚27ʹ04ʹʹW), Idaho (1 January 1989).

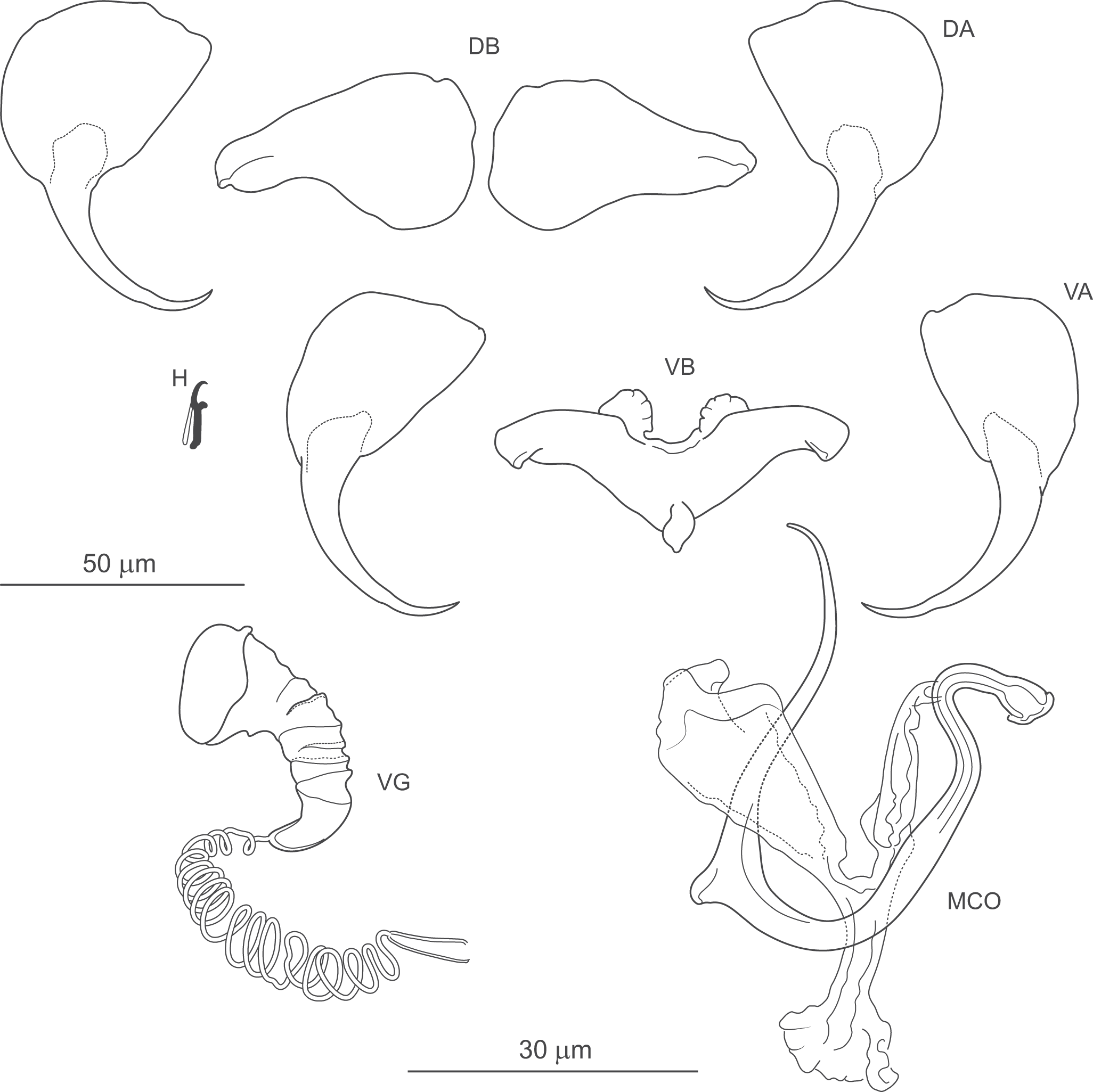
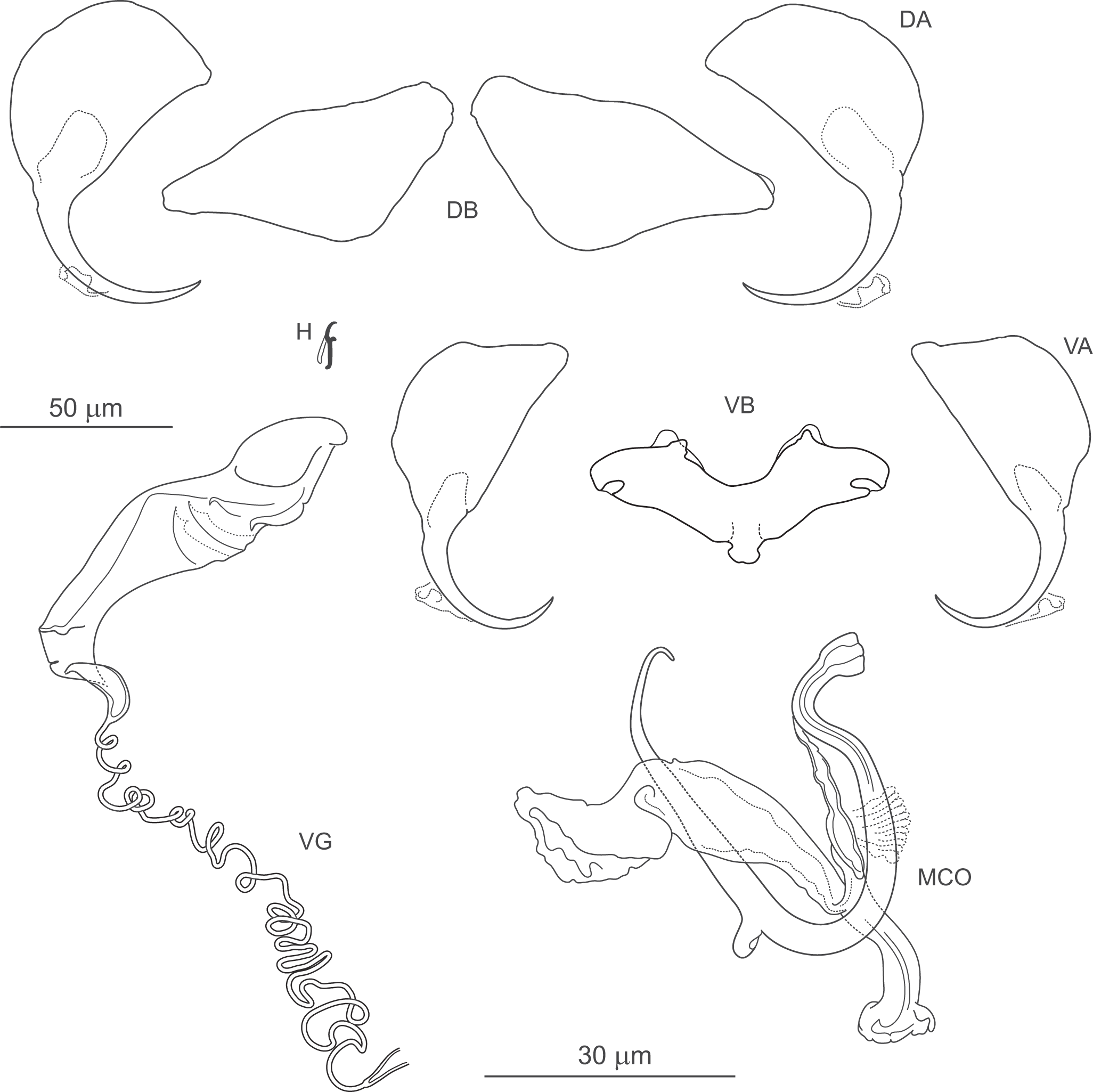
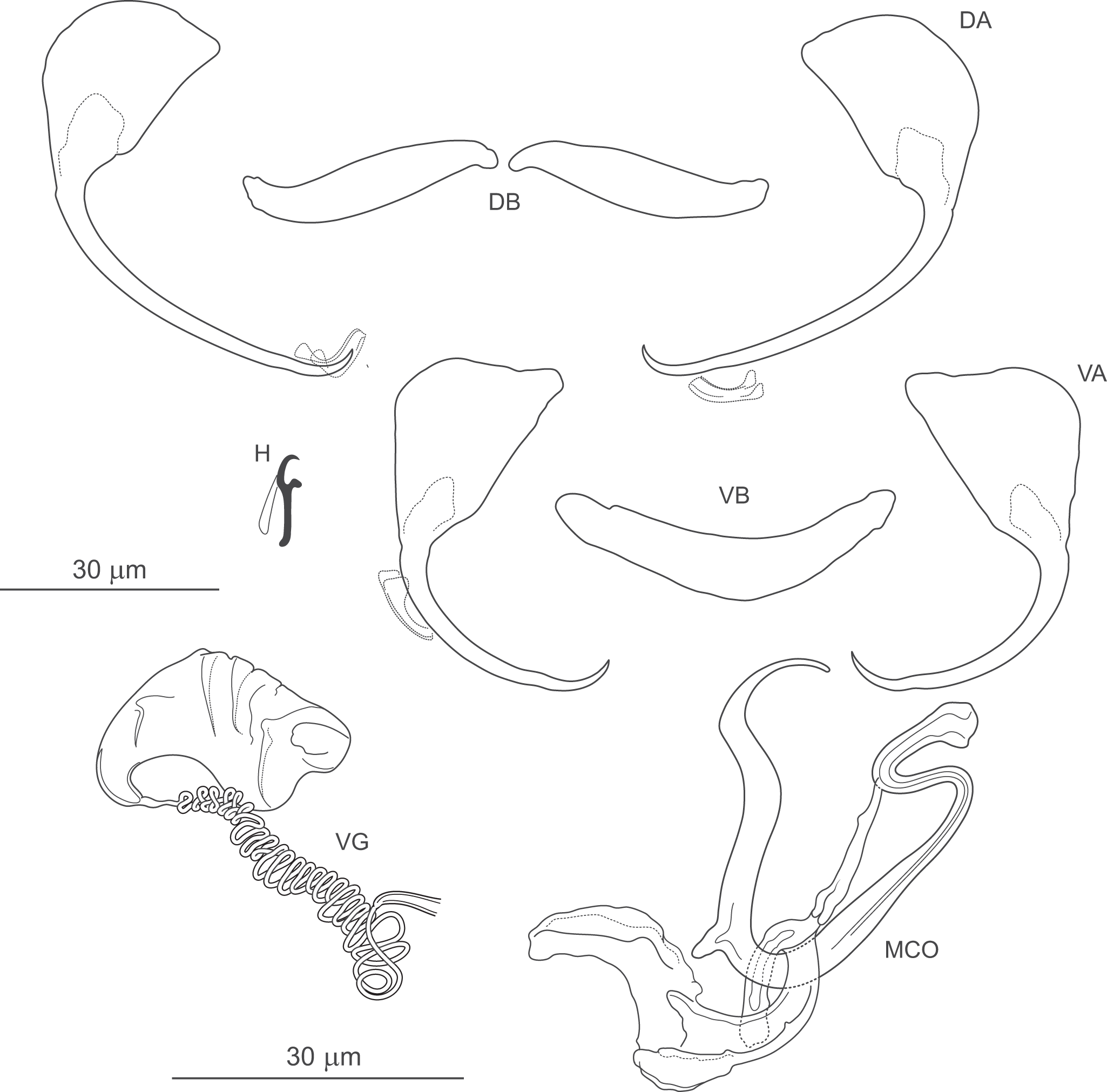
Figure 9. Pseudomurraytrema kritsdelani n. sp. ex Catostomus ardens (Idaho): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Site of infection: Gills.
Etymology: This species is named for Delane C. Kritsky in recognition of his enormous contributions to the taxonomy and systematics of monopisthocotylans, and for the support he provided for this study.
ZooBank registration (LSID): urn:lsid:zoobank.org:act:33A4C552-A3DF-45FB-8A83-A7001AA69932.
Prevalence and intensity of infection: Not available.
Type specimens: Holotype (USNM 1762103); 5 paratypes (USNM 1762104–1762108).
Description: Body elongate to fusiform, dorsoventrally flattened. Cephalic region slightly narrower than trunk, with poorly differentiated cephalic lobes. Trunk widest at or slightly posterior to level of testis. Peduncle short. Haptor subhexagonal in dorsoventral view, wider than long, small relative to trunk size. Head organs not observed. Two pairs of eyespots with poorly associated chromatic granules; members of posterior pair slightly farther apart than those of anterior pair. Accessory chromatic granules sparse, scattered in cephalic and anterior trunk region. Ventral mouth located between anterior eyespots. Pharynx subovate to spherical. Oesophagus short. Intestinal caeca confluent posterior to gonads.
Gonads tandem or with testis partly overlapping ovary. Ovary looping dorsoventrally around right intestinal caecum. Ootype and uterus not observed. Vagina dextroventral, located slightly anterior to body mid-length, composed of trumpet-shaped distal part and multi-coiled vaginal tube with proximal fork. Vaginal canal and seminal receptacle not observed. Vitellarium follicular, extending from level of oesophagus to near haptor; absent in region of other reproductive organs. Transverse vitelline ducts not observed. Testis postovarian. Vas deferens looping around left intestinal caecum; seminal vesicle a simple dilation of distal vas deferens, located to left and posterior to MCO. Two prostatic reservoirs unequal in size. MCO composed of articulated copulatory tube and accessory piece. Copulatory tube U-shaped, with thin, sharply S-shaped proximal portion (2 distinct bends in proximal third), separated by submedial spine from narrowed distal part, usually curved outwards at tip. Accessory piece with 3 rami: proximal ramus articulated to base; distal ramus largest, forming V-shaped structure with proximal one; medial ramus arising externally to their junction, with frilled terminal margin.
Haptor armed with dorsal and ventral anchor–bar complexes and 7 pairs of hooks. Ventral and dorsal anchors dissimilar in shape. Ventral anchors with terminally flattened base and elongate curved shaft with slight undulation near junction with short point. Dorsal anchors with broad fan-shaped base and elongate curved shaft with slight undulation near junction with short point. Ventral bar saddle-shaped, with 2 large submedial protuberances along anterior margin and posteromedial process; ends slightly recurved posteriorly. Paired dorsal bar robust, with markedly broad, bulbous medial end. Hooks similar in shape and size; each with slightly erect, hooked thumb; shank slightly inflated along inner side except for curved terminal portion; FH loop nearly entire shank length.
Measurements: Body 1040 (582–1416; n = 5) long; greatest width 235 (199–300; n = 5). Pharynx 99 (87–112; n = 5) long, 92 (88–99; n = 5) wide. Haptor 95 (70–117; n = 5) long, 160 (112–200; n = 5) wide. Ventral anchor 67 (65–70; n = 5) long; base width 29 (27–32; n = 5). Dorsal anchor 70 (67–73; n = 5) long; base width 34 (33–35; n = 5). Ventral bar 76 (71–81; n = 5) long. Paired dorsal bar 55 (53–58; n = 5) long. Hooks 14 (14–15; n = 3) long. MCO – tube curved length 98 (95–101; n = 5); tube height 40 (37–42; n = 5).
Remarks: This species is described here based on 6 specimens collected from Catostomus ardens in Idaho by Delane Kritsky (1989), to whom we are grateful for kindly providing the material. The specimens were of sufficient quality to confirm their status as a new species; however, some internal characters could not be assessed, precluding the preparation of a complete body illustration. Pseudomurraytrema kritsdelani n. sp. is most similar to P. ardens n. sp., both of which parasitize the gills of C. ardens. A detailed comparison and differential diagnosis are provided in the Remarks section for P. ardens n. sp. Although molecular data are not available for P. kritsdelani n. sp., and we acknowledge that some differences between these 2 species may reflect intraspecific variability or artefacts of fixation, the observed morphological distinctions are consistent across most available specimens. We therefore regard the 2 taxa as representing separate species, while recognizing that future sequencing of P. kritsdelani n. sp. will be essential to confirm this taxonomic distinction.
Pseudomurraytrema species parasitizing species of Moxostoma (Moxostomatini)
Pseudomurraytrema cf. coosense Rogers, 1969 (Figure 10)
Type host and locality: Moxostoma duquesnei (Lesueur) – Coosawattee River (Gilmer Co.), Georgia.

