Introduction
Toxoplasma gondii (T. gondii) is a protozoan parasite that causes toxoplasmosis, a widespread zoonotic disease diagnosed worldwide (Hamilton et al., Reference Hamilton, Black, Oliveira, Burrells, Bartley, Melo, Chianini, Palarea-Albaladejo, Innes, Kelly and Katzer2019a) with approximately one third of the human population likely to have been exposed to T. gondii (Hill and Dubey, Reference Hill and Dubey2002). Toxoplasma gondii can infect most warm-blooded animals, including humans, small ruminants, rodents and birds (Buxton and Rodger, Reference Buxton, Rodger and Aitken2007; Hamilton et al., Reference Hamilton, Black, Oliveira, Burrells, Bartley, Melo, Chianini, Palarea-Albaladejo, Innes, Kelly and Katzer2019a). Toxoplasma gondii is transmitted between hosts orally through consumption of food or water contaminated with oocysts (shed by infected cats) or by ingestion of tissue cysts found in raw or undercooked meat. Additionally, T. gondii can be transmitted transplacentally from mother to offspring (Hill and Dubey, Reference Hill and Dubey2002).
So far, at least 292 restriction fragment length polymorphism (RFLP) Toxoplasma genotypes have been reported worldwide (Valenzuela-Moreno et al., Reference Valenzuela-Moreno, Rico-Torres, Cedillo-Peláez, Luna-Pastén, Méndez-Cruz, Reyes-García, Correa, Alves, Pena and Caballero-Ortega2020), causing different degrees of pathology in different hosts. Strains can be separated into 2 main groups based on parasite genetics: canonical, and non-canonical (Pena et al., Reference Pena, Gennari, Dubey and Su2008; Chiebao et al., Reference Chiebao, Pena, Cabral, Rocca, Lopes, Valadas, Keid, Grisi Filho and Soares2016). Currently, 12 lineages have been identified in different parts of the world, but some non-canonical strains are highly diverse with unique polymorphisms that mean they cannot be clustered and assigned to a specific lineage (Robert-Gangneux and Darde, Reference Robert-Gangneux and Darde2012). Genotypes #1, #2 and #10 are clonal lineages and are classified as canonical (Robert-Gangneux and Darde, Reference Robert-Gangneux and Darde2012). Genotype #10 strains are generally the most virulent canonical strains, while genotypes #1 and #2 are less virulent in mice and humans (Howe and Sibley, Reference Howe and Sibley1995; Su et al., Reference Su, Shwab, Zhou, Zhu and Dubey2010; Dvorakova-Hortova et al., Reference Dvorakova-Hortova, Sidlova, Ded, Hladovcova, Vieweg, Weidner, Steger, Stopka and Paradowska-Dogan2014; Liu et al., Reference Liu, Wang, Huang and Zhu2015). Genotypes #6, #11 and #8 form the Brazilian clonal lineages and are examples of non-canonical genotypes with #6 being the most virulent, #11 being moderately virulent and most #8 isolates being avirulent or non-lethal (Pena et al., Reference Pena, Gennari, Dubey and Su2008). Human toxoplasmosis is generally not severe and potentially asymptomatic in the immunocompetent. In immunocompromised or congenitally infected individuals, toxoplasmosis can be severe, potentially resulting in encephalitis or death (Remington et al., Reference Remington, McLeod, Thulliez, Desmonts, Remington, Klein, Wilson and Baker2006; Ajzenberg et al., Reference Ajzenberg, Yera, Marty, Paris, Dalle, Menotti, Aubert, Franck, Bessieres, Quinio, Pelloux, Delhaes, Desbois, Thulliez, Robert-Gangneux, Kauffmann-Lacroix, Pujol, Rabodonirina, Bougnoux, Cuisenier, Duhamel, Duong, Filisetti, Flori, Gay-Andrieu, Pratlong, Nevez, Totet, Carme, Bonnabau, Darde and Villena2009). Toxoplasmosis caused by non-canonical strains can result in severe disease that can cause death in immunocompetent individuals (Chiebao et al., Reference Chiebao, Pena, Cabral, Rocca, Lopes, Valadas, Keid, Grisi Filho and Soares2016; da Silva Jr et al., Reference da Silva, Maciel, de Santana Souza Santos, Carvalho, de Santana Rocha, Lopes and Albuquerque2017; Hamilton et al., Reference Hamilton, Robins, Thomas, Oura, Oliveira, Villena, Innes, Katzer and Kelly2019b).
In addition to T. gondii genotype, factors including parasite load (Saeij et al., Reference Saeij, Boyle and Boothroyd2005), T. gondii life stage (Chiebao et al., Reference Chiebao, Pena, Cabral, Rocca, Lopes, Valadas, Keid, Grisi Filho and Soares2016; Dubey et al., Reference Dubey, Ferreira, Alsaad, Verma, Alves, Holland and McConkey2016), host species and immune response can influence infection outcome (Gazzinelli et al., Reference Gazzinelli, Talvani, Camargo, Santiago, Oliveira, Vieira, Martins, Aliberti and Silva1998; Johnson, Reference Johnson1998; Carruthers and Suzuki, Reference Carruthers and Suzuki2007). Mice are often used to determine the virulence and disease outcome when infected with Toxoplasma strains as toxoplasmosis in this species often correlates with the same disease in humans (Hamilton et al., Reference Hamilton, Black, Oliveira, Burrells, Bartley, Melo, Chianini, Palarea-Albaladejo, Innes, Kelly and Katzer2019a) and they show associated lesions in many organs (Pinheiro et al., Reference Pinheiro, Noviello Mde, Cunha, Tavares, Carneiro, Arantes and Vitor2015). The diversity of mouse virulence profiles within the same genotype has previously been shown (Chiebao et al., Reference Chiebao, Pena, Cabral, Rocca, Lopes, Valadas, Keid, Grisi Filho and Soares2016; Calero-Bernal et al., Reference Calero-Bernal, Fernández-Escobar, Katzer, Su and Ortega-Mora2022), which elicits the need for more studies associating a broader set of phenotypic traits with genotypic prediction for virulence.
The aim of this study was to characterize and compare lesions and describe parasite distribution in murine tissues infected with isolates of T. gondii of varying virulence to better understand the relationship between virulence and pathology.
Materials and methods
Tissue samples
Tissues used in this investigation were selected from the Pathology Histology Archive from the Moredun Research Institute (Edinburgh, UK) and were originally collected during 2 previously published studies that will be referred to as Study A (Chiebao et al., Reference Chiebao, Bartley, Chianini, Black, Burrells, Pena, Soares, Innes and Katzer2021) and Study B (Hamilton et al., Reference Hamilton, Black, Oliveira, Burrells, Bartley, Melo, Chianini, Palarea-Albaladejo, Innes, Kelly and Katzer2019a). All animal procedures complied with the Animals (Scientific Procedures) Act 1986, conducted at Home Office registered premises, and were approved by the Moredun Research Institute Experimental and Ethical Review Committee (Study A: E22/14; Study B: E60/15).
Briefly, in Study A, 110 adult female Swiss-Webster mice (Hsd:ND4; Harlan Laboratories, UK) were assigned to 1 of 4 groups: the negative control group A1 (n = 5), A2 (n = 35), A3 (n = 35) and A4 (n = 35). Mice in groups A2, A3 and A4 were inoculated orally with 50 T. gondii oocysts (Table 1). For A1, all mice were humanely euthanised at day 21 post inoculation (p.i.). For A2, A3 and A4, 5 mice were scheduled to be humanely euthanised at days 1, 2, 3, 4, 7, 14 and 21 p.i. Mice were monitored at least 2 times daily for clinical signs of toxoplasmosis (including ruffled coat, hunched demeanour, dehydration and dyspnoea). These signs were scored and if they were above a predetermined score, mice were humanely euthanised (see Chiebao et al. (Reference Chiebao, Bartley, Chianini, Black, Burrells, Pena, Soares, Innes and Katzer2021) for details). Brain and peripheral organs (lung, liver, kidney, diaphragm, stomach and intestine) were collected at postmortem and fixed in 10% buffered formalin.
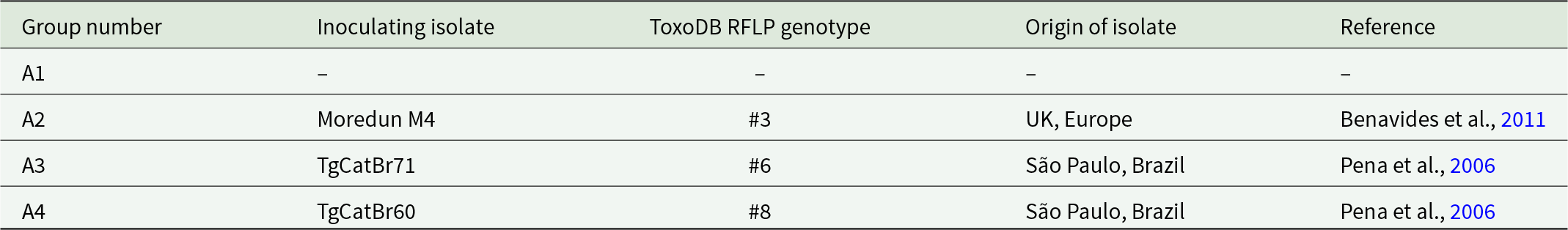
Table 1. Study A groups and corresponding T. gondii isolates

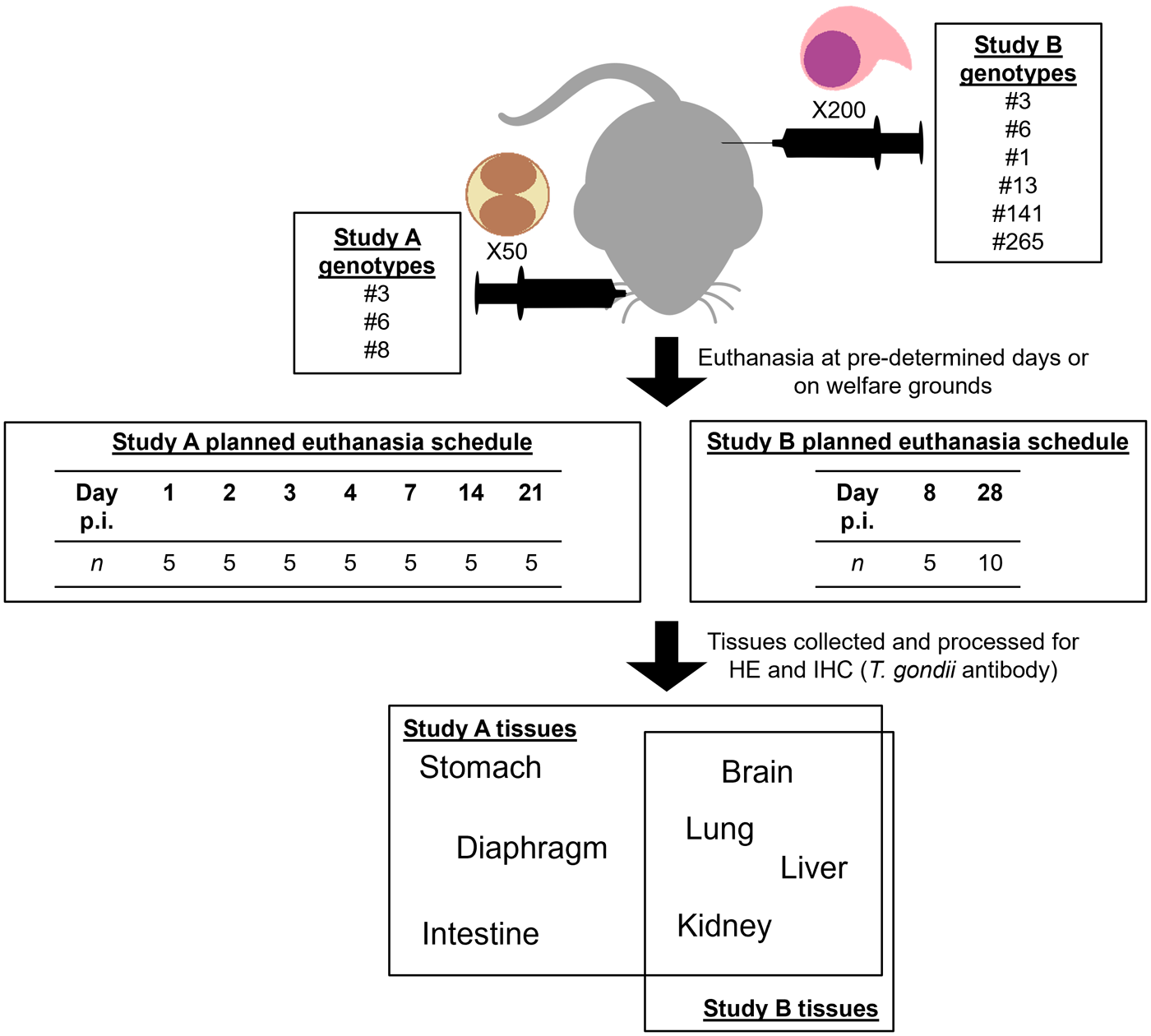
For Study B, 120 adult female Swiss CD-1 mice (Hsd:ICR; Envigo RMS, UK) were assigned to 1 of 8 groups (15 mice per group) as shown in Table 2. European genotype #3, and Brazilian genotype #6 described in Study A were used as controls. Mice were intraperitoneally inoculated with 200 T. gondii tachyzoites and 5 animals from each group were humanely euthanised at day 8 p.i. The remaining 10 mice from each group were monitored until day 28 p.i. Mice were monitored at least 2 times daily for signs of clinical toxoplasmosis, which were subsequently scored. When signs persisted at the maximum permissible score for 2 consecutive days and recovery was deemed not possible, mice were humanely euthanised (see Hamilton et al. (Reference Hamilton, Black, Oliveira, Burrells, Bartley, Melo, Chianini, Palarea-Albaladejo, Innes, Kelly and Katzer2019a) for details). Brain and peripheral organs (lung, liver and kidney) were collected at postmortem and fixed in 10% buffered formalin. A graphical summary of the workflows is provided in Figure 1.