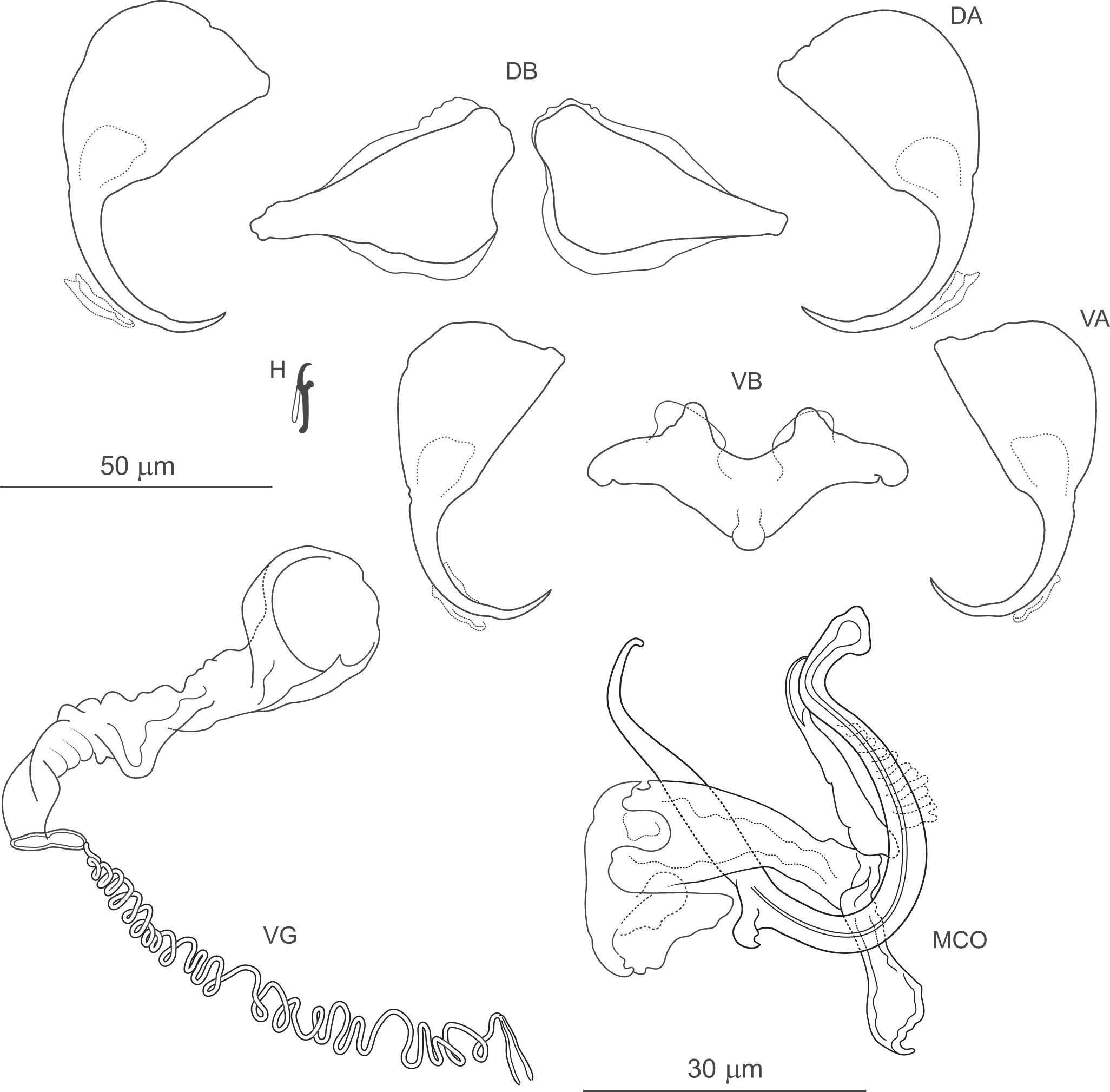
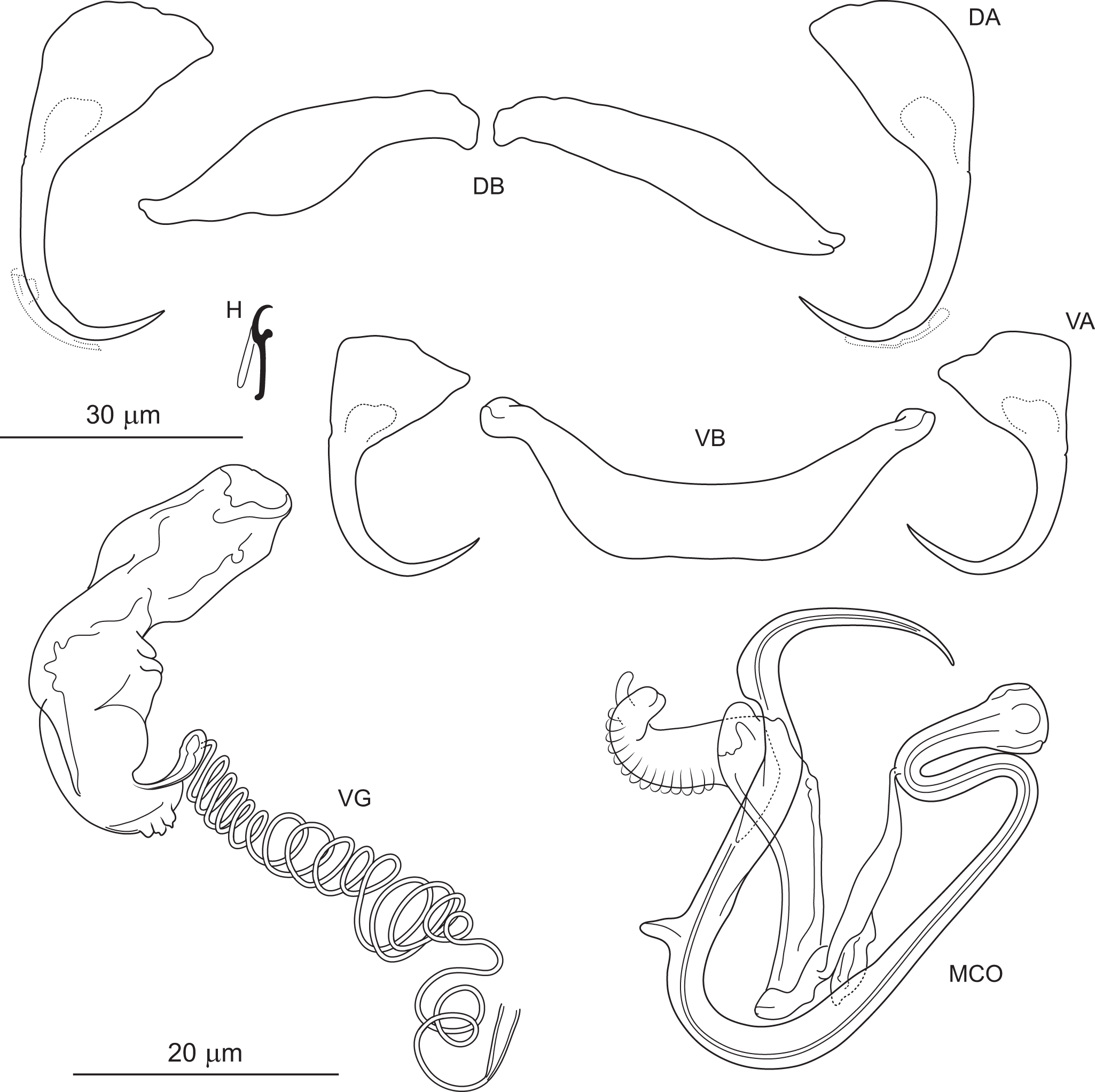
Figure 10. Pseudomurraytrema cf. coosense ex Moxostoma macrolepidotum (Wisconsin): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Current records: Moxostoma macrolepidotum (Lesueur) – Mississippi River, Main Channel (43°51′35.94″N; 91°18′8.65″W), Wisconsin (6 July 2022).
Previous records: See type host species and locality (Rogers Reference Rogers1969); Hillabee Creek (Clay Co.), Alabama (Rogers Reference Rogers1969).
Site of infection: Gills.
Prevalence and intensity of infection: 67% (2 fish infected/3 fish examined); 1 parasite per infected host.
Voucher specimens: One hologenophore (USNM 1762086).
Specimens studied for comparison: P. coosense (Rogers Reference Rogers1969) (USNM 1366719, paratype).
Measurements: Haptor 167 (n = 1) long, 275 (n = 1) wide. Ventral anchor 99 (n = 1) long; base width 37 (n = 1). Dorsal anchor 101 (n = 1) long; base width 38 (n = 1). Ventral bar 92 (n = 1) long. Paired dorsal bar 83 (n = 1) long. Hooks 15 (14–15; n = 1) long. MCO – tube curved length 96 (94–97; n = 2); tube height 39 (n = 2).
Remarks: Pseudomurraytrema coosense was described by Rogers (Reference Rogers1969) from the gills of M. duquesnei collected in the Coosa River system in Alabama and Georgia. It clearly differs from all other congeners parasitizing Moxostoma species by having anchors with a markedly elongate, bent shaft and a short, recurved point (vs more arched and moderately long or short shaft and point in P. fergusoni, Pseudomurraytrema fluviatile, Pseudomurraytrema milleri and Pseudomurraytrema swinglei). Examination of 1 paratype (USNM 1366719) confirmed that the measurements and illustration of the specimen correspond to the original description. While the Wisconsin specimens generally conform to the morphological features described for P. coosense, they are tentatively assigned to this species as P. cf. coosense. This conditional assignment is based on the following considerations: (1) the anchors of the Wisconsin specimens are longer than those reported in the original description (ventral anchor: 99 vs 55–84; dorsal anchor: 101 vs 56–72); (2) the ventral bar in the Wisconsin specimens is slightly deformed due to mechanical distortion during trisection of the specimen for DNA analysis and is therefore not suitable for detailed comparison; and (3) the specimens were collected from a different host (M. macrolepidotum) and in a different river system (Mississippi River drainage, Wisconsin) than the type locality (Coosawattee River, Georgia). Although the specimens are tentatively assigned to P. cf. coosense, they are compared here to P. milleri due to shared morphological features of the haptoral bars. In both species, the paired dorsal bar is subfusiform, and the ventral bar possesses a dorsally located membrane protruding as 2 processes from its anteromedial margin. However, the ends of the paired dorsal bar are uniformly narrowed in P. cf. coosense, whereas in P. milleri, the medial end is wider than the lateral one. In addition, the ventral bar of P. cf. coosense lacks the posteromedial shield-like process present in P. milleri.
Until more comprehensive material becomes available, including specimens in better condition and from a broader host and geographic range, the identification as P. cf. coosense should be retained.
Pseudomurraytrema milleri Mergo & White, 1982 (Figure 11)
Type host and locality: Moxostoma anisurum (Rafinesque) – Salt Creek, Eagle Twp. (Vinton Co.), Ohio.