Figure 1. Summary of the workflows for Studies A and B.
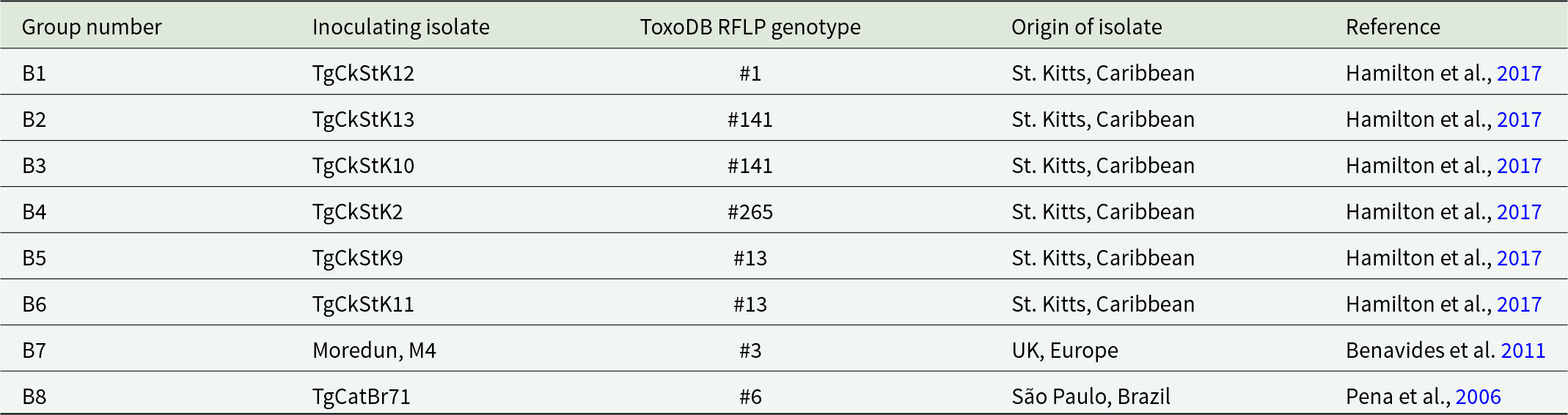
Table 2. Study B groups and corresponding T. gondii isolates

Histopathology and immunohistochemistry
Formalin-fixed tissues from both studies were routinely processed and embedded in paraffin wax. Blocks were processed for histology (haemotoxylin and eosin [HE]) and immunohistochemistry (IHC) as previously described (Hamilton et al., Reference Hamilton, Black, Oliveira, Burrells, Bartley, Melo, Chianini, Palarea-Albaladejo, Innes, Kelly and Katzer2019a; Chiebao et al., Reference Chiebao, Bartley, Chianini, Black, Burrells, Pena, Soares, Innes and Katzer2021). Briefly, sections were cut (5 μm) and stained with HE for histological examination. For IHC, serial sections were cut (5 μm), dried, dewaxed in xylene and taken through alcohols to rehydrate tissues. Slides were then treated with citrate buffer (0.01 M citric acid, pH 6) for heat-induced epitope retrieval. Sections were incubated with normal goat serum (25%) then T. gondii rabbit polyclonal antibody (#PA5-16638, Thermo-Fisher Scientific, USA. Immunogen was intact T. gondii and used at 1:600) before goat anti-rabbit HRP-labelled polymer (DAKO EnVision+, USA) was applied. AEC (3-amino-9-ethylcarbazole) chromogen solution (Vector Laboratories, UK) was then used to label T. gondii red. All tissues were counterstained with haematoxylin and mounted with cover slips. A murine liver infected with T. gondii was included as a control, and sections were incubated with T. gondii antibody as described above, or with normal rabbit serum (HKV1; 1:500) replacing the T. gondii antibody.
Scoring system for lesions and parasites
Whole sections of brain and peripheral organs were scored blindly using 2 different schemes to account for differences in the pathology observed in different organs. The brain scoring scheme assessed perivascular cuffing (PC), glial foci (GF) and necrotic foci (NF) while the peripheral organ scoring scheme assessed inflammatory infiltrate (II) and NF. In the brain, the number and size of PCs, GF and NF were combined to assess lesions. The frequency of PCs, GF and NF was scored as follows: 0 = absent, 1 = 1–3 foci, 2 = 4–7 foci and 3 = 8 or more foci. The size of PCs, GF and NF was scored based on the proportion of microglia as follows: 0 = not present, 1 = sparse, 2 = moderate and 3 = abundant. The presence of meningitis and any other significant changes were also recorded. In the peripheral organs, the II score was based on the presence of inflammatory cells as follows: 0 = not present, 1 = sparse, 2 = moderate and 3 = abundant. The score for NF was determined by the number found as follows: 0 = absent, 1 = 1–3 foci, 2 = 4–7 foci and 3 = 8 or more foci.
T. gondii parasites were labelled and scored in tissues using IHC. A cyst wall–specific antibody was not used in this study meaning it was not possible to determine if tight groups of parasites were cysts containing bradyzoites or parasitophorous vacuoles containing tachyzoites; therefore, tight groups of parasites are referred to as pseudocysts herein. T. gondii presence was scored as 0 = absent, 1 = 1–3 pseudocysts or groups of individual tachyzoites, 2 = 4–7 pseudocysts or groups of individual tachyzoites and 3 = 8 or more pseudocysts or groups of individual tachyzoites.
Statistical analysis
The lesions scored for brain and peripheral organs were different; therefore, the brain and peripheral organs were analysed separately for both studies. The scores for each animal across the measured attributes described above were jointly considered within a multivariate data analysis framework to explore overall patterns and associations according to the groups defined. In particular, principal component analysis (PCA) (Jolliffe and Cadima, Reference Jolliffe and Cadima2016) was used to determine optimal combinations of the attributes (principal components; as many as original attributes) providing overall indexes of scores for each animal sample. By construction, the first principle component dimensions account for the highest fraction of total variance in the original data. Thus, the first 2 (denoted Dim1 and Dim2 in graphics) were used to obtain a graphical representation in 2 dimensions known as a biplot. Here, both samples and attributes are respectively represented by points and arrows from the origin, which facilitates a visual assessment of relationships and comparisons at the expense of losing some percentage of information contained in the subsequent principle components. The proximity between the points represents the similarity between samples according to their overall scores across attributes. The arrows indicate directions of increasing scores in the corresponding attributes, with the origin of the biplot (point (0, 0)) representing their average in the original multi-attribute data set. The angle between arrows is proportional to the association, in terms of scores, between the corresponding attributes. As the score data in this work correspond to discrete ordinal variables taking values from 0 to 3, a specialized (nonlinear) variant of PCA was required (unlike ordinary PCA designed for continuous numerical variables). Namely, an optimal scaling procedure (Goodall and Gifi, Reference Goodall and Gifi1992) was applied, by which the ordinal variables were quantified into a continuous numerical scale making use of step functions in such a way that (1) the variance accounted for in the quantified variables was maximized, and (2) the original order was respected. The resulting transformed (quantified) variables were then used as input in ordinary PCA. Any attribute for which the scores were only zero across all days were not included in the analysis. Moreover, some random noise was added to identical points to facilitate visual distinction in the graphics. Finally, additional information regarding meningitis was included in the brain data sets by projecting its categories (‘Yes’, ‘No’) onto the PCA biplots as supplementary variables. Their coordinates in the graph were determined by the barycentre of the samples belonging to each category.
All data analyses and graphical representations were conducted on the R system for statistical computing v4.4.1 (R Core Team, 2024), using the specialized packages Gifi (Mair et al., Reference Mair, De Leeuw and Groenen2022) and factoMineR (Lê et al., Reference Lê, Josse and Husson2008).
Results
Study A: A1 (negative) and A2 (genotype #3) pathology
Peyer’s patches (PPs) of the intestine were developed and active within normal histological limits, in all 5 uninfected mice (A1). No pathological changes or T. gondii were observed in group A1 during the study.
Mildly depleted PPs of the intestine were noted in 1 A2 mouse at day 7 p.i. accompanied by mild hepatitis of the liver (semi-suppurative inflammation, eosinophil rich) whilst all other animals had no pathological changes. PPs were active in the majority of A2 mice and T. gondii was not identified.
Study A: A3 (genotype #6) pathology
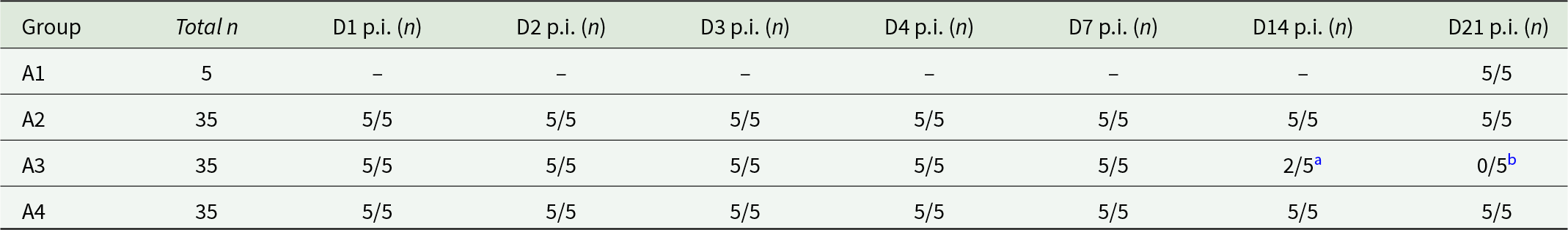
Group A3 mice (n = 5) were euthanised at days 1, 2, 3, 4 and 7 p.i. as planned. Three mice scheduled to be euthanised at day 14 p.i. had to be euthanised at days 8 (n = 1) and 10 (n = 2) p.i. due to signs of toxoplasmosis (described in Chiebao et al. (Reference Chiebao, Bartley, Chianini, Black, Burrells, Pena, Soares, Innes and Katzer2021)). Two mice were euthanised as scheduled on day 14 p.i. as they recovered from signs of toxoplasmosis (Table 3). No samples were collected at day 21 p.i. as all mice had to be euthanised between days 8 (n = 1), 9 (n = 3) and 11 (n = 1) p.i. due to signs of toxoplasmosis. Observations for group A3 are summarized below and in Table 5, but specific scores are presented in Fig. S1.
Table 3. Number of animals that reached scheduled euthanasia time points in Study A