Figure 11. Pseudomurraytrema milleri ex Moxostoma anisurum (Wisconsin): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Current records: Moxostoma anisurum – Mississippi River, Main Channel (43°51′35.94″N; 91°18′8.65″W), Wisconsin (7 July 2022).
Previous records: No other records, apart from the original description by Mergo and White (Reference Mergo and White1982).
Site of infection: Gills.
Prevalence and intensity of infection: 100% (2 fish infected/2 fish examined); 6–22 parasites per infected host.
Voucher specimens: Three hologenophores (USNM 1762109–1762111); 3 morphological vouchers (USNM 1762112).
Specimens studied for comparison: P. milleri (Mergo and White Reference Mergo and White1982) (USNM 1371986, 3 paratypes).
Measurements: Body 2526 (1697–3544; n = 4) long; greatest width 373 (337–448; n = 4). Pharynx 148 (128–160; n = 4) long, 124 (107–148; n = 4) wide. Haptor 154 (141–161; n = 3) long, 242 (218–273; n = 3) wide. Ventral anchor 59 (55–63; n = 7) long; base width 27 (25–27; n = 7). Dorsal anchor 71 (68–74; n = 7) long; base width 32 (31–33; n = 7). Ventral bar 66 (62–72; n = 7) long. Paired dorsal bar 63 (58–67; n = 7) long. Hooks 15 (14–15; n = 5) long. MCO – tube curved length 109 (106–112; n = 5); tube height 44 (42–46; n = 5).
Remarks: While in need of redescription, P. milleri differs from its congeners primarily by possessing a saddle-shaped ventral bar with a shield-like posteromedial process that is divided medially (in other species, this process is either knob-like or absent). Mergo and White (Reference Mergo and White1982) described this process as consisting of 2 closely associated plates. The original illustrations of the sclerotized structures are highly schematic (likely drawn freehand) and are insufficient for accurate species identification. In their description of P. milleri, Mergo and White (Reference Mergo and White1982) characterized the paired dorsal bar as ‘angular’ and depicted it with 1 end angularly recurved. The morphology of the paired dorsal bar in P. milleri is somewhat variable among our specimens, which may reflect artefacts of mounting technique. Its shape ranges from relatively straight to recurved in the lateral third. The latter condition may correspond to the character reported by Mergo and White (Reference Mergo and White1982, their Figure 3), although these authors illustrated the paired dorsal bar with both ends similarly tapered, whereas in our specimens, the medial end is distinctly broader than the lateral one. Additionally, P. milleri resembles P. swinglei in the morphology of the ventral and dorsal anchors, which in both species possess a shaft exhibiting slight undulation near its junction with the point. This undulation was neither described nor illustrated in the original description of P. milleri (Mergo and White Reference Mergo and White1982; Figures 1 and 4). Moreover, the illustration of the hook by Mergo and White (Reference Mergo and White1982) does not accurately reflect the morphology typical of Dactylogyridea. The thumb is entirely absent in their Figure 2, rendering the illustration unsuitable for diagnostic purposes. In this character, P. milleri resembles P. kritsdelani n. sp., as both species possess hooks with a shank that is slightly inflated along the inner side, except for the curved terminal portion (see Figure 7). However, the hook of P. milleri differs from that of P. kritsdelani n. sp. by having a thumb that is robust and projects perpendicularly from the shank, whereas in P. kritsdelani n. sp. the thumb is more slender and erect.
Comparison of 3 paratypes (USNM 1371986) with our specimens collected from the same host species in Wisconsin revealed no morphological differences in the sclerotized structures of the haptor. However, comparison of the morphology of the MCO was not possible due to the poor condition of the available paratypes.
The occurrence of P. milleri on Moxostoma anisurum in Wisconsin represents a new geographic record for this species.
Pseudomurraytrema fluviatile Rogers, 1969 (Figure 12)
Type host and locality: Moxostoma carinatum (Cope) – Cahaba River (Perry Co.), Alabama.

Figure 12. Pseudomurraytrema fluviatile ex Moxostoma erythrurum (Wisconsin): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Current records: Moxostoma erythrurum (Rafinesque) – Mississippi River, Main Channel (43°51′35.94″N; 91°18′8.65″W), Wisconsin (10 July 2022).
Previous records: See type host species and locality (Rogers Reference Rogers1969); Tallapoosa River (Elmore Co.), Tombigbee River (Sumter Co.), Alabama (Rogers Reference Rogers1969).
Site of infection: Gills.
Prevalence and intensity of infection: 100% (1 fish infected/1 fish examined); 10 parasites per infected host.
Voucher specimens: Two hologenophores (USNM 1762095, 1762096); 2 morphological vouchers (USNM 1762097).
Measurements: Body 1203 (1167–1284; n = 4) long; greatest width 198 (158–241; n = 4). Pharynx 98 (92–106; n = 3) long, 83 (81–85; n = 3) wide. Haptor 119 (81–152; n = 4) long, 201 (178–230; n = 4) wide. Ventral anchor 79 (75–84; n = 6) long; base width 32 (29–34; n = 6). Dorsal anchor 83 (81–87; n = 6) long; base width 36 (34–37; n = 6). Ventral bar 78 (72–86; n = 6) long. Paired dorsal bar 78 (72–88; n = 6) long. Hooks 14 (13–15; n = 4) long. MCO – tube curved length 92 (90–96; n = 4); tube height 39 (37–40; n = 4).
Remarks: Rogers (Reference Rogers1969) described P. fluviatile from M. carinatum in Alabama. Although his illustrations do not include the vagina and his depiction of the MCO is too schematic for reliable species differentiation, his drawings of the sclerotized structures of the haptor correspond well with our specimens collected from M. erythrurum in Wisconsin. The respective dimensions of the hard parts in our material are generally larger than those reported by Rogers (Reference Rogers1969), but mostly fall within the range provided in his original description of P. fluviatile. A more pronounced difference was observed in the length of the paired dorsal bar, which is considerably greater in our specimens than that reported by Rogers (Reference Rogers1969) (72–88 vs 57–63). However, given the wide intraspecific variation in dorsal bar length observed across all species of Pseudomurraytrema examined in this study, we do not consider this difference sufficient to warrant recognition of a separate species at this time. Pseudomurraytrema fluviatile is most similar to P. swinglei. In both species, the anchors possess a moderately long, arcuate shaft and point; the paired dorsal bar is subtriangular; and the ventral bar is saddle-shaped with 2 pairs of anteromedial and 1 posteromedial processes. Pseudomurraytrema fluviatile differs from P. swinglei by the following characters: (1) a dorsal anchor with a crescent-shaped base (vs a base with a straight inner margin, not resembling a crescent, in P. swinglei), (2) dorsal and ventral anchors lacking distal shaft undulation (vs both anchors with distal shaft undulation in P. swinglei), (3) a ventral bar with small anteromedial processes (vs more prominent anteromedial processes in P. swinglei) and (4) a paired dorsal bar with a smoothly convex posterior margin (vs an angularly convex margin in P. swinglei).
The occurrence of P. fluviatile on the golden redhorse, Moxostoma erythrurum, from Wisconsin represents both a new host and a new locality record for this species.
Pseudomurraytrema swinglei Rogers, 1966 (Figure 13)
Type host and locality: Moxostoma duquesnei – Chewacla Creek (Lee Co.), Alabama.

Figure 13. Pseudomurraytrema swinglei ex Moxostoma congestum (Texas): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Current records: Moxostoma congestum (Baird & Girard) – Colorado River, between Austin and Bastrop (30°11′15.4″N 97°28′37.1″W), Texas (31 May 2023).
Previous records: No other records, apart from the original description by Rogers (Reference Rogers1966).
Site of infection: Gills.
Prevalence and intensity of infection: 100% (3 fish infected/3 fish examined); 1–12 parasites per infected host.
Voucher specimens: Three hologenophores (USNM 1762120–1762122); 2 morphological vouchers (USNM 1762123).
Measurements: Body 890 (n = 1) long; greatest width 98 (n = 1). Pharynx 100 (98–104; n = 5) long, 92 (88–93; n = 5) wide. Haptor 165 (155–186; n = 5) long, 292 (267–310; n = 5) wide. Ventral anchor 53 (51–55; n = 6) long; base width 23 (22–24; n = 6). Dorsal anchor 58 (56–59; n = 6) long; base width 27 (25–27; n = 6). Ventral bar 57 (55–59; n = 6) long. Paired dorsal bar 51 (47–54; n = 6) long. Hooks 13 (12–14; n = 6) long. MCO – tube curved length 90 (88–92; n = 6); tube height 37 (30–39; n = 6).
Remarks: The original description of Pseudomurraytrema swinglei by Rogers (Reference Rogers1966) generally agrees with the characters exhibited by our specimens. In Rogers’ Figure 2, the ventral bar is illustrated as saddle-shaped with 2 distinct anteromedial rectangular processes, which likely correspond to the 2 pairs of anteromedial processes observed in our specimens: 1 pair is rectangular and projects dorsally, while the other is rounded and protrudes from the ventral surface. These 2 pairs may overlap and appear as a single rectangular structure under low magnification. Pseudomurraytrema fluviatile also possesses a ventral bar with 2 pairs of anteromedial processes, but these are rounded and considerably smaller than those in P. swinglei. For a detailed comparison between P. swinglei and its closest congener, P. fluviatile, refer to the Remarks under the description of P. fluviatile.
The occurrence of P. swinglei on the grey redhorse, M. congestum, in Texas represents a new host and geographic record for this species.
Pseudomurraytrema fergusoni McAllister, Leis, Cloutman, Woodyard, Camus & Robinson, 2022 (Figure 14)
Type host and locality: Moxostoma pisolabrum – White River drainage, Black River, Black Rock (Lawrence Co.), Arkansas.