a Three A3 animals assigned to day 14 p.i were euthanised prematurely at days 8 (n = 1) and 10 (n = 2) p.i. on welfare grounds.
b All 5 A3 animals scheduled to be euthanised at day 21 p.i. were euthanised early on welfare grounds at days 8 (n = 1), 9 (n = 3) and 11 (n = 1) p.i.
Pathological changes and T. gondii were usually observed from day 7 p.i., although mild intestinal inflammation was present in 1 mouse at day 1 p.i. At day 3 p.i., mild nephritis in the kidney (n = 1), inflammation of the diaphragm (n = 1), and PP depletion (n = 1) were noted in 3 mice. Paneth cell depletion was noted at day 4 p.i. in 1 mouse.
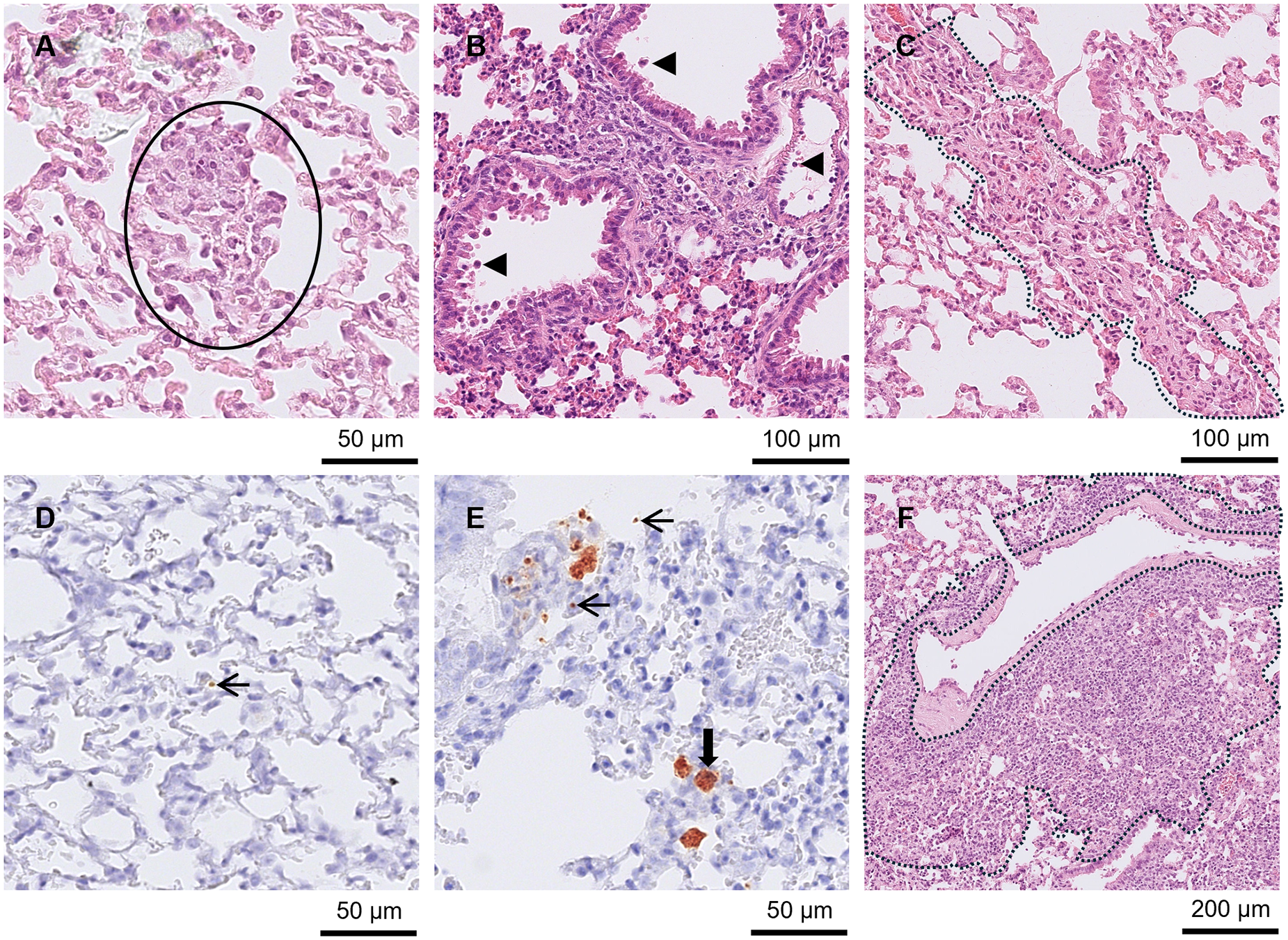
Lesions were found in the brain from day 9 p.i. Five out of the 6 mice euthanised between days 9 and 11 p.i. had meningitis (Figure 2B and 2E). One of those mice also had large NF (Figure 2A and Figure 2D) and small to moderately sized GF, while 4 other mice had low numbers of small PCs. Between days 8 and 11 p.i. (n = 8), all brains had low to high numbers of tachyzoites. Additionally, between days 8 and 11 p.i., 7 of the brains had low to high numbers of pseudocysts. No pathological changes were observed in the brain at day 7 p.i., but 2 mice had low to moderate numbers of tachyzoites. No changes were observed in the 2 brains from mice euthanised at day 14 p.i.

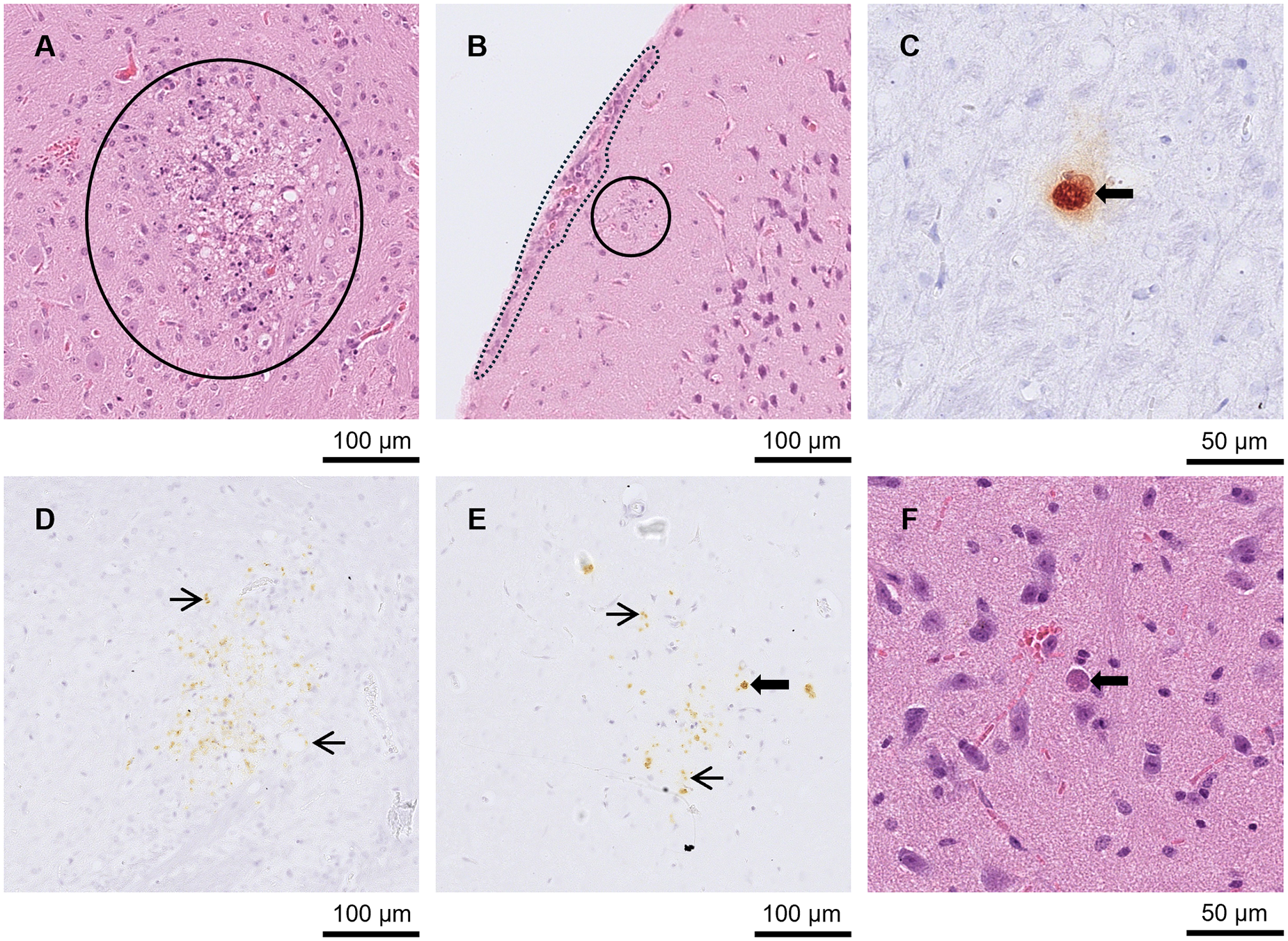
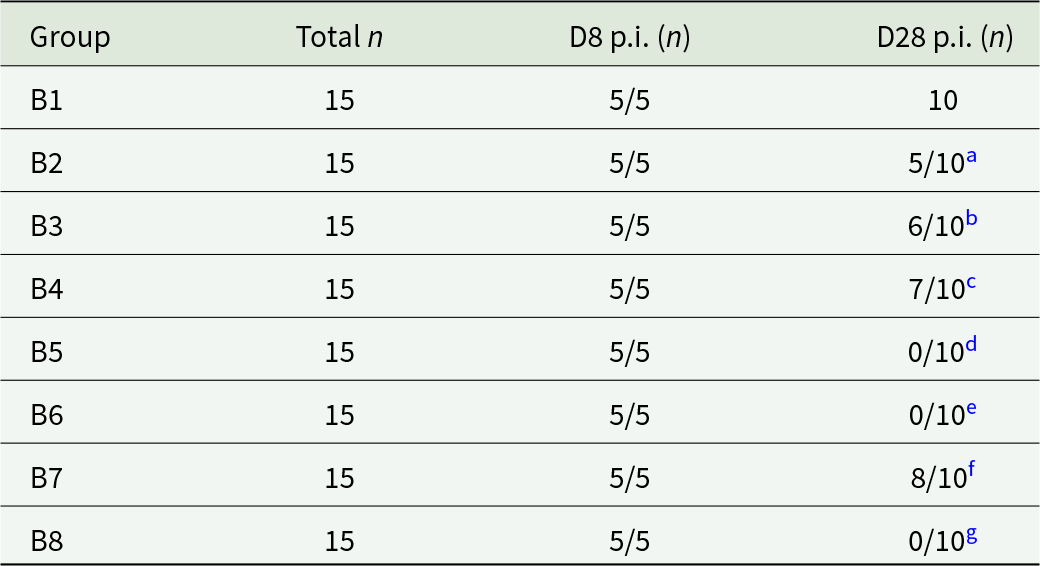
Figure 2. Brain from A3 and A4 mice. (A) and (D) A3 mouse orally inoculated with genotype #6 and euthanised at day 9 p.i. (A) Large area of necrotising inflammation (circled) in HE and (D) tachyzoites labelled by IHC in the same area after serial cutting (arrows). (B) and (E) A3 mouse orally inoculated with genotype #6 and euthanised at day 9 p.i. (B) Moderate meningitis (dotted line) and tachyzoites (solid circle) in HE with the same area (after serial cutting) shown in IHC (E)-Labelled tachyzoites (arrows). (C) and (F) A4 mouse orally inoculated with genotype #8 and euthanised at day 21 p.i. Toxoplasma gondii labelled by IHC, (C), was not always directly associated with lesions shown in HE (F).
Multiple peripheral organs were affected in group A3 mice between days 7 and 11 p.i. During this period, mild to severe hepatitis (n = 12) and NF (n = 11) were found in the liver. In 1 liver with NF, mineralisation was also present (day 10 p.i.; Figure 3C and 3F). Mild to severe pneumonia (n = 10) and moderate to mild NF (n = 6) were found in the lung. Pulmonary oedema was reported in 4 mice at days 8 (n = 2), 9 (n = 1) and 10 (n = 1) p.i. and bronchus-associated lymphoid tissue (BALT) induction could be seen in 1 animal at day 9 p.i. Mild to severe nephritis (n = 10) and mild NF (n = 2) were present in the kidneys (Figure 3A). Low to high numbers of tachyzoites (n = 12) and low numbers of pseudocysts (n = 11) were also observed. Mild to severe inflammation (n = 9), moderate to severe NF (n = 4; between day 9 and 11 p.i. only) and mineralisation (n = 5; between day 9 and 11 p.i. only) were found in the diaphragm (Figure 4A). Eight diaphragms had low to high numbers of tachyzoites and 6 of these tissues also had low to moderate levels of pseudocysts. Mild to severe inflammation (n = 11) and mild to moderate NF were found in the intestine (n = 5). Additionally, Paneth cell depletion (n = 9) and mild to severe depletion of PPs (n = 10) was observed between days 7 and 11 p.i. (Figure 4B and 4E). Tachyzoites were found at low to high numbers (n = 12) and pseudocysts were found at moderate to high numbers in the intestine. T. gondii was frequently found concentrated at the PPs, villi and lamina propria. Mild to moderate gastritis of the stomach (n = 4; only available from 11 mice) and moderate NF (n = 1) were present in the stomach (Figure 4C and 4F). Mucosa-associated lymphoid tissue (MALT) was active in a stomach at day 3 p.i. and 2 stomachs at day 9 p.i. In the stomach, tachyzoites ranged from low to high in 6 mice and pseudocysts were mainly low in 5 of these mice. At day 14 p.i., 1 mouse had mild pneumonia in the lung and parasites were absent. In the other mouse, no pathological changes were observed, but a low number of tachyzoites were found in the liver.

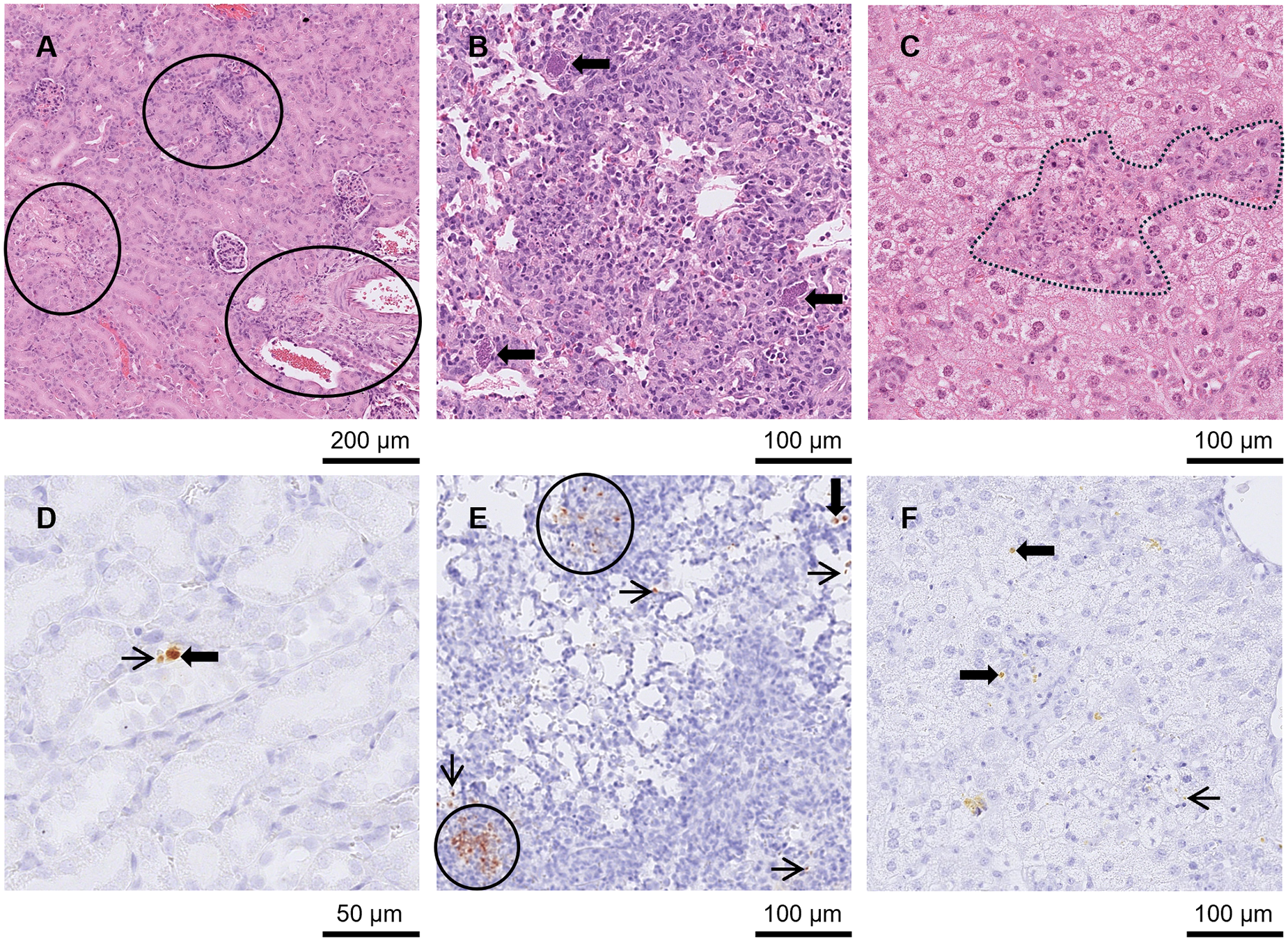
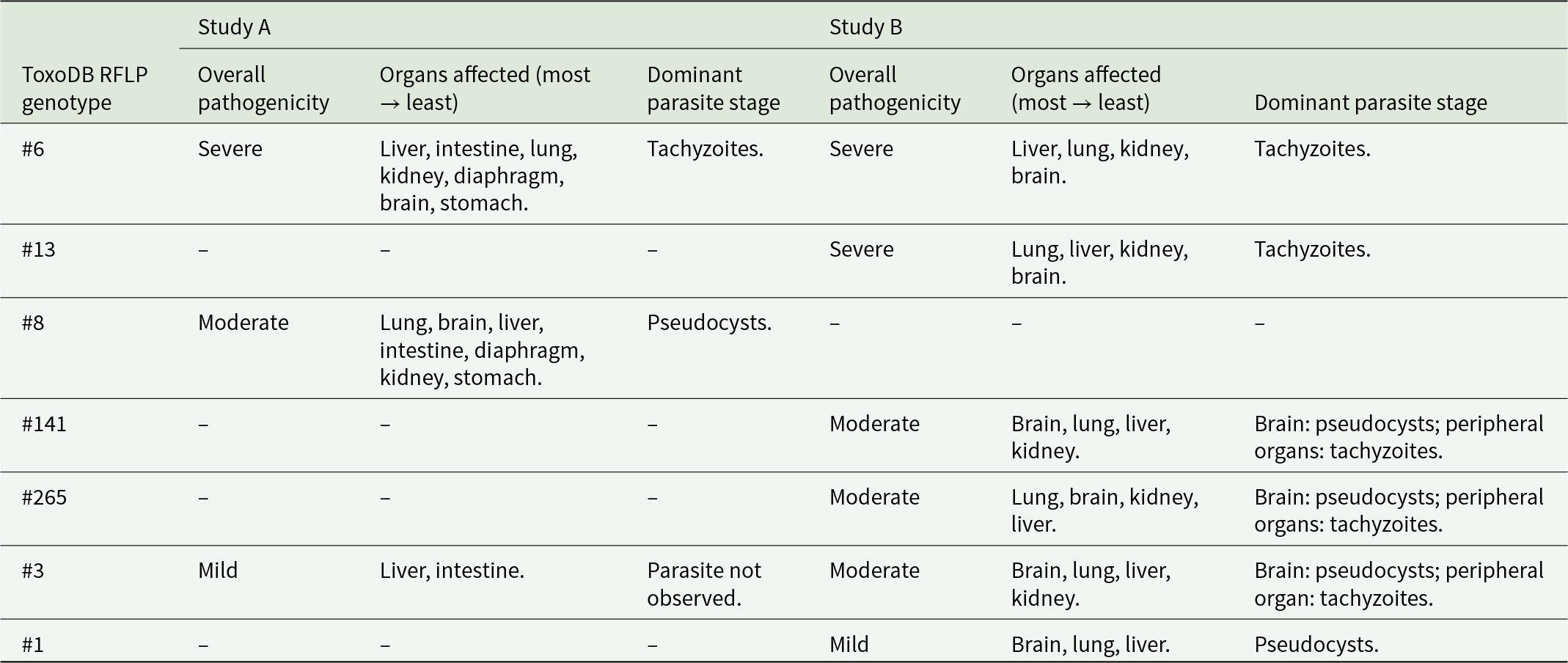
Figure 3. Kidney, lung and liver from groups A3 and A4 mice in Study A. (A) Kidney from an A3 mouse orally inoculated with genotype #6 and euthanised at day 9 p.i. Displaying nephritis and moderate necrotic foci (circled) in HE. (D) Kidney from an A4 mouse orally inoculated with genotype #8 and euthanised at day 14 p.i. With a small pseudocyst (solid arrow) and tachyzoite (thin arrow) in IHC. (B) and (E) Lung from an A4 mouse orally inoculated with genotype #8 and euthanised at day 14 p.i. (B) Necrosis and pneumonia in lung HE surrounded by 3 large pseudocysts (arrows). (E) shows the same area in IHC where pseudocysts (examples given with solid arrows) and groups (outlined in black) or single tachyzoites (examples given with thin arrows) were observed. (C) and (F) Liver from A3 mouse orally inoculated with genotype #6 and euthanised at day 10 p.i. (C) Hepatitis with necrosis and mineralisation in HE (outlined in black) and examples of tachyzoites (thin arrows) and small pseudocysts (solid arrows) in similar area of IHC in (F).

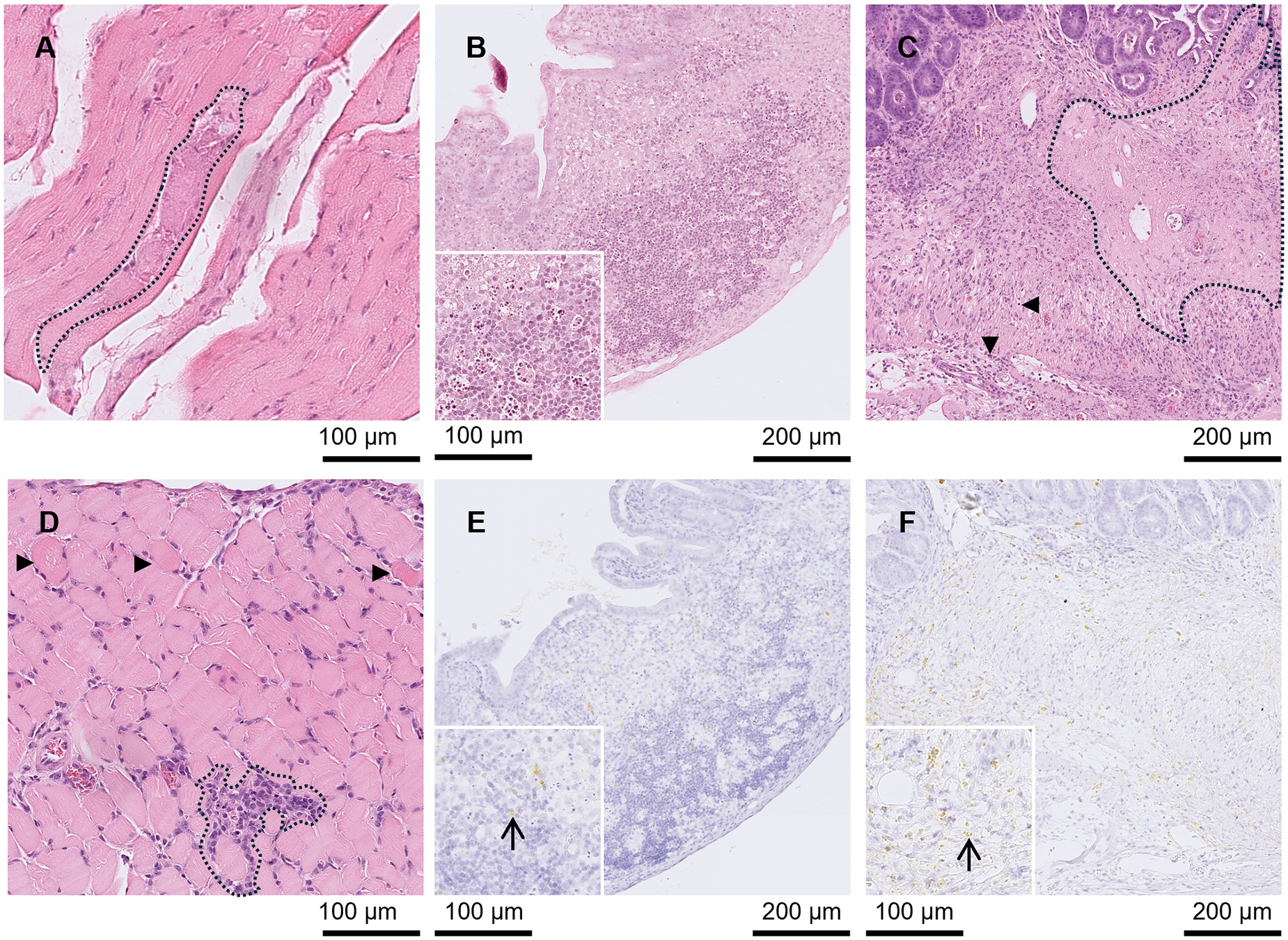
Figure 4. Diaphragm, intestine and stomach from groups A3 and A4 mice. (A) Diaphragm from an A3 mouse orally inoculated with genotype #6 and euthanised at day 10 p.i. Showing mineralisation in HE (outlined in black). (B) and (E) intestinal Peyer’s patches from an A3 mouse orally inoculated with genotype #6 (and euthanised at day 11 p.i. (B) Peyer’s patch induction and depletion in HE and (E) IHC showing various pseudocysts and tachyzoites throughout the same area. Inserts in (B) and (E) show PPs at a higher magnification. A tachyzoite is highlighted by the arrow in (E). Scale bars for the inserts at to the left-hand side of the image. (C) and (F) Stomach from an A3 mouse orally inoculated with genotype #6 and euthanised at day 9 p.i. (C) Gastritis and necrosis in stomach HE. A large area of necrosis was present in the submucosa and the most severely affected area is surrounded by a dotted line. Mononuclear inflammatory cells also infiltrated the submucosa (arrowheads). A large number of tachyzoites were present in IHC, (F), and are highlighted with an arrow in the insert. Scale bars for the inserts at the left-hand side of the image. (D) Diaphragm HE from an A4 mouse orally inoculated with genotype #8 and euthanised at day 14 p.i. Generalized inflammatory cell infiltration with the most severely affected area outlined in black to highlight the presence of inflammatory and necrotic cells. Additionally, several degenerating fibres were present (arrowheads).
Study A: A4 (genotype #8) pathology
In group A4, 5 mice were euthanised at days 1, 2, 3, 4, 7, 14 and 21 p.i. as planned (Table 3). Observations for group A4 are summarized below and in Table 5, while specific scores are presented in Fig. S2. Pathological changes and T. gondii were mainly observed in the peripheral organs from day 7 p.i. and in the brain from day 14 p.i.; however, it was noted that BALT was activated in 3 mice between days 2 and 3 p.i.
At day 7 p.i., the liver was the most affected organ with all mice displaying mild to moderate hepatitis and 1 with multifocal necrosis. Low numbers of tachyzoites were found in 2 livers. Mild intestinal inflammation was observed in 3 mice and PP depletion affected 1 of them. Moderate to high numbers of tachyzoites (n = 3) and low to high numbers of pseudocysts (n = 4) were also found in some intestines. In the lung, occasional mild pneumonia was observed (n = 2) accompanied by BALT induction (n = 1) and low numbers of tachyzoites (n = 2) and pseudocysts (n = 4). The brain, kidney, diaphragm and stomach were unaffected at day 7 p.i.
At day 14 p.i., all brains (n = 5) displayed meningitis, low to high numbers of small PCs and low to moderate numbers of small to large GF. In 2 of the mice, the size of GF were variable. In most mice, low to moderate numbers of tachyzoites (n = 4) and low to high numbers of pseudocysts (n = 5) were present. Severe oedema and pneumonia with moderate to severe necrosis was present in all 5 mice euthanised at day 14 p.i. (Figure 3B) and BALT was induced in 1 case. High numbers of tachyzoites and pseudocysts were found in all lungs examined (Figure 3E). In the liver, mild to severe hepatitis was observed in all animals in addition to mild necrosis in 3 cases, but T. gondii was not observed. In the kidney, mild to moderate nephritis occurred in all 5 mice and mild NF was present in 1. One mouse had low numbers of tachyzoites and another had low numbers of pseudocysts (Figure 3D). Mild to severe inflammation of the diaphragm was present in all animals and in 2 animals skeletal muscle degeneration was detected (Figure 4D). A low number of pseudocysts were found in 1 diaphragm. In the intestine mild inflammation was observed in 3 mice and mild depletion of the PP (n = 2) or Paneth cells (n = 1) was noted. Mild gastritis (n = 1) and MALT induction (n = 2) was found in the stomach at day 14 p.i. T. gondii was not found in the intestine or stomach.
At day 21 p.i., 3 brains had moderate to low numbers of medium to small sized PCs and low numbers of medium to small sized GF. Additionally, 2 were affected by meningitis. Low to high numbers of pseudocysts were found in the 3 brains and low to moderate numbers of tachyzoites were present in 2 (Figure 2C and 2F). The 3 mice with brain pathology also had severe pneumonia with mild to moderate necrosis, oedema and active BALT in the lung. Low numbers of tachyzoites (n = 2) and moderate numbers of pseudocysts (n = 3) were also identified in the lungs. All mice had mild to moderate hepatitis in the liver and 1 mouse with moderate hepatitis also had mild NF, but no T. gondii was found in any case. Mild to moderate nephritis was present in 3 out of the 4 kidneys available for analysis, but T. gondii was not observed. Moderate inflammation (n = 2) and low numbers of pseudocysts (n = 1) were found in the diaphragm. Only 4 diaphragms were available for assessment. Mild PP depletion (n = 2) and mild inflammation (n = 1) were found in the intestine.
Study A: Multi-attribute score data analysis for pathology
As most mice in groups A1 and A2 had zero scores, and due to the variability in euthanasia time points for some isolates, it was not feasible to assess scores across T. gondii isolates by day; therefore, scores for all time points were combined and assessed by the nonlinear PCA method described above (Figure 5). If a full panel of peripheral organ samples was not available for histopathological analysis, scores for the whole animal were removed from this analysis (n = 18). Also, the scoring system used for lesions in the brain (PC, GF, NF) was more complex than that used for peripheral organ analysis as 2 factors, number and size, were included; therefore, this PCA approach was particularly effective for assessing the brain. Upon further investigation (Fig. S3), tachyzoites were found to be the most abundant parasite stage in A3; however, pseudocyst values were not much lower. This analysis also showed that the lung, liver and intestine displayed higher parasite loads than other organs. In A4, pseudocysts appeared more abundant than tachyzoites and they mainly affected the lung, brain and intestine.