Figure 14. Pseudomurraytrema fergusoni n. sp. ex Moxostoma macrolepidotum (Wisconsin): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Current records: Moxostoma macrolepidotum – Mississippi River, Main Channel (43°51′35.94″N; 91°18′8.65″W), Wisconsin (11 July 2022).
Previous records: No other records, apart from the original description by McAllister et al. (Reference McAllister, Leis, Cloutman, Woodyard, Camus and Robison2022).
Site of infection: Gills.
Prevalence and intensity of infection: 100% (2 fish infected/2 fish examined); 1–3 parasites per infected host.
Voucher specimens: Two hologenophores (USNM 1762092, 1762093); 1 morphological voucher (USNM 1762094).
Measurements: Body 2620 (n = 1) long; greatest width 380 (n = 1). Pharynx 149 (n = 1) long, 135 (n = 1) wide. Haptor 290 (n = 1) long, 440 (n = 1) wide. Ventral anchor 82 (n = 1) long; base width 39 (n = 1). Dorsal anchor 82 (n = 1) long; base width 45 (n = 1). Ventral bar 102 (n = 1) long. Paired dorsal bar 118 (n = 1) long. Hook 15 (n = 1) long. MCO – tube curved length 46 (45–47; n = 2); tube height 27 (26–27; n = 2).
Remarks: Pseudomurraytrema fergusoni was recently described and sequenced by McAllister et al. (Reference McAllister, Leis, Cloutman, Woodyard, Camus and Robison2022) from the gills of M. pisolabrum in Arkansas. This species differs markedly from its congeners and from all other members of Pseudomurraytrematidae by the unique morphology of both the MCO and the vagina. A characteristic feature observed in all previously known members of the family is the U-shaped configuration of the copulatory tube, which resembles the frame of a lyre. In P. fergusoni, however, the distal end of the copulatory tube appears truncated, which represents a striking departure from the curved and distinctly narrowed distal end observed in other pseudomurraytrematids. In addition, the base of the copulatory tube is poorly defined and merges with the proximal portion of the accessory piece, forming a bulbous structure. This contrasts with other species of the family, in which the base is S-shaped and remains separate from the accessory piece.Another distinguishing feature of P. fergusoni is the presence of a tubular vagina located in a small bulb that protrudes from the right body margin. In contrast, all other known members of Pseudomurraytrematidae possess a vagina composed of a weakly sclerotized distal funnel and a proximal coiled tube, as noted in the family diagnoses provided by Kritsky et al. (Reference Kritsky, Mizelle and Bilqees1978) and Beverley-Burton (Reference Beverley-Burton, Margolis and Kabata1984). Moreover, in all Pseudomurraytrema species examined during the present study, the vagina is situated on the dextroventral side of the body, not at the margin as in P. fergusoni.
The original description of P. fergusoni, supported by drawings and microphotographs of the haptoral and copulatory sclerotized structures, is generally adequate. However, McAllister et al. (Reference McAllister, Leis, Cloutman, Woodyard, Camus and Robison2022) illustrated the hooks as having a straight, proximally extending shank and an upright thumb. Based on their illustrations (Figures 1D, 3C, D), it appears that these authors overlooked the proximal knob-like termination of the shank. Examination of GAP-fixed specimens collected during the present study revealed that P. fergusoni possesses hooks with the shank inflated along its inner margin, except for the proximal third, which ends in a curved, knob-like base. The thumb of the hook is dome-shaped and either slightly erect or projecting perpendicularly from the shank (Figure 7). The ventral bar in our specimens of P. fergusoni from M. macrolepidotum exhibits more pointed lateral ends than those illustrated by McAllister et al. (Reference McAllister, Leis, Cloutman, Woodyard, Camus and Robison2022); however, this difference may be attributed to variation in the orientation of the ventral bar in the compared specimens. McAllister et al. (Reference McAllister, Leis, Cloutman, Woodyard, Camus and Robison2022) also reported that the vagina was not observed. In their description, they stated that measurements were based on 10 formalin-fixed specimens (holotype and 9 paratypes). However, it remains uncertain how many of these individuals were examined in detail for internal morphology. In the present study, the vagina was not detected in 1 of the 3 examined specimens, despite the presence of the MCO in all of them.
The occurrence of P. fergusoni on M. macrolepidotum in Wisconsin represents a new host and geographic record for this species.
Pseudomurraytrema species parasitizing Erimyzon claviformis and Minytrema melanops (Erimyzonini)
Pseudomurraytrema alabarrum Rogers, 1966 (Figure 15)
Type host and locality: Minytrema melanops (Rafinesque) – Chewacla Creek (Lee Co.), Alabama.

Figure 15. Pseudomurraytrema alabarrum ex Minytrema melanops (Michigan): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Current records: Minytrema melanops – Pascagoula River, Moon Lake near Poticaw Landing (30°28′18″N, 88°36′45″W), Mississippi (15 June 2019); Lake Saint Claire (42°35′32.9″N 82°46′15.7″W), Michigan (19 July 2022); UMR 7, Lake Onalaska, Blackdeer area (43°56′0.21″N; 91°18′41.43″W), Wisconsin (8 July 2022).
Previous records: See type host species and locality (Rogers Reference Rogers1966); Bridge Creek (Geauga Co.), Ohio (Mergo and White Reference Mergo and White1984); Crow Creek at Madison (St. Francis Co.), Arkansas (McAllister et al. Reference McAllister, Cloutman, Choudhury, Scholz, Trauth, Fayton and Robison2018).
Unconfirmed host and locality records: Catostomus commersonii – Georgia (Price Reference Price1967); Milk River, Alberta (Price and Arai Reference Price and Arai1967). The assignment of these records to P. alabarrum is tentative and based solely on historical synonymy with Pseudomurraytrema muelleri (Chien Reference Chien1969), which is not accepted in the present study.
Site of infection: Gills.
Prevalence and intensity of infection: Mississippi – 33% (1 fish infected/3 fish examined); 2 parasites per fish. Michigan – 50% (1 fish infected/2 fish examined); 8 parasites per infected host. Wisconsin – 67% (2 fish infected/3 fish examined); 5–6 parasites per infected host.
Voucher specimens: Three hologenophores (USNM 1762078–1762080).
Specimens studied for comparison: P. alabarrum (Rogers, Reference Rogers1966) (USNM 1356456, paratype).
Measurements: Body 1507 (1200–1750; n = 4) long; greatest width 242 (165–294; n = 4). Pharynx 84 (69–97; n = 4) long, 74 (68–80; n = 4) wide. Haptor 117 (110–123; n = 4) long, 189 (184–194; n = 4) wide. Ventral anchor 80 (74–83; n = 5) long; base width 29 (29–31; n = 5). Dorsal anchor 79 (71–85; n = 5) long; base width 32 (29–35; n = 5). Ventral bar 74 (67–82; n = 5) long. Dorsal bar 82 (74–87; n = 5) long. Hooks 14 (13–14; n = 5) long. MCO – tube curved length 88 (88–89; n = 5); tube height 34 (34–35; n = 5).
Remarks: Pseudomurraytrema alabarrum was described by Rogers (Reference Rogers1966) from the gills of Minytrema melanops in Alabama, later recorded from the same host species in Ohio by Mergo and White (Reference Mergo and White1984), and more recently in Arkansas by McAllister et al. (Reference McAllister, Cloutman, Choudhury, Scholz, Trauth, Fayton and Robison2018). Examination of 1 paratype (USNM 1356456) from Minitrema melanops confirmed that our specimens collected from the same host in Mississippi, Michigan and Wisconsin are conspecific with P. alabarrum. Although the MCO was not clearly visible in the paratype, the morphology of the haptoral structures corresponded well with that of our material. However, some ambiguity exists regarding the morphology of the hooks. Rogers (Reference Rogers1966) described them as lacking inflated bases, yet his Figure 14 depicts a hook with a slightly enlarged posterior end. In all specimens available to us, including the paratype, the hooks exhibit a uniformly slender shank with a recurved, knob-like proximal end (Figure 7) and are nearly identical in size within individual specimens.
The presence of P. alabarrum on C. commersonii is, however, questionable. When re-examining the holotype of P. muelleri from C. commersonii (see Price Reference Price1967), Rogers (Reference Rogers1969) found it to be morphologically indistinguishable from P. alabarrum, a conclusion that was further supported by Chien (Reference Chien1969), who synonymized the 2 species in the same year. Kritsky and Leiby (Reference Kritsky and Leiby1973) later added complexity to this taxonomic issue by suggesting that the specimens identified as P. copulatum by Mizelle and Klucka (Reference Mizelle and Klucka1953) from C. commersonii in Wisconsin were likely misidentified P. alabarrum. Consequently, they proposed that earlier records of P. copulatum on C. commersonii from New York (Mueller Reference Mueller1938) and Wisconsin (Mizelle and Klucka Reference Mizelle and Klucka1953) should be re-assigned to P. alabarrum. In the present study, these previously published records of P. copulatum on C. commersonii have been reclassified as P. commersoni n. sp. (see Remarks under the new species and P. copulatum for details). However, the synonymy of P. alabarrum and P. muelleri remains unresolved.
Our examination of the holotype of P. muelleri (USNM 1357082) revealed several differences compared to the original illustrations by Price (Reference Price1967). Notably, the shaft of the dorsal anchor appears straight in its proximal half, whereas in the original Figure 2, it is depicted as sharply curved from the base (compare Price’s illustration with Figure 16 herein). Additionally, the lengths of the ventral and paired dorsal bars in the holotype are greater than those reported in the original description, measuring 55 and 69 compared to 42–49 and 51–57, respectively. A comparison of Price’s specimen with the available paratype and our specimens of P. alabarrum did not confirm the conspecificity of P. muelleri and the latter species. Although the holotype is in poor condition, the hooks are sufficiently visible and differ from those in P. alabarrum. In P. muelleri, the hooks show slight variation in size (12–14) and shape: the inner side of the shank is inflated either along its entire length or excluding the proximal end (Figure 16). In contrast, the hooks of P. alabarrum have a uniformly slender shank terminating in a knob-like, curved proximal end.

Figure 16. Pseudomurraytrema muelleri ex Catostomus commersonii (Georgia), holotype: VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook.
While the ventral and dorsal anchor–bar complexes are morphologically similar in both species, the measurements reported by Price (Reference Price1967) for P. muelleri, as well as those obtained from the holotype in the present study, are considerably smaller than those given for P. alabarrum by Rogers (Reference Rogers1966) and confirmed by our own data. For comparison, Price (Reference Price1967) reported the following ranges (n = 5): ventral anchor 49–56, dorsal anchor 45–51, ventral bar 42–49 and paired dorsal bar 52–57. Unfortunately, the morphology of the ventral bar, 1 of the primary diagnostic structures in species of Pseudomurraytrema, could not be reliably assessed in the holotype of P. muelleri due to distortion of its median portion. Nonetheless, its 3-lobed posteromedial process resembles that observed in P. alabarrum. Comparative analysis of the MCO was also inconclusive due to the schematic nature of Price’s drawings (Figures 8–11) and the twisted orientation of the MCO in the holotype. It remains unclear whether the observed differences in hook morphology and the size of anchor–bar complexes reflect intraspecific variation related to host association (i.e. Minytrema melanops vs C. commersonii) or represent true species-level differences. Given the morphological differences observed in the holotype of P. muelleri, particularly in hook shape and sclerite dimensions, as well as unresolved discrepancies in previous descriptions, we believe that the conspecificity of P. muelleri and P. alabarrum requires further investigation. Until additional material becomes available, preferably including new collections from C. commersonii, we refrain from formally treating P. muelleri as a junior synonym of P. alabarrum.
Records of P. alabarrum from Minytrema melanops in Michigan, Mississippi and Wisconsin extend the known geographic distribution of this species.
Pseudomurraytrema janullae n. sp. (Figures 17 and 18)
Type host and locality: Erimyzon claviformis (Girard) – Caddo River (Montgomery Co.), Arkansas (15 June 2019).

Figure 17. Pseudomurraytrema janullae n. sp. ex Erimyzon claviformis (Arkansas): whole body, ventral view.