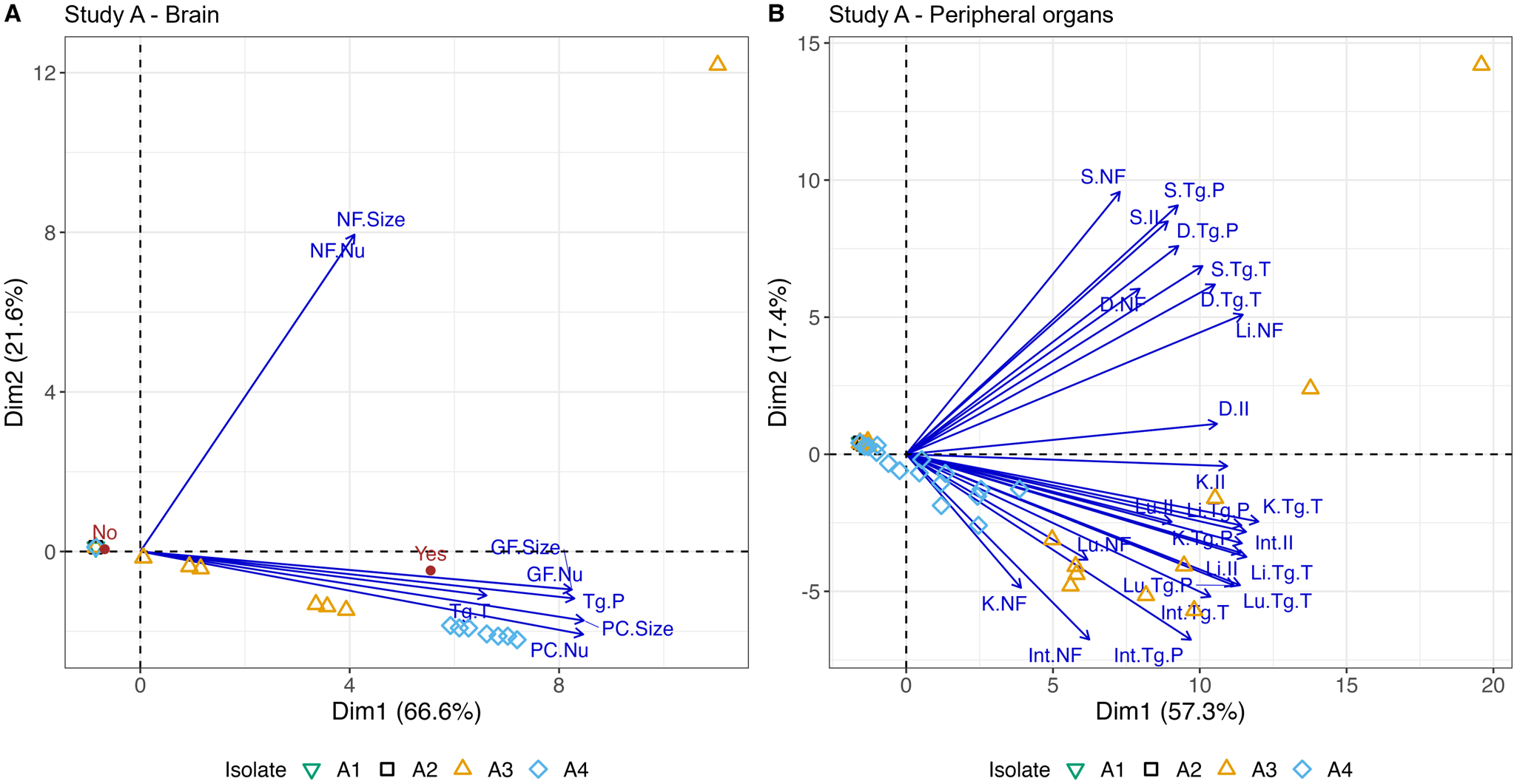
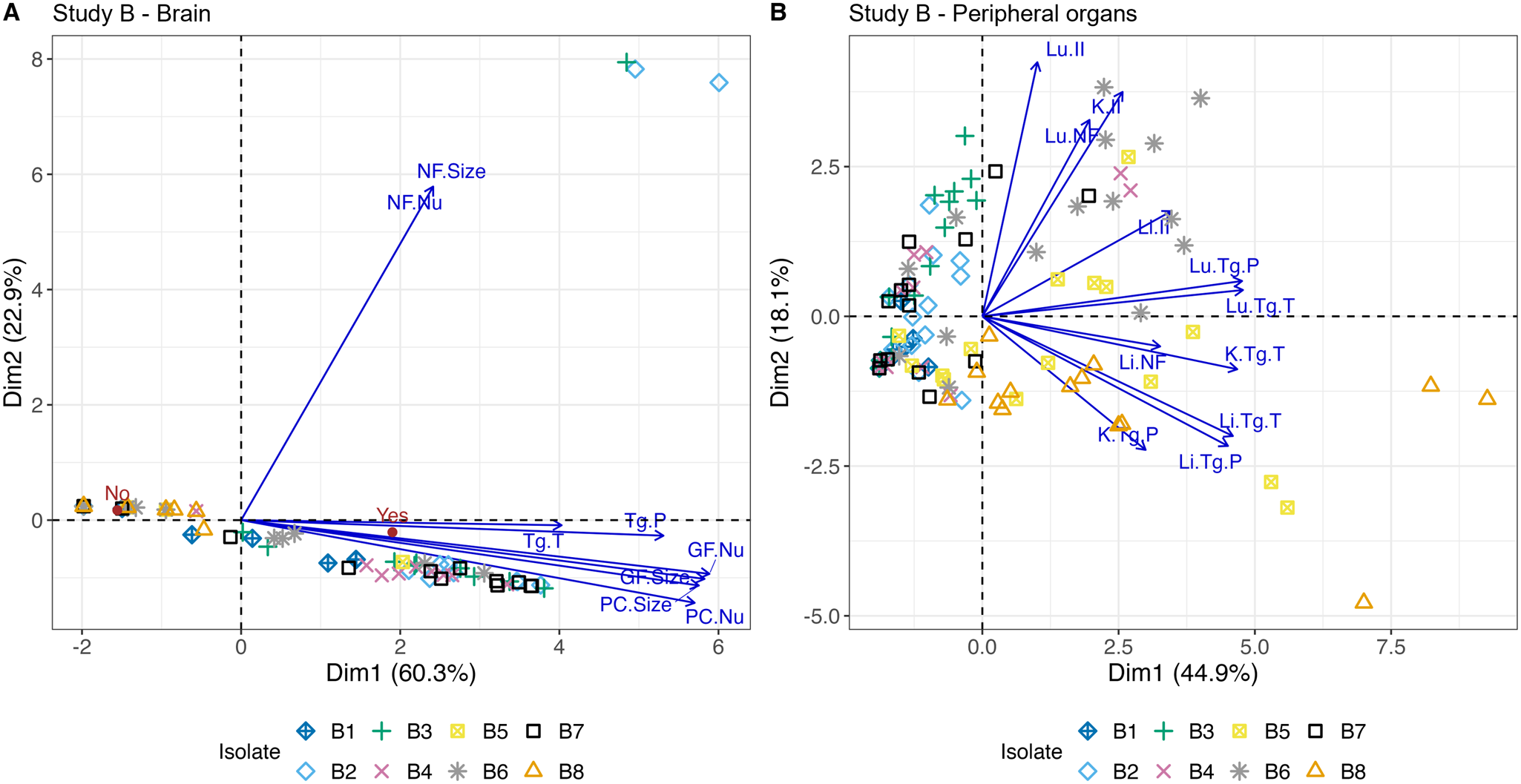
Figure 5. Nonlinear PCA biplots for brain and peripheral organ samples from Study A. Presented in (A) and (B) are the nonlinear PCA biplots for brain (A) and peripheral organs (B) from mice in groups A1, A2, A3 and A4. Scores from all days, lesions and parasite were combined. In the PCA biplot for the brain (A), 2 clear clusters of points were observed, and these corresponded to groups A3 and A4. The distance between the 2 groups suggests the combined scores for A4 were higher than those found in A3 in the brain, and this can be attributed to the increased inflammatory responses (PC, GF) observed in A4 discussed earlier. Data points to the left of the PCA biplot represent zero scores. The arrows show that tachyzoites (Tg.T) had a greater association with A3 while pseudocysts (Tg.P) were associated with both Brazilian isolates but were more abundant in A4. An indication of the average level of meningitis is given by the red points labelled ‘Yes’ and ‘No’. PC.Nu = perivascular cuff (PC) number; PC.Size = size of PC; GF.Nu = glial foci (GF) number; GF.Size = size of GF; NF.Nu = necrotic foci (NF) number; NF.Size = size of NF. In the peripheral organs (B), there were also 2 clear groups that were associated with A3 and A4. Overall scores were higher for A3 than A4 (A3 further to the right of A4). This is likely driven by the often severe levels of pathology and high parasite burdens in A3 mice between days 7 and 11 p.i. Both isolates had a predilection for the lung, liver and intestine of animals and high scores for the stomach, kidney and diaphragm were frequently observed in group A3 and less frequently in A4 as indicated by the arrows. There was a cluster of points to the left of the y-axis which are indicative of data points with zero scores and some random noise was added so they were visible.
Study B: B1 (genotype #1) pathology
All mice from B1 were euthanised as planned on days 8 (n = 5) and 28 (n = 10) p.i. (Table 4). A summary of observations for group B1 is given below and in Table 5, while specific scores are presented in Fig. S4.
Table 4. Number of animals that reached scheduled euthanasia time points in Study B

Several animals were euthanised before day 28 p.i. on welfare grounds.
a B2 animals euthanised at days 12 (n = 1), 23 (n = 2) and 26 (n = 2) p.i.
b B3 animals euthanised at days 15 (n = 2), 16 (n = 1) and 21 (n = 1).
c B4 animals euthanised at days 11 (n = 2) and 26 (n = 1) p.i.
d B5 animals euthanised at days 7 (n = 3), 8 (n = 6) and 12 (n = 1) p.i.
e B6 animals euthanised at days 11 (n = 9) and 16 (n = 1) p.i.
f B7 animals euthanised at days 16 (n = 1) and 21 (n = 1).
g B8 animals euthanised at days 8 (n = 7), 11 (n = 1), 12 (n = 1) and 14 (n = 1) p.i.
Table 5. Summary of organs affected by each T. gondii genotype used in this investigation