Figure 18. Pseudomurraytrema janullae n. sp. ex Erimyzon claviformis (Arkansas): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Site of infection: Gills.
Etymology: The specific name janullae is a Latinized form of Jana, dedicated in memory of the sister of the first author, as a tribute to her life, strength and spirit.
ZooBank registration (LSID): urn:lsid:zoobank.org:act:6DBE5C5D-A515-45B3-BBAC-CA19427E9164.
Prevalence and intensity of infection: 67% (2 fish infected/3 fish examined); 1–4 parasites per infected host.
Type and voucher specimens: Holotype (USNM 1762098); 1 paratype (USNM 1762099); 3 hologenophores (USNM 1762100–1762102).
Description: Body elongated, dorsoventrally flattened. Cephalic region slightly narrower than trunk; trunk width relatively uniform. Peduncle moderately long, tapering slightly posteriorly. Haptor rectangular in dorsoventral view, relatively small compared to body size. Head organs indistinct, about 3 paris. Two pairs of eyespots with poorly associated chromatic granules; members of posterior pair slightly farther apart than those of anterior pair, located at level of anterior pharynx. Accessory chromatic granules sparse, scattered in cephalic region, especially near eyespots, and in anterior part of trunk. Ventral mouth situated between anterior pair of eyespots. Pharynx pyriform. Oesophagus long, bifurcating at about one-quarter of digestive tract length into 2 intestinal caeca. Caeca confluent posteriorly, well behind gonads. Gonads tandem or with testis slightly overlapping ovary dorsally. Ovary looping dorsoventrally around right caecum. Ootype situated anterior to oviduct, surrounded by numerous unicellular glands (Mehlis’ gland?). Uterus usually containing single oval egg with subterminal lateral spine, extending anteriorly, slightly to right of midline, towards common genital pore. Vagina dextroventral. Vaginal pore situated in wrinkled tegumental indentation, at approximately three-quarters of distance from anterior extremity to body midpoint. Pouch thin-walled, wrinkled, with proximal fork giving rise to tightly coiled vaginal tube (more than 20 coils); tube terminating in secondary fork. Vaginal canal thin and delicate, continuing from secondary fork, directed posterodorsally into seminal receptacle. Seminal receptacle pyriform, situated anterior to ootype and to right of proximal portion of uterus. Vitellarium follicular, extending from level of oesophagus to just posterior to termination of digestive tract; absent in region of other reproductive organs. Transverse vitelline ducts not observed. Testis postovarian, subovate, distinctly smaller than ovary. Vas deferens emerging from anteromedial margin of testis, passing dorsally over ovary, crossing body intercaecally, looping around left caecum, and proceeding anteromedially to form seminal vesicle. Seminal vesicle located to the left and posterior to MCO. MCO composed of articulated copulatory tube and accessory piece. Copulatory tube U-shaped, with sharply S-shaped proximal portion (2 distinct bends located in proximal third), separated by submedial spine from narrowed, sickle-shaped distal part. Accessory piece with 3 rami: proximal ramus articulated to base; distal ramus largest, forming V-shaped structure with proximal one; medial ramus arising externally to their junction. Prostatic glands located intercaecally, posterior to intestinal bifurcation. Two prostatic reservoirs entering base of copulatory tube, unequal in size; larger one about twice as long, with thick wall.
Haptor armed with ventral and dorsal anchor–bar complexes and 7 pairs of hooks. Ventral bar widely V-shaped, with posteromedial bulbous process. Paired dorsal bar large relative to dorsal anchors, fusiform, with markedly tapered medial end; maximum width at approximately inner one-third. Ventral anchor slightly larger than dorsal anchor; both anchors similar in shape: base undivided into roots, fan-shaped; shaft bent, moderately long, clearly delimited from short recurved point. Anchor filaments inconspicuous, resembling an open sheath, often lying close to outer margin of distal shaft. Hook distribution following Mizelle (Reference Mizelle1936); hooks with upright, rounded thumb; shank variable in diameter, slightly inflated along inner side except for proximal knob-like termination; FH loop about three-quarters of shank length.
Measurements: Body 1759 (n = 1) long; greatest width 304 (n = 1). Pharynx 106 (n = 1) long, 105 (n = 1) wide. Haptor 138 (n = 1) long, 254 (n = 1) wide. Ventral anchor 62 (60–63; n = 3) long; base width 22 (19–15; n = 3). Dorsal anchor 57 (56–57; n = 3) long; base width 24 (23–25; n = 3). Ventral bar 56 (52–63; n = 3) long. Paired dorsal bar 65 (57–70; n = 3) long. Hooks 14 (13–15; n = 2) long. MCO – tube curved length 88 (85–89; n = 4); tube height 34 (33–34; n = 4).
Remarks: Pseudomurraytrema janullae n. sp. differs from all known congeners by possessing a saddle-shaped ventral bar with a robust, bulbous posteromedial process. In other congeners, when present, the posteromedial process is smaller and knob-like (e.g. P. alabarrum, P. ardens n. sp., P. commersoni n. sp., P. coosense, P. fluviatile, P. kritsdelani n. sp., P. muelleri, P. swinglei) or shield-like (P. milleri). It is most similar to P. alabarrum in having: (1) a fusiform paired dorsal bar with a markedly tapered medial end and (2) anchors with a fan-shaped base, a bent shaft and a short recurved point. In addition to the ventral bar with a prominent bulbous posteromedial process, P. janullae n. sp. further differs from P. alabarrum by having: (1) ventral anchors with a terminally rounded base (vs terminally flattened in P. alabarrum) and (2) hooks with a thick thumb and a shank inflated along the inner side, except for the proximal knob-like termination (vs a delicate thumb and a slender shank of uniform diameter in P. alabarrum; compare in Figure 7).
Pseudomurraytrema species parasitizing Hypentelium nigricans (Thoburniini)
Pseudomurraytrema copulatum (Mueller, 1938) Bychowsky, 1957 (Figure 19)
Synonym: Murraytrema copulata Mueller, 1938 (Bychowsky, Reference Bychowsky1957)

Figure 19. Pseudomurraytrema copulatum ex Hypentelium nigricans (Arkansas): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Type host and locality: Hypentelium nigricans – French Creek, near Panama, New York.
Current records: Hypentelium nigricans – Huddleston Creek (Montgomery Co.), Arkansas (12 June 2019).
Previous records: See type host species and locality (as Murraytrema copulata; Mueller Reference Mueller1938); Chautaqua Lake, New York (as Murraytrema copulata; Mueller Reference Mueller1938); Sangamon River near Lake of the Woods (Champaign Co.), Little Vermilion River near Sidell (Vermilion Co.), Illinois (Kritsky and Hathaway Reference Kritsky and Hathaway1969); Sangamon River near Foosland (Champaign Co.), Illinois (Kritsky and Leiby Reference Kritsky and Leiby1973).
Unconfirmed and erroneous (*) host and locality records: Catostomus catostomus – not specified, British Columbia (Anonymous 1978, 1981, 1984 in McDonald and Margolis Reference McDonald and Margolis1995); McGregor River and Parsnip River, British Columbia (Arai and Mudry Reference Arai and Mudry1983); Lake Superior, Ontario (Dechtiar and Lawrie Reference Dechtiar and Lawrie1988). Catostomus columbianus (Eigenmann & Eigenmann) – not specified, British Columbia (Anonymous 1984 in McDonald and Margolis Reference McDonald and Margolis1995). Catostomus commersonii – Chautaqua Lake and French Creek, New York (as Murraytrema copulata; Mueller Reference Mueller1938*); not specified, British Columbia (Anonymous 1978 in McDonald and Margolis Reference McDonald and Margolis1995); McGregor River and Parsnip River, British Columbia (Arai and Mudry Reference Arai and Mudry1983); Lake Erie, Lake Ontario, Lake Superior, Lake of the Woods, Algonquin Park Lakes, Ontario (Dechtiar Reference Dechtiar1972a, Reference Dechtiar1972b; Dechtiar and Christie Reference Dechtiar and Christie1988; Dechtiar and Lawrie Reference Dechtiar and Lawrie1988; Dechtiar and Nepszy Reference Dechtiar and Nepszy1988; Dechtiar et al. Reference Dechtiar, MacLean and Nepszy1989); Silver Creek (Fond du Lac Co.), St. Croix River (Burnett Co.), Wisconsin (as Murraytrema copulata; Mizelle and Klucka Reference Mizelle and Klucka1953*). Catostomus macrocheilus – not specified, British Columbia (Anonymous 1978, 1981, 1984 in McDonald and Margolis Reference McDonald and Margolis1995); McGregor River and Parsnip River, British Columbia (Arai and Mudry Reference Arai and Mudry1983). Chasmistes fecundus (Cope & Yarrow) (syn. Catostomus fecundus) – Spring Lake, Wyoming (as Murraytrema copulata; Mizelle and Webb Reference Mizelle and Webb1953). Moxostoma anisurum – Lake Erie and Lake of the Woods, Ontario (Dechtiar Reference Dechtiar1972a, Reference Dechtiar1972b); Chautaqua Lake and French Creek, New York (as Murraytrema copulata; Mueller Reference Mueller1938). Moxostoma erythrurum – Lake Erie, Ontario (Dechtiar Reference Dechtiar1972a); Chautaqua Lake and French Creek, New York (as Murraytrema copulata; Mueller Reference Mueller1938). Moxostoma macrolepidotum – Lake Erie, Lake Ontario, Lake Huron, Ontario (Dechtiar Reference Dechtiar1972a; Dechtiar and Christie Reference Dechtiar and Christie1988; Dechtiar et al. Reference Dechtiar, Collins and Reckahn1988). Moxostoma pisolabrum (syn. M. aureolum) – Silver Creek (Fond du Lac Co.), St. Croix River (Burnett Co.), Wisconsin (as Murraytrema copulata; Mizelle and Klucka Reference Mizelle and Klucka1953*).
Site of infection: Gills.
Prevalence and intensity of infection: 33% (1 fish infected/3 fish examined); 2 parasites per infected host.
Voucher specimens: One hologenophore (USNM 1762089); 1 morphological voucher (USNM 1762090).
Specimens studied for comparison: M. copulata (Mueller Reference Mueller1938) (USNM 1367014; 8 slides).
Measurements: Body 445 (n = 1) long; greatest width 65 (n = 1). Pharynx 40 (n = 1) long, 39 (n = 1) wide. Haptor 85 (n = 1) long, 121 (n = 1) wide. Ventral anchor 36 (n = 1) long; base width 11 (n = 1). Dorsal anchor 41 (n = 1) long; base width 13 (n = 1). Ventral bar 39 (n = 1) long. Paired dorsal bar 27 (n = 1) long. Hooks 12 (11–12; n = 1) long. MCO – tube curved length 91 (n = 1); tube height 34 (n = 1).
Remarks: Pseudomurraytrema copulatum, the type species of the genus, has been reported from 10 catostomid species representing 4 genera (Catostomus, Chasmistes, Hypentelium and Moxostoma) within the subfamily Catostominae, across 4 US states (Illinois, New York, Wisconsin and Wyoming) and 2 Canadian provinces (British Columbia and Ontario). Such a broad host range is highly unusual for monopisthocotylan parasites of catostomids, and it is likely that some of the records summarized under the species account are erroneous or represent misidentifications. Unfortunately, because the majority of these host–parasite records are not supported by illustrations or deposited voucher specimens, they cannot be critically re-evaluated.
Mueller (Reference Mueller1938) described P. copulatum from the gills of H. nigricans, M. anisurum, M. erythrurum and C. commersonii. Our examination of a large collection of cotypes (8 slides, each containing more than 2 specimens) from H. nigricans revealed that the type series includes individuals belonging to 3 other species of Pseudomurraytrema described after the original designation of P. copulatum: Pseudomurraytrema etowanum, Pseudomurraytrema paradoxum and Pseudomurraytrema rogersi. Unfortunately, many of the cotypes were in such poor condition that specific identification was not possible, and the specimen used by Mueller (Reference Mueller1938) to illustrate his Figures 7, 8 and 11 could not be located. However, his illustrations of the haptoral and copulatory structures are of good quality and correspond well with subsequent redescriptions of P. copulatum by Kritsky and Hathaway (Reference Kritsky and Hathaway1969) and Kritsky and Leiby (Reference Kritsky and Leiby1973), as well as with our own specimens collected from the same host species in Arkansas.
In contrast, Mueller’s (Reference Mueller1938) report of P. copulatum on C. commersonii is demonstrably erroneous. His Figures 9, 12 and 13 clearly depict a different species, here described as P. commersoni n. sp. (see Remarks for the new species). It is also evident that Mizelle and Klucka (Reference Mizelle and Klucka1953) misidentified their material as P. copulatum. Although they did not illustrate the haptoral bars or the vagina, and their depiction of the MCO is insufficient for diagnostic purposes, the anchors in their figures closely resemble those of P. commersoni n. sp. However, because they did not indicate whether the illustrated sclerites originated from specimens collected on C. commersonii or on M. pisolabrum (syn. M. aureolum), the occurrence of P. commersoni n. sp. on M. pisolabrum remains unconfirmed.
Pseudomurraytrema copulatum is 1 of 4 Pseudomurraytrema species currently recorded from the gills of H. nigricans, along with P. etowanum, P. paradoxum and P. rogersi. Among these, P. copulatum most closely resembles P. etowanum in the morphology of both haptoral and copulatory sclerites (see Remarks under P. etowanum for detailed differentiation).
The occurrence of P. copulatum on H. nigricans in Arkansas represents a new geographic record for this parasite.
Pseudomurraytrema etowanum Rogers, 1966 (Figure 20)
Type host and locality: Hypentelium etowanum (Jordan) – Mooreʼs Mill Creek (Lee Co.), Alabama.

Figure 20. Pseudomurraytrema etowanum ex Hypentelium nigricans (Arkansas): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Current records: Hypentelium nigricans – Huddleston Creek (Montgomery Co.), Arkansas (12 June 2019).
Previous records: See type host species and locality (Rogers Reference Rogers1966). Hypentelium nigricans – Little Vermilion River near Sidell (Vermilion Co.), Illinois (Kritsky and Hathaway Reference Kritsky and Hathaway1969); Grand River at the Ashtabula-Lake Co., Ohio (Mergo and White Reference Mergo and White1984).
Site of infection: Gills.
Prevalence and intensity of infection: 67% (2 fish infected/3 fish examined); 1–3 parasites per infected host.
Voucher specimens: One hologenophore (USNM 1762091).
Measurements: Body 1040 (546–1264; n = 4) long; greatest width 169 (155–177; n = 4). Pharynx 69 (65–72; n = 3) long, 61 (58–63; n = 3) wide. Haptor 109 (94–119; n = 4) long, 158 (129–170; n = 4) wide. Ventral anchor 38 (33–42; n = 4) long; base width 17 (16–18; n = 4). Dorsal anchor 50 (44–56; n = 4) long; base width 16 (15–17; n = 4). Ventral bar 39 (33–42; n = 4) long. Paired dorsal bar 30 (25–35; n = 4) long. Hooks 13 (12–14; n = 3) long. MCO – tube curved length 89 (86–92; n = 6); tube height 33 (31–34; n = 6).
Remarks: Pseudomurraytrema etowanum was described on the gills of H. etowanum in Alabama by Rogers (Reference Rogers1966), and later reported on H. nigricans in Illinois (Kritsky and Hathaway Reference Kritsky and Hathaway1969) and Ohio (Mergo and White Reference Mergo and White1984). The morphology of the haptoral sclerites in our specimens from H. nigricans (Arkansas) corresponds to that depicted by Rogers (Reference Rogers1966), except that the dorsal and ventral anchors have a slight undulation near the junction of the shaft and point, the character also described by Kritsky and Hathaway (Reference Kritsky and Hathaway1969).
Pseudomurraytrema etowanum could be confused with P. copulatum, as both species have very similar morphology of haptoral and copulatory sclerites. The dorsal anchor of both species has markedly elongate shaft and short point. The dorsal anchor of P. etowanum, however, differs from that of P. copulatum by its slightly bent shaft (vs arcuate shaft in P. copulatum) and shorter sharply recurved point. It further clearly differs from P. copulatum in that the ventral anchor has a terminally flattened base (vs diagonally truncated termination of base in P. copulatum), a slightly bent shaft and a shorter sharply recurved point (vs arcuate shaft poorly delimited from moderately long point in P. copulatum).
The occurrence of P. etowanum on H. nigricans in Arkansas represents a new geographic record for this parasite.
Pseudomurraytrema paradoxum Kritsky & Hathaway, 1969 (Figure 21)
Type host and locality: Hypentelium nigricans – Sangamon River near Lake of the Woods, Mahomet (Champaign Co.), Illinois.

Figure 21. Pseudomurraytrema paradoxum ex Hypentelium nigricans (Arkansas): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Current records: Hypentelium nigricans – Walnut Creek (Garland Co.), Arkansas (16 June 2019).
Previous records: See type host species and locality (Kritsky and Hathaway Reference Kritsky and Hathaway1969); Little Vermilion River near Sidell (Vermilion Co.), Illinois (Kritsky and Hathaway Reference Kritsky and Hathaway1969); Grand River at the Ashtabula-Lake Co., Ohio (Mergo and White Reference Mergo and White1984).
Site of infection: Gills.
Prevalence and intensity of infection: 100% (3 fish infected/3 fish examined); 1–7 parasites per infected host.
Voucher specimens: Three hologenophores (USNM 1762113–1762115); 1 morphological voucher (USNM 1762116).
Measurements: Body 868 (n = 1) long; greatest width 94 (n = 1). Pharynx 46 (n = 1) long, 39 (n = 1) wide. Haptor 105 (n = 1) long, 125 (n = 1) wide. Ventral anchor 31 (29–35; n = 4) long; base width 14 (13–16; n = 4). Dorsal anchor 43 (38–50; n = 4) long; base width 14 (12–16; n = 4). Ventral bar 60 (56–68; n = 4) long. Paired dorsal bar 49 (42–57; n = 4) long. Hooks 12 (11–13; n = 4) long. MCO – tube curved length 103 (101–104; n = 5); tube height 36 (35–37; n = 5).
Remarks: Pseudomurraytrema paradoxum was collected from the gills of Hypentelium nigricans in association with P. copulatum, P. etowanum and P. rogersi. Of these species, P. paradoxum and P. rogersi differ from all other congeners by the presence of a unique haptoral structure: a pair of bilateral, unarmed, rounded plates arising from the anteroventral haptoral surface and ventrally overlapping the posterior part of the peduncle.
Specimens collected from H. nigricans in Arkansas during the present study correspond well to the original descriptions of P. paradoxum (Kritsky and Hathaway Reference Kritsky and Hathaway1969) and P. rogersi (Chien Reference Chien1969), with the exception that those authors described the haptor as bearing 2 plates on the ventral side and 1 on the dorsal side. In our material, the presence of a dorsal plate could not be confirmed in either species. Instead, we observed a semicircular thick band of muscle, the ends of which are oriented toward the ventral anchor–bar complex and, in combination with that complex, give the appearance of a closed central structure. This configuration may have been interpreted as a dorsal plate in the earlier descriptions.
The ambiguity surrounding this haptoral region was also noted by Chien (Reference Chien1969), who, after examining type specimens of P. paradoxum, concluded that the 3 plates described by Kritsky and Hathaway (Reference Kritsky and Hathaway1969) most likely represent a single large sucker. Based on our observations, we suggest that scanning electron microscopy (SEM) should be employed to clarify the structural configuration of the haptor and to resolve the existence and nature of the 3 previously described plates in P. paradoxum and P. rogersi. Pseudomurraytrema paradoxum differs from P. rogersi by the following characters: (1) a subrectangular haptor with 2 large anteroventral plates (vs an anteriorly elongated, subhexagonal haptor with 2 small anteroventral plates in P. rogersi), (2) a ventral bar that is proportionally huge relative to the ventral anchors (vs proportionally small relative to the ventral anchors in P. rogersi), (3) a paired dorsal bar lacking a segmented medial end (vs a dorsal bar with a segmented medial end in P. rogersi), (4) a dorsal anchor with a long, straight shaft and a relatively short base with a flattened proximal margin (vs a shorter shaft and a large, fan-shaped base with a rounded proximal margin in P. rogersi), (5) a ventral anchor with a short, proximally widened and flattened base (vs a fan-shaped base with a proximally elongated outer margin in P. rogersi), and (6) a vagina with a longer and more tightly coiled tube.
The occurrence of P. paradoxum on H. nigricans in Arkansas represents a new geographic record for this species.
Pseudomurraytrema rogersi Chien, 1969 (Figure 22)
Type host and locality: Hypentelium nigricans – Rock Creek, near Chattahoochee Forest National Fish Hatchery (Fannin Co.), Georgia.

Figure 22. Pseudomurraytrema rogersi ex Hypentelium nigricans (Arkansas): VA – ventral anchor, VB – ventral bar; DA – dorsal anchor; DB – dorsal bar; H – hook; MCO – male copulatory organ; VG – vagina.
Current records: Hypentelium nigricans – Walnut Creek (Garland Co.), Huddleston Creek (Montgomery Co.), Arkansas (16 June 2019).
Previous records: See type host species and locality (Chien Reference Chien1969); Little Vermilion River, 3 mi NE of Allerton (Vermilion Co.), Illinois (Kritsky and Leiby Reference Kritsky and Leiby1973); Grand River at the Ashtabula-Lake Co., Ohio (Mergo and White Reference Mergo and White1984).
Site of infection: Gills.
Prevalence and intensity of infection: 100% (3 fish infected/3 fish examined); 1–6 parasites per infected host.
Voucher specimens: Two hologenophores (USNM 1762117, 1762118); 1 morphological voucher (USNM 1762119).
Measurements: Body 2231 (1268–2990; n = 3) long; greatest width 224 (164–260; n = 3). Pharynx 110 (104–118; n = 3) long, 93 (85–97; n = 3) wide. Haptor 195 (182–208; n = 2) long, 187 (180–193; n = 2) wide. Ventral anchor 61 (51–71; n = 6) long; base width 26 (23–30; n = 6). Dorsal anchor 71 (61–82; n = 6) long; base width 32 (24–39; n = 6). Ventral bar 37 (31–42; n = 6) long. Paired dorsal bar 54 (44–63; n = 6) long. Hooks 15 (15–16; n = 5) long. MCO – tube curved length 84 (83–86; n = 3); tube height 33 (32–34; n = 3).
Remarks: The unique configuration of the haptor and the presence of a paired dorsal bar with a segmented medial end are the main features distinguishing Pseudomurraytrema rogersi from all other known congeners. The occurrence of P. rogersi on H. nigricans in Arkansas represents a new geographic record for this species.
Molecular data and phylogenetic inference
New DNA sequences of 3 nuclear markers (i.e. 28S rDNA, 18S rDNA and ITS1) were generated for 21 species of Pseudomurraytrematidae. BLAST searches confirmed that among the newly collected specimens, 4 belong to 2 species – Pseudomurraytrema sp. ‘ardens’ and P. fergusoni – which are currently the only representatives of Pseudomurraytrema available in GenBank. BI and ML analyses yielding nearly identical topologies. Therefore, only the ML tree with nodal support values from both analyses was selected for presentation of each of 2 datasets – 28S (Figure 23) and concatenated 18S, ITS1 and 28S (Figure 24).

Figure 23. Molecular phylogeny of Dactylogyridea inferred by Maximum Likelihood (ML) analysis from an alignment of partial 28S rDNA sequences (789 bp). Species newly described in this study are shown in bold. Numbers along the branches indicate Posterior Probabilities (PP)/Shimodaira–Hasegawa approximate likelihood ratio test (SH-aLRT)/Ultrafast Booststrap (UFBoot) values resulting from BI and ML analyses. Only high support values are shown; dashes (-) indicate support below the threshold (PP ≤ 0.95, SH-aLRT ≤ 80, UFBoot ≤ 95). *Taxon traditionally placed within Gyrodactylidea, but here recovered within Dactylogyridea as sister to Tetraonchinea.