In B1, only moderate to mild hepatitis was observed at day 8 p.i. (n = 3) and no T. gondii was identified. At day 28 p.i., mild mononuclear meningitis (n = 8), moderate to mild numbers of small PCs (n = 6) and low numbers of moderately to small sized GF (n = 3) were observed in the brain. Moderate to low numbers of T. gondii pseudocysts (n = 4) and low numbers of tachyzoites (n = 2) were discovered in the brain. Mild pneumonia (n = 8), mild necrosis (n = 1) and BALT activation (n = 1) was present in the lung. A low number of pseudocysts were found in 1 lung. Mild hepatitis (n = 7) and mild NF (n = 1) were observed in the liver. No lesions or parasite were found in B1 kidneys.
Study B: B2 and B3 (genotype #141) pathology
B2 animals were euthanised at days 8 (n = 5) 12 (n = 1), 23 (n = 2), 26 (n = 2) and 28 (n = 5) p.i. B3 animals were euthanised at days 8 (n = 5) 15 (n = 2), 16 (n = 1), 21 (n = 1) and 28 (n = 6) (Table 4). Summaries of observations for groups B2 and B3 are given below and in Table 5, while scores are shown in Figs S5 and S6.
At day 8 p.i. in B2 and B3, no pathology was observed in brains, but a low number of pseudocysts were identified in 1 B2 brain. In B2 peripheral organs at day 8 p.i., mild to moderate pneumonia (n = 2) and low numbers of tachyzoites (n = 5) and pseudocysts (n = 2) were found in the lung. Mild hepatitis (n = 2), low numbers of tachyzoites (n = 3) and low numbers of pseudocysts (n = 2) were found in the liver. It was noted that the hepatic Glisson’s capsule was particularly affected by tachyzoites in 2 cases. Mild nephritis (n = 2) and low numbers of tachyzoites (n = 1) were found in 2 of the 4 kidneys available for analysis. At day 8 p.i. B3 mice, mild hepatitis (n = 2), pneumonia (n = 1) and nephritis (n = 1) were found in the liver, lung and kidney respectively, but no T. gondii was identified.
In the animal from B2 euthanised at day 12 p.i., meningitis, low numbers of small PCs and GFs were observed accompanied by high numbers of T. gondii pseudocysts and tachyzoites. Severe pneumonia with mild necrosis and high numbers of pseudocysts and tachyzoites were found in the lung. Moderate hepatitis was found in liver and the kidney was not available for assessment.
In B2 mice euthanised at days 23 and 26 p.i. (n = 4), mononuclear meningitis (n = 3) and moderate to high numbers of moderate to large PCs (n = 4) that often varied in size (n = 3) were observed along with low to high numbers of small (Figure 6A) or large GF (n = 4). A large area of necrosis was observed in 1 case (day 23 p.i.) and mineralisation was reported in 3 brains. Low to high numbers of tachyzoites and moderate to high numbers of T. gondii pseudocysts were found in all brains (Figure 6D). Mild to severe pneumonia (n = 4), pulmonary oedema (n = 3), low to moderate numbers of tachyzoites (n = 4) and low numbers of pseudocysts (n = 2) were reported in lungs. Mild to moderate nephritis (n = 2) and low numbers of tachyzoites were observed in kidney (n = 1). Mild to moderate hepatitis was noted in 4 livers.

Figure 6. Brain from group B2 and B7 mice. (A) and (D) Brain from a B2 mouse intraperitoneally inoculated with #141 and euthanised at day 23 p.i. (A) Mild necrotizing encephalitis (outlined in black) in HE. (D) IHC-labelled small T. gondii pseudocysts (solid arrows) and tachyzoites (groups or singles, thin arrows). (B) and (E) Brain from a B7 mouse intraperitoneally inoculated with genotype #3 and euthanised at day 28 p.i. (B) Moderate glial focus (circled) and mild perivascular cuff (arrow) in HE with IHC-labelled T. gondii pseudocysts (arrows) in the same area in (E). (C) and (F) Brain from a B2 mouse intraperitoneally inoculated with #141 and euthanised at day 28 p.i. (C) HE shows a large area of necrosis and severe meningitis (outlined in black) in the brain which was highly populated with T. gondii tachyzoites (thin arrows) and pseudocysts (thick arrow) in IHC, (F).
At day 28 p.i., all B2 brains (n = 5) showed mononuclear meningitis, mild to moderate numbers of small to large PCs and low to high numbers of small to large GF. One brain also had a low number of large NF (Figure 6C and 6F) and mineralisation was noted in 3 brains. Low to high numbers of pseudocysts were identified in all mice whereas low to high numbers of tachyzoites were present in 3. Mild to severe pneumonia (n = 5) with mild necrosis (n = 1) or pulmonary oedema (n = 1) were identified in addition to low numbers of tachyzoites (n = 3) in the lung. Mild hepatitis (n = 3) and a low number of pseudocysts (n = 1) were found in the liver. Mild nephritis was found in 1 kidney.
B3 mice euthanised at days 15, 16 and 21 p.i. (n = 4) had meningitis, low numbers of small PCs and low to moderate numbers of small to moderately sized GF. One brain also displayed low numbers of small NF. Low to high numbers of pseudocysts and tachyzoites were present in all brains.
Pneumonia was severe in all lungs and mild to moderate necrosis (n = 2) accompanied by pulmonary oedema (n = 1) was observed in addition to low numbers of tachyzoites (n = 3) and pseudocysts (n = 2). Mild to moderate hepatitis (n = 3) and low numbers of pseudocysts were found in the liver. Mild nephritis was also observed in 2 kidneys.
At day 28 p.i. in B3 (n = 6), meningitis (n = 6), low to high numbers of small (n = 1) or large (n = 4) PCs and low to high numbers of small (n = 1), moderate (n = 3) or large (n = 1) GF were present in the brain. Low numbers of tachyzoites (n = 4) and low to high numbers of pseudocysts (n = 6) were also found in the brains. Moderate to severe pneumonia affected all mice and at times was associated with mild to moderate necrosis (n = 5), pulmonary oedema (n = 3) and BALT induction (n = 2). Low numbers of tachyzoites were present in 2 lungs. Mild to moderate hepatitis (n = 6) and low numbers of tachyzoites were found in the liver. Mild to moderate nephritis (n = 4) was observed in the kidney.
Study B: B4 (genotype #265) pathology
B4 animals were euthanised at days 8 (n = 5), 11 (n = 2), 26 (n = 1) and 28 (n = 7) p.i. (Table 4). A summary of observations for group B4 is given below and in Table 5, while specific scores are shown in Fig. S7. Mice euthanised at day 8 p.i. (n = 5) displayed pathology in the lung and liver. Mild pneumonia (n = 2), mild pulmonary oedema (n = 1) and low numbers of tachyzoites (n = 2) were present in the lung. Mild hepatitis (n = 1), low numbers of tachyzoites and 1 pseudocyst were identified in the liver. Lesions were not present in the brain or kidney, but low numbers of tachyzoites were present in both (brain n = 1; kidney n = 2).
At day 11 p.i. (n = 2), both mice presented with meningitis, with 1 also showing low numbers of small PCs and GF. High numbers of pseudocysts and low to moderate numbers of tachyzoites were identified in both brains. Moderate and severe pneumonia affected both lungs and 1 also had mild necrosis. Moderate or high numbers of tachyzoites and low and moderate numbers of pseudocysts were found in each lung. Moderate and severe nephritis and low or moderate numbers of tachyzoites were observed in the kidney (Figure 7B). It was noted that the renal capsule was concentrated with T. gondii (Figure 7E). Moderate hepatitis was present in both mice, and 1 also had mild necrosis. Low or moderate numbers of tachyzoites (n = 2) low numbers of pseudocysts (n = 1) were also identified in the liver. Toxoplasma gondii was abundant in the Glisson’s capsule.

Figure 7. Liver and kidney from groups B4–B6 mice. (A) and (D) Liver from a B5 mouse intraperitoneally inoculated with #13 and euthanised at day 8 p.i. (A) Moderate hepatitis at edge of liver HE section (arrowheads) with inflammation of the Glisson’s capsule (large arrows) which had a high T. gondii tachyzoite presence in IHC (arrows), (D). (B) and (E) Kidney from a B4 mouse intraperitoneally inoculated with #265 and euthanised at day 11 p.i. (B) Moderate necrotizing nephritis (solid circle) with inflammatory infiltrate (dotted circle) in HE. (E) Small cluster of IHC labelled T. gondii tachyzoites (arrow) in renal capsule. (C) and (F) Kidneys from 2 B6 mice intraperitoneally inoculated with #13 and euthanised at day 11 p.i. (C) necrotizing nephritis (circled) in the kidney HE. (F) Tachyzoite (arrow) IHC labelled in the main body of the kidney.
At day 26 and 28 p.i. (n = 8), all brains displayed meningitis, low to high numbers of small to large PCs and low to moderate numbers of small to large GF. Pseudocysts were present in the brain at low to moderate numbers (n = 7), and few tachyzoites were present (n = 5). Mild to severe pneumonia (n = 6), pulmonary oedema (n = 1), BALT induction (n = 2) and low numbers of T. gondii pseudocysts (n = 2) and tachyzoites (n = 1) were observed in the lung. Mild hepatitis (n = 4) and nephritis (n = 2) were observed in the liver and kidney, respectively.
Study B: B5 and B6 (genotype #13) pathology
B5 animals were euthanised at days 7 (n = 3), 8 (n = 11) and 12 (n = 1) p.i. B6 animals were euthanised at days 8 (n = 5), 11 (n = 9) and 16 (n = 1) p.i. (Table 4). A summary of observations for groups B5 and B6 are presented below and in Table 5, while specific scores are shown in Figs S8 and S9.
In B5 at days 7 and 8 p.i., low numbers of tachyzoites (n = 4) and pseudocysts (n = 1) were occasionally found in the brain, but no pathological changes were identified. At day 7 p.i. mild hepatitis (n = 3) with mild necrosis (n = 2), low to high numbers of tachyzoites (n = 3) and low to moderate numbers of pseudocysts (n = 2) were present in the liver. It was noted that T. gondii was often concentrated in the Glisson’s capsule. Low to high numbers of tachyzoites (n = 2), low to moderate numbers of pseudocysts (n = 1) and mild nephritis (n = 1) were found in the kidney. The lung had low to high numbers of tachyzoites (n = 3) and high numbers of pseudocysts (n = 1) but no lesions. At day 8 p.i., there was moderate to severe hepatitis (n = 10) with mild necrosis (n = 2) and low to high numbers of tachyzoites (n = 10) and pseudocysts (n = 8) in the liver. Inflammation and T. gondii were concentrated at the periphery of the tissue and Glisson’s capsule of 4 livers (Figure 7A and 7D). Moderate to severe pneumonia (n = 7) with mild necrosis (n = 3), pulmonary oedema (n = 1) and BALT induction (n = 2) were identified in the lung along with low to high numbers of tachyzoites (n = 11) and pseudocysts (n = 8; Figure 8B and 8E). Mild or moderate nephritis was present in 6 animals. Low to moderate numbers of tachyzoites (n = 7) and low numbers of pseudocysts (n = 3) were found in the kidney.