Figure 24. Phylogenetic relationships within Pseudomurraytrematidae inferred from Maximum Likelihood (ML) analysis based on an alignment of concatenated 18S rDNA, ITS1 and 28S rDNA sequences (1558 bp). Species newly described in this study are shown in bold. Numbers along the branches indicate Posterior Probabilities (PP)/Shimodaira–Hasegawa approximate likelihood ratio test (SH-aLRT)/Ultrafast Booststrap (UFBoot) values resulting from BI and ML analyses. Only high support values are shown; dashes (-) indicate support below the threshold (PP ≤ 0.95, SH-aLRT ≤ 80, UFBoot ≤ 95).
Position of Pseudomurraytrematidae within Dactylogyridea using 28S rDNA
In the phylogenetic tree based on 28S rDNA (Figure 23), Dactylogyrinea was well supported within Dactylogyridea. Anoplodiscus cirrusspiralis (Anoplodiscidae) was inferred as the sister lineage to Tetraonchus monenteron (Tetraonchidae), suggesting a close phylogenetic relationship. Earlier morphological studies placed A. cirrusspiralis within Gyrodactylidea (Boeger and Kritsky Reference Boeger and Kritsky1993, Reference Boeger, Kritsky, Littlewood and Bray2001), a view also reflected in the WoRMS database. In contrast, molecular analyses (Boeger et al. Reference Boeger, Kritsky, Domingues and Bueno-Silva2014; present study) consistently support its position within Dactylogyridea. This conflict highlights the need for additional data before its systematic placement can be reliably resolved. Within Dactylogyrinea, 3 families were clearly circumscribed: Dactylogyridae, Diplectanidae and Pseudomurraytrematidae. The monophyly of Pseudomurraytrematidae was strongly supported (PP/SH-aLRT/UFBoot = 1/100/100), as was the monophyly of the included species of Dactylogyridae (PP/UFBS = 1/100/100) and Diplectanidae (PP/SH-aLRT/UFBoot = 1/96/99). Pseudomurraytrematidae formed a clade sister to Diplectanidae (PP/SH-aLRT/UFBoot = 0.97/87/95), with Pseudomurraytrema asiaticum, a parasite of the Chinese sucker (Myxocyprinus asiaticus), recovered in a basal position relative to all Nearctic species within the family. Nearctic pseudomurraytrematids (PP/SH-aLRT/UFBoot = 0.84/90/87) formed a clade within which 2 lineages were recovered. The first lineage, comprising species of Pseudomurraytrema, was well supported (PP/SH-aLRT/UFBoot = 0.99/92/95), whereas the second, with limited support (PP/SH-aLRT/UFBoot = 0.88/97/87), included species of Anonchohaptor and Icelanonchohaptor.
Interspecific relationships of the Nearctic pseudomurraytrematids using concatenated 18S rDNA, ITS1 and 28S rDNA
Using concatenated data set, the same 2 lineages within Pseudomurraytrematidae are present (Clades A and B), using both BI and ML analyses (Figure 24). Clade A comprises 13 species of Pseudomurraytrema, grouped into 2 well supported subclades (A1 and A2), with the exception of P. fergusoni, which occupies a basal position relative to the remaining 12 species (i.e. subclades A1 and A2). The separation of P. fergusoni from the remaining species of Pseudomurraytrema is supported morphologically by the unique structure of the MCO and vagina (see Figure 14). In P. fergusoni, the copulatory tube’s distal end is truncated (vs curved and narrowed in other family members), and the base of the tube merges with the proximal portion of the accessory piece, creating a bulb-like appearance (vs S-shaped base in other pseudomurraytrematids). The vagina is tubular and located in a small bulb protruding from the right margin of the body in P. fergusoni (vs a vagina with a distally weakly sclerotized funnel and a proximally coiled tube in other pseudomurraytrematids).
The first subclade (A1) is strongly supported by both BI and ML analyses (PP/SH-aLRT/UFBoot = 1/100/100) and includes 4 species of Pseudomurraytrema host specific to Hypentelium nigricans (P. copulatum, P. etowanum, P. paradoxum and P. rogersi), P. alabarrum from M. melanops and P. janullae n. sp. from E. claviformis. Two species from H. nigricans, namely P. copulatum and P. etowanum, occupy the sister position relative to the clade including 4 remaining species. The sister relationship of P. copulatum and P. etowanum supported by BI analysis (PP/SH-aLRT/UFBoot = 0.92/80/81) is corroborated by high similarity in haptoral morphology and nearly identical reproductive structures in both species (see Remarks for P. etowanum). The other 4 species of Pseudomurraytrema form a group well supported by BI analysis (PP/SH-aLRT/UFBoot = 0.99/90/77). Within this group, P. rogersi from H. nigricans occupies the basal position. The last species of Pseudomurraytrema from H. nigricans, P. paradoxum, appears as the sister to P. alabarrum from M. melanops; however, this relationship is variably supported and recovered only in the BI analysis (PP/SH-aLRT/UFBoot = 0.94/84/64). The relationship of P. janullae n. sp. from E. claviformis within this group remains unresolved.
The second subclade (A2) is strongly supported (PP/SH-aLRT/UFBoot = 1/96/97) and includes 4 Pseudomurraytrema species (P. fluviatile, P. swinglei, P. cf. coosense and P. milleri) from Moxostoma species and 2 Pseudomurraytrema species (P. ardens n. sp. and P. commersoni n. sp.) from Catostomus species. Pseudomurraytrema commersoni n. sp. from C. commersonii is recovered in a basal position relative to a clade (PP/SH-aLRT/UFBoot = 0.98/85/76) comprising the remaining Pseudomurraytrema species, predominantly parasitizing Moxostoma hosts; within this clade, P. ardens n. sp. from C. ardens is basal. The position of P. milleri from M. anisurum was unresolved (PP/SH-aLRT/UFBoot = 0.69/19/62).
Clade B includes 6 species of Anonchohaptor and 2 species of Icelanonchohaptor, with the latter recovered in a basal position. The sister relationship between I. cherubinus n. sp. and I. seraphinus n. sp., both parasitizing species of Ictiobus (I. bubalus and I. niger, respectively), is strongly supported by BI and ML analyses (PP/SH-aLRT/UFBoot = 1/99/100). The subclade containing Anonchohaptor species lacks support in both BI and ML analyses (PP/SH-aLRT/UFBoot = 0.78/32/65). Within this subclade, A. muelleri from Carpiodes carpio is sister to the remaining taxa, although this relationship is unsupported (PP/SH-aLRT/UFBoot = 0.69/19/58). The sister relationships between A. meganbeanae n. sp. and Anonchohaptor sp. 4, both from I. bubalus, as well as between Anonchohaptor sp. 1 from M. poecilurum and Anonchohaptor sp. 2 from M. macrolepidotum, are strongly supported (PP/SH-aLRT/UFBoot = 1/96/95 and 1/98/99, respectively). The relationship of Anonchohaptor sp. 3 from C. commersonii and Minytrema melanops relative to the 2 latter Anonchohaptor species is unresolved in BI and ML analyses.
The genetic differences among Pseudomurraytrema species (excluding P. asiaticum) based on 28S rDNA sequences ranged from 0.53% (between P. fluviatile and P. swinglei) to 5.26% (between P. copulatum and P. cf. coosense). For the 18S and ITS1 fragment, the lowest genetic distance was observed between P. etowanum and P. rogersi (1.01%), while the highest divergence was found between P. ardens n. sp. and P. copulatum (4.56%) (Table S1).
Discussion
Species of Pseudomurraytrematidae are parasitic flatworms that exclusively inhabit the gills and/or skin of suckers (Catostomidae, Cypriniformes). Prior to this study, 20 nominal species had been described within the family, distributed across 4 genera (Anonchohaptor, Icelanonchohaptor, Myzotrema and Pseudomurraytrema), and recorded from 19 species of Nearctic suckers and a single species of Eastern Asian suckers. Here, we describe 1 new species of Anonchohaptor, 2 new species of Icelanonchohaptor, and 4 new species of Pseudomurraytrema, increasing the number of recognized pseudomurraytrematid species to 27 and extending the host range to 22 catostomid species across both continents.
Monophyly of Pseudomurraytrematidae and relationships within Dactylogyridea
Our phylogenetic analyses based on 28S rDNA sequences, including representatives of major dactylogyridean lineages, support the monophyly of Pseudomurraytrematidae. All analysed members, including species of Anonchohaptor, Icelanonchohaptor and Pseudomurraytrema, form a well-supported clade. Morphological evidence provides additional support for the monophyly of the family, highlighted by a synapomorphy of the MCO: a U-shaped copulatory tube with a submedial spine and a 3-ramus accessory piece. The phylogenetic tree also confirms a close phylogenetic relationship between Pseudomurraytrematidae and Diplectanidae. These 2 families form a strongly supported cluster within Dactylogyridea, reinforcing their shared evolutionary history. This result is consistent with previous morphological and molecular studies (e.g. Kritsky and Boeger Reference Kritsky and Boeger1989; Boeger and Kritsky Reference Boeger and Kritsky1993, Reference Boeger and Kritsky1997, Reference Boeger, Kritsky, Littlewood and Bray2001; Plaisance et al. Reference Plaisance, Littlewood, Olson and Morand2005; Ogawa and Itoh Reference Ogawa and Itoh2017; Soares et al. Reference Soares, Domingues and Adriano2021), which similarly suggested a phylogenetic affinity between the representatives of the 2 families based on shared morphological features and limited molecular data. Notably, pseudomurraytrematids and diplectanids share the feature of the ovary looping around the right intestinal caecum, consistently documented across all genera of both families. A paired dorsal bar is likewise present in all species of Pseudomurraytrema and in members of Diplectanidae. Similarities in the morphology of MCOs further suggest potential evolutionary connections between Pseudomurraytrematidae and certain subgroups within Diplectanidae, particularly the subfamily Lamellodiscinae Oliver, 1969. According to Oliver (Reference Oliver1987), species of Lamellodiscus can be categorized into 3 morphological types. One of them, the ‘lyre type’, includes species with an MCO that features a slightly curved or S-shaped copulatory tube and an accessory piece shaped like a lyre, tuning fork, or Y (Domingues and Boeger Reference Domingues and Boeger2008). These species, parasitizing sparid hosts in Mediterranean and Atlantic waters (Oliver Reference Oliver1987; Neifar et al. Reference Neifar, Euzet and Oliver2004; Amine et al. Reference Amine, Neifar and Euzet2006, Reference Amine, Euzet and Kechemir-Issad2007), share with pseudomurraytrematids the 3-ramus configuration of the accessory piece. This character may represent a synapomorphy of Pseudomurraytrematidae or of a broader lineage that includes ‘lyre type’ Lamellodiscus, although further phylogenetic analyses are needed to clarify its evolutionary significance. These similarities suggest either convergent evolution or shared ancestry and underscore the need for more detailed molecular and morphological investigations of these groups.
Phylogenetic relationships within Pseudomurraytrematidae and the position of Pseudomurraytrema asiaticum
The phylogenetic analyses support the monophyly of Pseudomurraytrematidae, but do not support the monophyly of either Pseudomurraytrema or Anonchohaptor. More specifically, analyses based on 28S rDNA sequences suggest that Pseudomurraytrema may be paraphyletic because P. asiaticum, the sole Asian representative of the genus, is recovered as sister to the Nearctic pseudomurraytrematid clade (i.e. the lineage comprising Pseudomurraytrema, Anonchohaptor and Icelanonchohaptor). In the 28S-based tree, P. asiaticum branches separately from this Nearctic clade; within it, the Pseudomurraytrema subclade is well supported. This pattern suggests evolutionary divergence and challenges the current generic definition. This divergence likely reflects a combination of geographic isolation and host specificity. Pseudomurraytrema asiaticum parasitizes Myxocyprinus asiaticus, a basal East Asian member of Catostomidae (Bagley et al. Reference Bagley, Mayden and Harris2018), whereas all other species of Pseudomurraytrema infect North American catostomids. This host–parasite association between M. asiaticus and P. asiaticum is consistent with a long-term coevolutionary history and may indicate that P. asiaticum represents a relict lineage associated with the basal Asian host lineage of Catostomidae. Notably, P. asiaticum does not exhibit an exceptionally long branch in the phylogenetic tree (Figure 23), suggesting that its divergence is not simply due to elevated substitution rates but may instead reflect historical biogeographic and ecological processes.
Morphological data offer a contrasting perspective. Diagnostic structures such as the prohaptor, haptor and reproductive organs are conserved across the genus, supporting the monophyly of Pseudomurraytrema. The molecular placement of P. asiaticum outside the core Pseudomurraytrema clade, however, challenges this view and illustrates how molecular data may reveal cryptic divergence maintained by host-associated isolation and reinforced by biogeographic barriers. While morphology may reflect deeper evolutionary conservatism, reconciling these discrepancies will require expanded taxon sampling and additional molecular markers.
Similar phylogenetic uncertainty affects the relationship between Anonchohaptor and Icelanonchohaptor. In the 28S tree, species of Icelanonchohaptor cluster within Anonchohaptor, rendering the latter paraphyletic. In the concatenated dataset, species of Icelanonchohaptor and Anonchohaptor together form a monophyletic clade, with Icelanonchohaptor recovered as sister to the remaining Anonchohaptor species. These incongruences indicate that the 2 genera represent a closely related lineage, but the relationships among their species remain unresolved. In contrast to species of Pseudomurraytrema, which are morphologically distinct from both aforementioned genera, species of Anonchohaptor and Icelanonchohaptor are remarkably similar in morphology. Notably, they share the presence of a haptor bearing only 7 pairs of hooks and a similarly shaped proximal ramus of the accessory piece, which is expanded into wing-like lobes in both genera. The most prominent distinguishing feature between Anonchohaptor and Icelanonchohaptor lies in the soft morphology of the haptor. In Anonchohaptor, the haptor is broad, disc-shaped and distinctly wider than the body. In contrast, the haptor of Icelanonchohaptor is cup-shaped, highly muscular and does not exceed the body width.
Although Pseudomurraytrema, Anonchohaptor and Icelanonchohaptor are currently recognized as valid genera, the phylogenetic patterns observed in this study suggest that a revision of their generic boundaries may be warranted. Such a revision will require broader taxon sampling, particularly of species of Icelanonchohaptor, with special emphasis on sequencing its type species. This genus is currently represented by only 2 molecularly characterized species, which prevents a robust assessment of its distinctiveness. Additional sampling from undersurveyed host lineages, especially Ictiobinae and the inclusion of complementary data from mitochondrial genes may provide further resolution of the boundaries among these genera.