Figure 8. Lung from group B5 and B7 mice. (A), (C) and (D) Lung from a B7 mouse intraperitoneally inoculated with genotype #3 and euthanised at day 8 p.i. (A) Small necrotic foci in HE (outlined in black) with tachyzoite (arrow) labelled in the same area in IHC, (D). Also present in the HE section was vasculitis (outlined in black), (C). (B) and (E) Lung from a B5 mouse intraperitoneally inoculated with #13 and euthanised at day 8 p.i. (B) Moderate interstitial necrotizing pneumonia in HE with pseudocysts (solid arrow) and tachyzoites (thin arrow) labelled in the same tissue by IHC, (E). Additionally, there were inflammatory cells (arrowheads) in the bronchi in B). (F) Lung from a B7 mouse intraperitoneally inoculated with genotype #3 and euthanised at day 21 p.i. with induced and depleted BALT in HE (outlined in black).
At day 12 p.i. (n = 1) for B5, low numbers of small PCs and GF were present in the brain. High numbers of tachyzoites and low numbers of pseudocysts were also found. Severe pneumonia and high numbers of pseudocysts and tachyzoites were found in the lung. Moderate hepatitis and low numbers of pseudocysts and tachyzoites were observed in the liver. Low numbers of tachyzoites were found in the kidney, but no lesions were identified.
At day 8 p.i. (n = 5) in B6, mild to moderate pneumonia (n = 3), low to high numbers of tachyzoites (n = 5) and low to moderate numbers of pseudocysts (n = 3) were present in the lung. Mild to moderate hepatitis (n = 3) and low numbers of tachyzoites (n = 2) and pseudocysts (n = 1) were found in the liver. Mild nephritis (n = 2) and low numbers of tachyzoites (n = 1) were found in the kidney. Low numbers of tachyzoites were found in 2 brains and no pathological changes were noted.
Mild neuropathology was observed in the brain from day 11 p.i. (n = 9) in B6. Mice presented with meningitis (n = 5), low numbers of small PCs (n = 3) and GF (n = 3) in the brain. Moderate to high numbers of tachyzoites were identified in all brains (n = 9) and low to moderate numbers of pseudocysts were found in most brains (n = 7). Moderate to severe pneumonia (n = 8) and mild to moderate necrosis (n = 6) could be seen in the lung. It was noted that in the lung without pneumonia, there was mild necrosis. Tachyzoites and pseudocysts were predominantly high in number in the lungs (n = 8 and n = 7, respectively) but moderate in a few cases (n = 1 and n = 2, respectively). Moderate to severe hepatitis (n = 7) was abundant in the liver but mild hepatitis was seen in 1 case. Mild necrosis (n = 2) was occasionally observed, including in 1 liver with no hepatitis. Low to moderate numbers of tachyzoites (n = 8) and pseudocysts (n = 6) were identified. Moderate to severe nephritis (n = 7) was observed in most kidneys (Figure 7C), but mild nephritis was found in 1 case. Low to moderate numbers of tachyzoites and a low number of pseudocysts were found in some kidneys (n = 6 and n = 1 respectively; Figure 7F).
At day 16 p.i. (n = 1), meningitis and a moderate number of small PCs and GF were found in the brain along with high numbers of pseudocysts and tachyzoites. Additionally, a large area of necrotising inflammation was identified in the brain. Severe pneumonia with mild necrosis and BALT activation was also observed in the lung. There was also moderate hepatitis in the liver and T. gondii was found in low numbers in the form of tachyzoites.
Study B: B7 (genotype #3) pathology
Group B7 animals were euthanised at days 8 (n = 5), 16 (n = 1), 21 (n = 1) and 28 (n = 8) p.i. (Table 4). A summary of observations for group B7 is given below and in Table 5, while specific scores are shown in Fig. S10.
Lesions were not observed in the brain at day 8 p.i. in B7, but pseudocysts were found at low numbers in 1 brain. Mild to moderate pneumonia (n = 3), occasionally with mild necrosis (n = 1) and low numbers of tachyzoites (n = 3) and pseudocysts (n = 1) were identified in the lung (Figure 8A, 8C and 8D). Mild hepatitis (n = 3) and mild necrosis (n = 1) were observed in the liver in addition to low numbers of tachyzoites (n = 1) and pseudocysts (n = 2). Mild nephritis (n = 1) was found in the kidney along with low numbers of tachyzoites (n = 1) and pseudocysts (n = 1).
The brain from the mouse euthanised at day 16 p.i. had meningitis, low numbers of small PCs, moderate numbers of small GF, moderate numbers of tachyzoites and high numbers of pseudocysts. There was also severe pneumonia and pulmonary oedema in the lung accompanied by high numbers of pseudocysts and tachyzoites. Moderate nephritis and low numbers of tachyzoites were present in the kidney. In the liver, mild hepatitis and low numbers of tachyzoites were found.
At day 21 p.i. (n = 1), the brain showed severe meningitis, a high number of moderately sized PCs and a moderate number of variably sized GF which could be large and associated with mineralisation. There were also a high number of pseudocysts and a low number of tachyzoites. Severe pneumonia, BALT activation and depletion and moderate numbers of tachyzoites and low numbers of pseudocysts were seen in the lung (Figure 8F). Moderate nephritis of the kidney and moderate hepatitis of the liver were also observed.
At day 28 p.i. (n = 8), all animals had mononuclear meningitis and in 3 of these animals, most cells resembled lymphocytes. Low to high numbers of small to large PCs were also found in all brains. Seven also had low to high numbers of small to large GF and in 4 cases, GF varied in size within the brain (Figure 6B and 6E). Low to high numbers of pseudocysts (n = 7) and low numbers of tachyzoites (n = 5) were present in some cases. Mild to severe pneumonia (n = 8) occasionally accompanied by mild necrosis (n = 1), BALT induction (n = 2) or oedema (n = 1) and low numbers of tachyzoites (n = 3) were present in lung. Mild hepatitis (n = 2) and low numbers of tachyzoites (n = 1) were present in the liver. No pathological changes or T. gondii were found in the kidney.
Study B: B8 (genotype #6) pathology
Group B8 animals were euthanised at days 8 (n = 12), 11 (n = 1), 12 (n = 1) and 14 (n = 1) p.i. (Table 4). A summary of observations for group B8 is given below and in Table 5, while scores are shown in Fig. S11. In group B8, brain lesions were present in 1 animal at day 8 p.i. in the form of a low number of small GF. Low numbers of tachyzoites and pseudocysts were found in another brain at day 8 p.i. At days 11, 12 and 14 p.i., moderate to high numbers of tachyzoites were found in all brains (n = 3), while low to moderate numbers of pseudocysts were found in 2.
Peripheral organs from mice euthanised at days 8 and 11 p.i. displayed similar pathology; therefore, they are described together. In the peripheral organs at days 8 and 11 p.i. (n = 13), mild to moderate pneumonia (n = 8), low to high numbers of tachyzoites (n = 13) and pseudocysts (n = 10) were present in lungs.
Mild to moderate hepatitis (n = 10) and low to high numbers of tachyzoites (n = 13) and pseudocysts (n = 8) were present in livers. It was noted that the Glisson’s capsule of most livers (n = 9) had a high proportion of labelled T. gondii. Only 12 kidney samples were available for histopathological assessment at days 8 and 11 p.i. In the kidney, mild nephritis (n = 1), low to moderate numbers of pseudocysts (n = 4) and low numbers of tachyzoites (n = 9) were present. The renal capsule of kidneys (n = 5) had a high proportion of labelled T. gondii.
At day 12 and 14 p.i. (n = 2), pathology was similar; therefore, the 2 animals are described together. Moderate to severe hepatitis with mild to moderate necrosis, high numbers of tachyzoites and pseudocysts were found in the livers. The Glisson’s capsule of 1 liver was particularly positive for T. gondii. In the lungs, there was mild or severe pneumonia with mild to moderate necrosis and high numbers of tachyzoites and pseudocysts. Mild nephritis and high numbers of tachyzoites were identified in both kidneys. One kidney also had low numbers of pseudocysts.
Study B: Multi-attribute score data analysis for pathology
Further assessment using combined scores and nonlinear PCA biplots were conducted for Study B samples (Figure 9). If a full panel of peripheral organ samples was not available for histopathological analysis, scores for the whole animal were removed from peripheral organ analysis (n = 4).

Figure 9. Nonlinear PCA biplot for brain and peripheral organ samples from Study B. Presented in (A) and (B) are the nonlinear PCA biplots for brain (A) and peripheral organs (B) from mice in groups B1 through to B8. Scores from all days, lesions and parasite were combined. In the brain (A), scores for B2, B3, B4, B6 and B7 were concentrated on the right of 0 on the x-axis indicating there were higher overall pathology and T. gondii presence compared to B5 and B8, where most points were located to the left. B1 were around zero on each side of the x-axis, consistent with mild to moderate pathology and parasite presence in the brain. Three outliers on the right belonging to B2 and B3 reflect the presence of NF. In the peripheral organs (B), many isolates that dominated the right-hand side of the graph in the brain were predominantly found on the left-hand side of the peripheral organ PCA biplot (B2, B3, B4 and B7). The opposite trend also appeared; those isolates that dominated the left-hand side of the brain PCA biplot were found more often on the right-hand side of the peripheral organ graph (B5, B6 and B8). B1 continued to be associated with low lesion and parasite scores in the peripheral organs and was positioned on the left of the x-axis.
Loosely, the nonlinear PCA panels in Figure 9 can be associated with organs, for example, points concentrated above 0 on the y-axis were often associated with higher overall lung scores, while points below 0 on the y-axis were often associated with higher scores in the liver. Additionally, points located on the left of 0 on the x-axis often had low to moderate scores while those found on the right of the x-axis often had moderate to severe scores. B1 was associated with low scores and points were found on either side of the y-axis close to 0. The points below 0 likely reflect the higher scores found in livers at day 8 p.i., while those slightly above 0 likely reflect an addition of scores in the lung at day 28 p.i. B2 and B3 scores were found on the left of the x-axis indicating mild to moderate scores. B2 scores were distributed above and below 0 of the y-axis as a result of mild to moderate scores in the liver and lung, while B3 appeared to dominantly affect the lungs although scores were mild to moderate in the liver on some occasions. B4 had higher scores in the lung, reflected in the presence of some points above the y-axis. Two points were found on the right of the x-axis and were likely driven by the presence of higher kidney scores in 2 animals. B5 affected the lung and liver most with some scores in the kidney, explaining the presence of data points in 3 panels of the plot. In this isolate, the scores were often higher because of parasite burden more than lesion severity. Most scores for B6 were concentrated in the upper left of the plot, probably because of high parasite and lesions scores found in the lung. The liver and kidney were often affected by moderate to severe pathology, but parasite scores were not as high potentially explaining the presence of some points to the left of the x-axis. Some animals in B7 had high scores in the lung, others had moderate scores in the lung and/or liver and/or kidney, while the rest had low or absent scores across the peripheral organs. This explains the range of points throughout 3 of the panels. B8 caused high overall scores in the lung and liver, but scores were consistently present in the kidney too, usually driven by high parasite loads. Scores were also often high in the other organs because of high parasite burdens. The addition of consistent scores in the kidney likely explains why data points for B8 were concentrated below 0 on the y-axis.
Further analysis of T. gondii life stage was assessed in Fig. S12. Pseudocysts were the dominant T. gondii life stage found in all B1 organs and B2, B3, B4 and B7 brains. Tachyzoites were the main life stage found in all tissues infected with B5, B6 and B8 and dominated the peripheral organs in B2, B3, B4 and B7.
Comparison of pathology caused by different parasite life stage
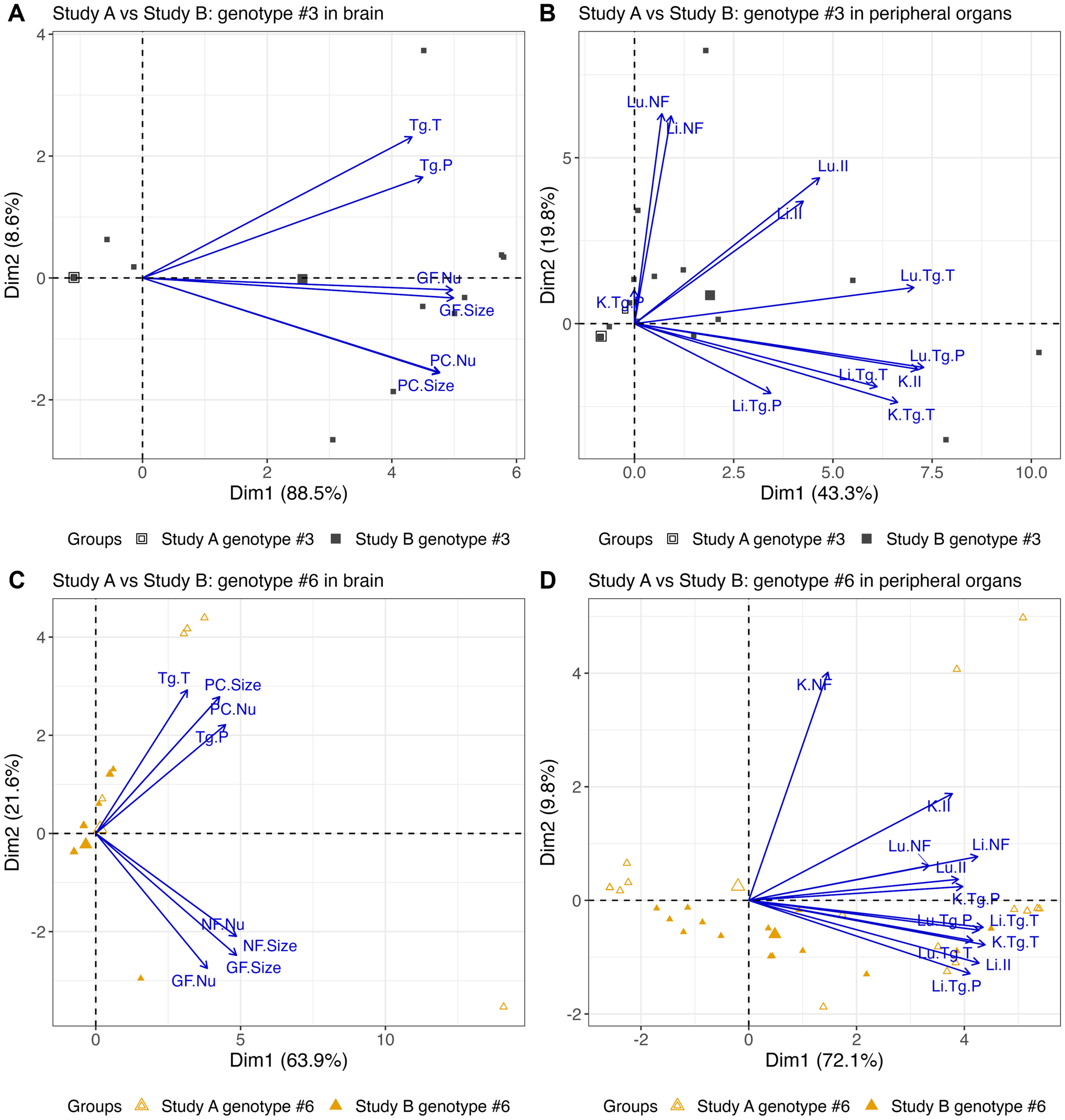
Nonlinear PCA biplots containing the brain or peripheral organ (lung, liver and kidney only) scores for genotype #3 from Studies A and B show there were differences in scores between the studies (Figure 10).

Figure 10. Nonlinear PCA biplots for brain and peripheral organs comparing scores for genotypes #3 and #6 in both studies. Genotypes #3 and #6 were used in both Studies A and B, but mice were orally inoculated with 50 oocysts in Study A and intraperitoneally inoculated with 200 tachyzoites in Study B. Additionally, the days at which the mice were euthanised at varied between studies and more organs were scored in Study A compared to Study B. Combining the scores allows preliminary assessment of genotypes #3 and #6 in 2 life stages. In the brain (A) and peripheral organs (B), genotype #3 from Study A, inoculated by ingestion of oocysts, produced zero or low scores while genotype #3 from Study B, inoculated by intraperitoneal injection of tachyzoites, caused higher scores for pathology and parasite burden in all organs. For genotype #6, in the brain (C), overall scores were higher in Study A than in Study B as it was more likely to see lesions and parasite. Parasite burdens, when present, appeared to be similar. In the peripheral organs present in both studies (lung, liver and kidney), Studies A and B showed moderate to severe lesion and parasite scores (D).
The apparent grouping that occurred in the PCA biplot may be explained by the difference in the days mice were euthanised on between the studies. For example, between days 8 and 12 p.i., scores were high in both studies and may explain the mix of data points towards the right of the x-axis, whereas the groups consisting primarily of Study A or Study B data points may relate to days out with this period where scores were lower.
Discussion
In this study, canonical and non-canonical strains of T. gondii showed visible differences in the severity of pathology observed in murine tissues, where genotype #6 and #13 isolates were most pathogenic, genotypes #8, #141 and #265 were moderately pathogenic and genotype #1 showed little pathogenicity. The pathogenicity for genotype #3 depended on route of infection, where an oral infection with oocysts resulted in mild pathogenicity and intraperitoneal infection with tachyzoites caused moderate pathogenicity.
The order in which organs were affected by T. gondii in both studies reflected the expected dissemination route the parasite would take from the respective inoculation sites. For those orally inoculated, T. gondii likely moved from the intestine to lymphatic and circulatory systems, through the portal blood stream to the liver, then into the systemic circulatory system (Denk et al., Reference Denk, De Neck, Khaliq and Stidworthy2022). Once in the bloodstream, it could be surmised that T. gondii tachyzoites left the circulation and entered organs in order of first contact beginning in the liver, then to the kidney, lung (Ajzenberg et al., Reference Ajzenberg, Yera, Marty, Paris, Dalle, Menotti, Aubert, Franck, Bessieres, Quinio, Pelloux, Delhaes, Desbois, Thulliez, Robert-Gangneux, Kauffmann-Lacroix, Pujol, Rabodonirina, Bougnoux, Cuisenier, Duhamel, Duong, Filisetti, Flori, Gay-Andrieu, Pratlong, Nevez, Totet, Carme, Bonnabau, Darde and Villena2009; Unno et al., Reference Unno, Kachi, Batanova, Ohno, Elhawary, Kitoh and Takashima2013; Jurankova et al., Reference Jurankova, Basso, Neumayerova, Frencova, Balaz, Deplazes and Koudela2015) and lastly the brain (Jurankova et al., Reference Jurankova, Basso, Neumayerova, Frencova, Balaz, Deplazes and Koudela2015).
In A3 mice inoculated with the genotype #6 isolate, damage in the intestine can be explained by the presence of excysted sporozoites infecting intestinal cells which subsequently rapidly proliferated. PP depletion indicates an active infection (Liesenfeld et al., Reference Liesenfeld, Kosek and Suzuki1997) and has previously been reported in association with severe intestinal pathology after oral ingestion of T. gondii (Suzuki et al., Reference Suzuki, Sher, Yap, Park, Neyer, Liesenfeld, Fort, Kang and Gufwoli2000) and lethal toxoplasmosis (Mordue et al., Reference Mordue, Monroy, La Regina, Dinarello and Sibley2001). Additional Paneth cell depletion observed can result in changes to the intestinal microbiota further consolidating intestinal inflammation and pathology (Raetz et al., Reference Raetz, Hwang, Wilhelm, Kirkland, Benson, Sturge, Mirpuri, Vaishnava, Hou, Defranco, Gilpin, Hooper and Yarovinsky2013).
Previously, enteric lesions associated with severe enteritis and mesenteric lymphadenitis were thought to cause early mortality in orally inoculated mice, while mice that survived succumbed to myocarditis, pneumonia and encephalitis (Dubey and Frenkel, Reference Dubey and Frenkel1973). While the previous reports account for some of the clinical and pathological findings in A3, systemic toxoplasmosis characterized by parasitaemia and overstimulation of the immune response, in addition to type of immune response, may also have contributed to morbidity and mortality (Chiebao et al., Reference Chiebao, Bartley, Chianini, Black, Burrells, Pena, Soares, Innes and Katzer2021; Denk et al., Reference Denk, De Neck, Khaliq and Stidworthy2022). This is proposed as, in most brains from A3 mice euthanised before their scheduled endpoints, lesions did not correspond to the severity of the parasite numbers suggesting genotype #6 was able to replicate substantially before a glial response could occur. Further, the high number of tachyzoites and severity of pathology throughout A3 tissues strongly suggest pseudocysts represented parasitophorous vacuoles, within which tachyzoites underwent rapid replication before lysis (Rodrigues Oliveira et al., Reference Rodrigues Oliveira, Ritter, Oliveira Dos Santos, Pizzolato de Lucena, Aquino de Mattos, Parente de Carvalho, Bullock, Giannini Alves Moreira, Magalhães Arthuso Vasconcelos, Barroso Costa, da Paixão T and Santos2022); however, further assessment is required to confirm the absence of a cyst wall.
In the 2 A3 mice that recovered by day 14 p.i, the immune response may have been adequate to control infection; however, methodological variations need to be considered: variation in viable oocyst numbers per inocula due to the inhomogeneous nature of an inoculating suspension; variation in oocyst excystation rate and infection rate of intestinal cells; and, given the use of outbred mice, genetic variability can allow mice to respond differently to infections.
A key difference in pathology between groups A3 and A4 was the apparent resolution of earlier liver and intestinal pathology as organs supplied later in the circularity system, including the brain and lungs, became more affected by genotype #8 T. gondii. This difference may be explained by T. gondii morphology; pseudocysts were the dominant life stage identified suggesting genotype #8 tachyzoites replicated slowly in parasitophorous vacuoles or they differentiated into bradyzoites in cysts, indicating the development of a chronic infection (Remington et al., Reference Remington, McLeod, Thulliez, Desmonts, Remington, Klein, Wilson and Baker2006). Meanwhile, genotype #6 sustained severe pathology throughout tissues.
In intraperitoneally inoculated mice, the origin of infection was reflected in the early pathology found in abdominal organs assessed; however, the subsequent spread of infection throughout organs was similar to those orally inoculated. For the highly pathogenic genotype #13 and #6 isolates inoculated into groups B5 and B8, respectively, the hepatic Glisson’s capsule was positive for T. gondii early in infection (days 8 and 12 p.i.) suggesting infiltration of tachyzoites from the peritoneal inoculation site to the liver. Additionally, pathology and parasite presence in the kidney was higher in groups inoculated with highly pathogenic isolates compared with other isolates investigated in this study.
For the moderately pathogenic T. gondii isolates, there were minor variations in the order in which organs were affected (Table 5), and mice presented with pseudocysts predominantly in the brain and tachyzoites predominantly in the peripheral organs. This contrasted with the pseudocyst dominant infection found throughout organs in the moderately pathogenic isolate in Study A suggesting that the inoculation method also influences pathology in moderately pathogenic T. gondii isolates. The continued observation of rapidly dividing tachyzoites in the periphery could account for the differences in mortality rates between moderately pathogenic isolates in Studies A and B.
Further evidence of the importance of inoculation route is highlighted by the change in genotype #3 pathogenicity from mild to moderate in Studies A and B, respectively. There are several reasons for increased pathogenicity when tachyzoites are intraperitoneally inoculated including: T. gondii bypassed gastric digestion and the intestinal barrier that could have prevented parasitic infection if inoculated orally; tachyzoites are more infectious or infectious for longer than sporozoites released from oocysts (Dubey and Frenkel, Reference Dubey and Frenkel1973, Reference Dubey and Frenkel1976); different breeds of outbred mice were used in the studies and the methodological variations discussed previously for oocysts must be considered.
Genotype #1 used to inoculate group B1 mice showed very mild pathogenicity. The low numbers of T. gondii observed in tissues suggests this isolate may not disseminate as much as more pathogenic strains (Gavrilescu and Denkers, Reference Gavrilescu and Denkers2001) and the presence of mild inflammatory lesions indicate the host immune response was adequate to control infection and limit secondary complications.
Genotype #3 and #6 isolates were used in Studies A and B, but the method of inoculation was different allowing a comparison of pathology induced by the different methods. The main limitations of comparing the results from the 2 studies were varying euthanasia schedules; differences in parasite stages and doses; different inoculation methods; and different organs examined. Each of the limitations were considered in turn when determining the conclusions.
To account for the differences in tissue availability, only tissues shared in both studies were compared in the analysis of genotypes #3 and #6. Using PCA as the statistical method allowed all pathology and parasite scores to be compared between isolates independent of euthanasia schedules. For genotype #6, animals in both studies were euthanised no later than day 14 p.i.; therefore, euthanasia schedules were not likely to affect assessment. For genotype #3, mice euthanised between day 8 and 28 p.i. in Study B had consistently higher scores than those in Study A at similar time points; therefore, it could be concluded that the differences in pathology were due to something other than euthanasia schedules, for example inoculation route, as described earlier, or the inoculant dose. A sporulated oocyst contains 2 sporocysts, which house 4 sporozoites each (Dubey, Reference Dubey1998). Inoculating 50 oocysts orally is the equivalent of 400 sporozoites being ingested; therefore, potentially 400 parasites able to infect intestinal cells, differentiate into tachyzoites, multiply and enter the circulation. Therefore, theoretically, the animals in Study A were inoculated with more T. gondii than animals in Study B. Despite this, at least for genotype #3, the mice inoculated with 200 tachyzoites had higher pathology and parasite scores in the brain and peripheral organs compared to those who received the higher infectious dose of oocysts, corroborating findings from an early paper (Dubey and Frenkel, Reference Dubey and Frenkel1973). For genotype #6, the differences between oocyst and tachyzoite infection were less obvious; however, it is known that highly virulent strains, like those in genotype #6, reach high parasite loads regardless of inoculation dose due to shorter doubling times, resistance to host immune system, reduced conversion to the bradyzoite stage and/or higher reinvasion rate (Saeij et al., Reference Saeij, Boyle and Boothroyd2005).
Regardless of the limitations, having formalin-fixed parafin-embedded material from 2 studies investigating multiple T. gondii isolates provided the unique opportunity to interrogate pathological changes caused by T. gondii. This study showed less pathogenic strains can establish infection in the brain in the form of pseudocysts/cysts and highly pathogenic strains can multiply so quickly they become systemic and cause widespread damage to tissues, parasitaemia and overstimulation of the immune response leading to death. Additionally, the route of inoculation/infective stage can influence the pathology caused by less virulent strains but has no apparent effect on the pathology caused by highly virulent strains. The results from the current study will improve the general understanding of the pathological changes induced by non-canonical strains and will help to refine the management and treatment of toxoplasmosis.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0031182026101589. Additional data can be made available upon request to the corresponding author.
Acknowledgements
The authors would like to thank Rodrigo M. Soares and Hilda F. J. Pena for allowing the use of the Brazilian isolates in the studies and Patrick J. Kelly for his part in the generation of the Saint Kitts isolates. The authors also thank the staff of the High Security Unit at the Moredun Research Institute and Jackie Thomson for their contribution to mouse work. A further thanks is extended to Jeanie Finlayson and Valerie Forbes for their assistance with histology and immunohistochemistry. For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission.
Author contribution
FC, JP and LEB conceived and designed this study. DPC, AB, FK and CU generated samples in Study A. CMH, PMB, AB, FK and CU generated samples in Study B. LEB conducted immunohistochemistry for Studies A and B. LEB and FC conducted histopathological analysis, image capture and interpreted results for Studies A and B. JP conducted statistical analysis and contributed to the relevant sections of the manuscript. LEB, FC, JP and FK prepared the manuscript, and the remaining authors reviewed and approved the final manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. LEB’s bench fees were funded by Student Awards Agency Scotland (SAAS). Tissue provided for Study A was collected under funding from CAPES (Higher Education Improvement Coordination; DPC, grant number 0382/14-0). Tissue provided for Study B was collected under funding from Ross University School of Veterinary Medicine, the Moredun Research Institute and the Scottish Government (Rural and Environment Science and Analytical Services Division). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The authors declare there are no conflicts of interest.
Ethical standards
All animal procedures complied with the Animals (Scientific Procedures) Act 1986 and were approved by the Moredun Research Institute Experimental and Ethical Review Committee (Study A: E22-14 and Study B: E60/15). The manuscript has been read and approved by all named authors and there are no other persons who satisfied the criteria for authorship. All contributors have approved the order of authors listed in the manuscript.