Host–parasite associations and diversification patterns
Our synthesis of new data and previous records indicates that host specificity is generally high in Nearctic species of Pseudomurraytrema: out of 15 recognized species, 9 are restricted to a single host species and 6 occur on 2 congeneric hosts (excluding unverified or erroneous older records; see above). In contrast, species of Anonchohaptor appear to have a broader host range, with A. anomalum reported from 4 host genera (e.g. Mueller Reference Mueller1938; Chien Reference Chien1969; Dechtiar and Dillon Reference Dechtiar and Dillon1974; Beverley-Burton Reference Beverley-Burton, Margolis and Kabata1984) and A. muelleri from 3 (e.g. Kritsky et al. Reference Kritsky, Leiby and Shelton1972; Combs et al. Reference Combs, Williams and Harley1976). However, such unusually wide host ranges should be interpreted with caution, as most published records lack illustrations or voucher specimens and therefore cannot be critically re-evaluated. A similar pattern of relatively narrow specificity is observed in Icelanonchohaptor, where 2 of the 3 known species were found on a single host species and I. microcotyle on 2 congeneric hosts (Kritsky et al. Reference Kritsky, Leiby and Shelton1972; Dechtiar and Nepszy Reference Dechtiar and Nepszy1988).
The present study reveals that phylogenetic relationships of pseudomurraytrematid parasites only partially reflect the phylogeny of their catostomid hosts. Three major parasite lineages are recovered, each associated with distinct sets of host taxa. Two of these lineages (A1 and A2) comprise species of Pseudomurraytrema parasitizing members of Catostominae, whereas a third lineage (Clade B) includes Anonchohaptor and Icelanonchohaptor primarily associated with Ictiobinae, with some occurrences on more distantly related catostomid hosts.
Subclade A1 includes species parasitizing H. nigricans (Thoburnini), M. melanops and E. claviformis (both Erimyzonini). However, these associations do not fully align with the phylogenetic relationships inferred for their catostomid hosts in previous studies (Chen and Mayden Reference Chen and Mayden2012; Bagley et al. Reference Bagley, Mayden and Harris2018; Yang et al. Reference Yang, Mayden and Naylor2024). According to the most recent host phylogeny (Yang et al. Reference Yang, Mayden and Naylor2024), Erimyzonini is variably recovered as sister to Catostomini (nuclear dataset) or as basal to the clade comprising Catostomini, Moxostomatini and Thoburnini (mitochondrial dataset). Despite this, Minytrema- and Erimyzon-associated monopisthocotylans are nested within the clade dominated by Hypentelium-associated parasites. This incongruence, which involves distantly related host genera, likely reflects historical host switching, ecological similarity, or the retention of ancestral associations across divergent host lineages.
Subclade A2 includes species parasitizing Moxostoma (Moxostomatini) and Catostomus (Catostomini). Although these hosts belong to different tribes and are placed in separate lineages within Catostominae, parasites from Catostomus are consistently embedded within the Moxostoma-dominated subclade. In various phylogenetic reconstructions, Catostomini is either sister to Erimyzonini (concatenated mitochondrial and nuclear datasets with morphological data; Bagley et al. Reference Bagley, Mayden and Harris2018), sister to Moxostomatini/Thoburnini (mitochondrial dataset; Yang et al. Reference Yang, Mayden and Naylor2024), or shows an unresolved position within Catostominae (nuclear datasets; Yang et al. Reference Yang, Mayden and Naylor2024). This again suggests that parasite diversification has not strictly mirrored host evolution and is consistent with host-switching events between distantly related host tribes.
Within clade A, P. fergusoni stands out from other Pseudomurraytrema species for its basal position and unusual genital morphology. This pattern is consistent with early divergence potentially linked to long-term ecological isolation or specialization on M. pisolabrum and M. macrolepidotum (McAllister et al. Reference McAllister, Leis, Cloutman, Woodyard, Camus and Robison2022; present study).
Clade B includes species of Anonchohaptor and Icelanonchohaptor, most of which parasitize fishes of Ictiobinae (Ictiobus and Carpiodes). The clade also includes 3 species infecting hosts outside Ictiobinae, namely M. melanops (Erimyzonini) and Moxostoma species (Moxostomatini). According to host phylogenies, Ictiobinae forms a clade together with Cycleptinae and Myxocyprininae, and this clade is sister to Catostominae (Bagley et al. Reference Bagley, Mayden and Harris2018; Yang et al. Reference Yang, Mayden and Naylor2024). The overall composition of Clade B suggests an origin on ictiobine hosts, followed by host-switching events leading to colonization of more distantly related catostomid lineages.
Although our sampling was not fully balanced across host lineages, the dataset includes multiple catostomin tribes (Catostomini, Moxostomatini, Thoburnini and Erimyzonini), as well as representatives of Ictiobinae. Moreover, similar host–parasite patterns are recovered across independent parasite lineages (A1, A2, B). Taken together, these observations indicate that the diversification of Pseudomurraytrema species and related taxa reflects the host phylogeny together with processes such as host switching and ecological similarity, rather than sampling bias alone. They are also consistent with comparative evidence linking host specificity and occasional host switching to diversification in dactylogyridean monopisthocotylans (Šimková et al. Reference Šimková, Verneau, Gelnar and Morand2006; Mendlová et al. Reference Mendlová, Desdevises, Civáňová, Pariselle and Šimková2012; Benovics et al., 2020).
Morphological traits and their phylogenetic relevance
The pattern of host specificity in Pseudomurraytrema species, evident in 2 subclades, may also reflect differences in haptoral morphology. Previous studies have demonstrated that hard haptoral structures, particularly anchors and bars, can provide a phylogenetic signal, while also being influenced by host-related factors (e.g. the gill attachment site) and by convergent evolution (e.g. Šimková et al. Reference Šimková, Verneau, Gelnar and Morand2006; Vignon et al. Reference Vignon, Pariselle and Vanhove2011; Khang et al. Reference Khang, Soo, Tan and Lim2016; Rodríguez-González et al. Reference Rodríguez-González, Míguez-Lozano, Sarabeev and Balbuena2016; Cruz-Laufer et al. Reference Cruz-Laufer, Pariselle, Jorissen, Muterezi Bukinga, Al Assadi, Van Steenberge, Koblmüller, Sturmbauer, Smeets, Huyse, Artois and Vanhove2022; Soares et al. Reference Soares, Cohen and Luque2024). Within Pseudomurraytrema, species infecting H. nigricans differ markedly from other congeners in ventral bar morphology. Their ventral bars are relatively simple – rod-like, slightly to broadly V-shaped – and lack the 2 anteromedial and 1 posteromedial processes typical of most other species. Phylogenetic analyses place the 4 species infecting H. nigricans, namely P. copulatum, P. etowanum, P. paradoxum and P. rogersi, in a subclade together with P. alabarrum (from M. melanops) and P. janullae n. sp. (from E. claviformis). All 6 species share a similar dorsal bar, which is elongate, fusiform and tapers to pointed ends. However, P. alabarrum and P. janullae n. sp. retain the more complex ventral bar seen in the second subclade, which includes species from Moxostoma and Catostomus. In this subclade, the ventral bar bears 2 anteromedial and 1 posteromedial process, and the dorsal bar is more robust with a broad medial end. Overall, both dorsal and ventral bar morphology shows patterns that appear consistent with phylogenetic relationships, as species with similar bar shapes tend to cluster together in phylogenetic trees. These observations indicate that haptoral structures, especially bars, might carry useful phylogenetic information within Pseudomurraytrema, a possibility that could be explicitly tested in future studies.
Regarding the sclerotized distal parts of the reproductive system, namely the MCO and the vagina, all representatives of Pseudomurraytrematidae (with the exception of P. fergusoni; see Remarks to the species) exhibit a consistent structural configuration of these organs across all 4 genera (including Myzotrema). This morphological uniformity may represent a synapomorphic trait of the family, reflecting evolutionary conservatism in reproductive structures. The MCO comprises a U-shaped copulatory tube with a submedial spine separating the proximal and distal portions, supported by a 3-armed accessory piece. The proximal portion of the tube is S-shaped, composed of 2 opposing curves, and the distal portion narrows into a blunt tip. The configuration of both parts shows species-level variation, often associated with host genus. For instance, Pseudomurraytrema species parasitizing H. nigricans (except P. rogersi) exhibit a sharply S-shaped proximal portion and a sickle-shaped distal end; Pseudomurraytrema species from species of Moxostoma, M. melanops and E. claviformis tend to have a moderately S-shaped proximal portion and a hook-shaped distal termination; whereas those from Catostomus display a moderately to loosely S-shaped proximal portion and an undulating distal portion, usually curved outward, in contrast to the inward-curved tips observed in the former groups. The vagina comprises a pouch-like distal part and a multiply coiled proximal tube, the number of coils of which is difficult to assess due to indistinct boundaries and uncertain interspecific stability. Although this feature may be species-specific, it is not practical for species delimitation based on light microscopy, with the exception of P. commersoni n. sp., where the number of coils is visibly low.
The coiled proximal tube of the vagina may serve a spring-like function, potentially facilitating prolonged copulation. This behaviour was noted in P. copulatum already in its original description (Mueller Reference Mueller1938) and was also confirmed in our material. We further observed prolonged copulation in P. etowanum and P. rogersi parasitizing H. nigricans, suggesting that this behaviour is more widespread within the genus. Prolonged mating may allow worms to feed or adjust their position on the gills while remaining in copula. In addition to prolonged copulation, reproductive success in monopisthocotylans has also been linked to microhabitat specificity – parasites occupying restricted positions on fish gills – which promotes frequent intraspecific encounters (Šimková Reference Šimková2024).
Acknowledgements
We are sincerely grateful to Delane Kritsky (Idaho State University, Pocatello, Idaho, United states) for sharing copies of earlier publications and for providing specimens of Pseudomurraytrema from Catostomus ardens collected in Idaho, which were described as a new species in this study. We also thank our colleagues from the United States, Canada and Mexico for their generous support during fieldwork, including logistical assistance, fish sampling and species identification. In particular, we are grateful to Megan Bean (Texas State University–San Marcos, San Marcos, Texas, United States), Anindo Choudhury (St. Norbert College, De Pere, Wisconsin, United States), Donald G. Cloutman (Burdett, Kansas, United States), Stephen S. Curran and the late Robin M. Overstreet (Gulf Coast Research Laboratory, Ocean Springs, Mississippi, United States), Eric Leis and his colleagues (La Crosse Fish Health Center, Onalaska, Wisconsin, United States), Chris T. McAllister (Eastern Oklahoma State College, Idabel, Oklahoma, United States), Shawn Meagher (Western Illinois University, Macomb, Illinois, United States), Melissa Pimentel and her colleagues (Ministry of Forests, Wildlife and Parks, Quebec, Canada), Rodolfo Pérez-Rodríguez (Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán, Mexico), Florian B. Reyda (State University of New York, Oneonta, New York, United States) and Edward F. Roseman (U.S. Geological Survey, Great Lakes Science Center, Michigan, United States). We thank our colleagues for their valuable help during dissections and parasite collection: Tomáš Scholz, Roman Kuchta and Carlos A. Mendoza-Palmero (Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice, Czech Republic), Markéta Ondračková (Institute of Vertebrate Biology, Czech Academy of Sciences, Brno, Czech Republic) and Lucie Seidlová (Faculty of Science, Masaryk University, Brno, Czech Republic). We are also grateful to Anna J. Phillips and Adam Stergis (Department of Invertebrate Zoology, Smithsonian National Museum of Natural History, Suitland, Maryland) for providing access to monopisthocotylan type material. We are deeply grateful to the 2 anonymous reviewers for their thoughtful reading and constructive suggestions, which substantially improved the manuscript.
Ethical standards
All field collections and specimen sampling were carried out under permits from the relevant local and regional authorities, facilitated by our partner institutions in the USA, Canada, and Mexico. All applicable institutional, national, and international guidelines for the care and use of animals in scientific research were followed. All authors involved in handling fish are certified for work with experimental animals under §15d of Act No. 246/1992 Coll. (Czech Republic, on the protection of animals against cruelty).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182025101017.
Data availability
Type and voucher specimens were deposited in the Smithsonian Institution, National Museum of Natural History (USNM), Washington, DC, USA. The nucleotide sequences generated in this study are available in GenBank (NCBI; https://www.ncbi.nlm.nih.gov); accession numbers are provided in Tables 2 and 3.
Author’s contribution
ER conceived and designed the study, collected and prepared parasite material, evaluated morphological and molecular data, and interpreted the results. MS carried out molecular sequencing and phylogenetic analyses. AS contributed to field collection and specimen preparation. All authors read and approved the final version of the manuscript.
Financial support
This study was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic, project no. LUAUS23080.
Competing interests
The authors declare there are no conflicts of interest.