Highlights
Anti-CD20 therapies are effective in relapsing multiple sclerosis, but discontinuation is understudied.
In our cohort of 881 patients followed for 2337 patient-years, 16.0% discontinued anti-CD20 therapies, most frequently due to side effects.
The discontinuation rate at 36 months was 12.5% for ocrelizumab and 22.2% for rituximab.
Introduction
Ocrelizumab (OCR) and rituximab (RTX) are anti-CD20 monoclonal antibodies (CD20Mabs) used in the treatment of relapsing multiple sclerosis (RMS). CD20Mabs deplete B cells, reducing pro-inflammatory cytokine production, T cell trafficking into the central nervous system and T cell activation. Reference Roach and Cross1 The pivotal phase III OPERA I and II trials with OCR showed significantly reduced annualized relapse rate, disability progression and number of new gadolinium-enhancing lesions on MRI compared to interferon beta-1a. Reference Hauser, Bar-Or and Comi2 In the phase III RIFUND-MS trial, RTX reduced risk of relapse and new T2 MRI lesions compared to dimethyl fumarate. Reference Svenningsson, Frisell and Burman3 Mild infusion reactions and infections were common in these trials, with low rates of serious adverse events. Given their favorable safety profile, efficacy and biannual dosing, these drugs are popular among RMS patients and clinicians, and consequently, utilization of CD20Mabs has increased compared to older platform injections or oral options. Reference Kwon, Sillau, Corboy, Nair and Carlson4
Accumulating evidence suggests that early initiation of treatment with high-efficacy therapies in RMS is associated with lower rates of disease activity and decreased risk of long-term disability progression. Reference Simonsen, Flemmen and Broch5,Reference He, Merkel and Brown6 Thus, adherence to treatment is important for abrogating inflammatory disease, particularly in a disease where risk of episodic clinical relapses can unpredictably recur for decades. A recent scoping review examined factors related to disease-modifying therapy (DMT) adherence and persistence, Reference Ben-Zacharia, Walker and Ross7 but data on rates and reasons for discontinuation in CD20Mabs are lacking.
In British Columbia, OCR and RTX are used interchangeably as first-line therapy for RMS, with access to OCR requiring private insurance coverage and RTX coverage via public provincial special authority access; this is unique to British Columbia, whereas other provinces use OCR almost exclusively. The aim of this study was to explore discontinuation of CD20Mabs in a real-world cohort of RMS patients with access to both OCR and RTX using real-world evidence.
Methods
Ethics approval and setting
This study was performed at the University of British Columbia (Vancouver, British Columbia). The study protocol was approved by the University of British Columbia Clinical Research Ethics Board and the University of Northern British Columbia Research Ethics Board (study ID H24-02237), with operational approval also from the Vancouver Coastal Health Research Institute and Northern Health. Strengthening the Reporting of Observational Studies in Epidemiology guidelines were followed.
Participants and data collection
Patient data were collected via retrospective chart review at two Canadian MS centers: the University of British Columbia MS clinic (Vancouver, British Columbia) and the Northern Health MS clinic (Prince George, British Columbia).
Inclusion criteria were: (1) patients with a diagnosis of RMS according to the 2010 McDonald Criteria Reference Polman, Reingold and Banwell8 for patients who started treatment prior to December 2017, and the 2017 McDonald Criteria Reference Thompson, Banwell and Barkhof9 for those who started treatment after December 2017 (including those with a diagnosis of secondary progressive multiple sclerosis), (2) initiation of RTX or OCR between January 2017 and March 2023 and (3) at least one completed infusion of OCR or RTX. Exclusion criteria were: (1) treatment with CD20Mabs prior to January 2017, (2) treatment with CD20Mabs for a reason other than MS, (3) incomplete clinical information, (4) simultaneous adjunct immunotherapy with CD20Mabs (excluding pulse steroids during acute relapses), (5) prior hematogenous stem cell transplant and (6) diagnosis of primary progressive multiple sclerosis or alternative central nervous system demyelinating or inflammatory disorder (e.g. neuromyelitis optica spectrum disorder, autoimmune encephalitis, neurosarcoidosis, etc.).
All patient data were assigned a unique patient identification number for anonymization. Patient data were screened for inclusion and exclusion criteria by D.J.H, N.Y.C and J.M, and if appropriate, electronic chart review was undertaken to abstract data. Disagreement between chart reviewers was adjudicated by senior author A.J.S. Data abstracted were: age, sex, date of MS onset (or if unknown, date of MS diagnosis), date of first infusion of CD20Mab therapy, date of last infusion of CD20Mab therapy (if applicable), date of last clinical follow-up, previous DMTs prior to CD20Mab therapy (acceptable prior DMTs include but were not limited to: platform injection therapy [glatiramer acetate, interferon-beta], teriflunomide, dimethyl fumarate, fingolimod, alemtuzumab, natalizumab, cyclophosphamide, mitoxantrone, azathioprine, mycophenolate and cladribine), treatment with OCR or RTX (no discrimination was made between RTX and its biosimilars), planned dosing intervals of CD20Mabs, relapses in the 12 months before CD20Mab initiation, Expanded Disability Status Score (EDSS) at time of CD20Mab initiation and reason for starting CD20Mabs. For patients with or without interruption of therapy, total duration on CD20Mabs was calculated based on the difference between the first infusion date and the date of last follow-up visit. For patients who discontinued CD20Mabs, time to discontinuation was calculated based on the duration from the date of the first infusion to the date of the last infusion, rounded to the nearest month. Analysis was only performed on the first CD20Mab that a patient was treated with. For example, if a patient switched from RTX to OCR, they were only counted once (in the RTX group) and were not analyzed for reasons for discontinuation or time to discontinuation in the OCR group.
Patients were considered to have discontinued CD20Mab therapy if any of the following were fulfilled: time elapsed from last CD20Mab infusion was >12 months (without subsequent restart of CD20Mab at any future recorded time), CD20Mab therapy was changed to a subsequent different DMT and/or a definitive decision to stop therapy was verbalized in documentation. Reasons for discontinuation of CD20Mabs (age, side effects, lymphopenia/laboratory abnormalities, new inflammatory disease activity [new clinical relapses and/or MRI lesions], disease progression, disease stability, insurance coverage, patient preference, pregnancy, lost to follow-up and other [with text descriptor]) were recorded from documentation.
Study outcomes
The primary study outcomes were reasons for discontinuation and discontinuation rates of CD20Mabs. Secondary outcomes of interest were time to discontinuation of CD20Mabs and hazard ratios of treatment discontinuation between OCR-treated and RTX-treated patients.
Statistical analysis
Baseline demographic information was reported as counts with percentages, means with standardized deviations (SD) and medians with interquartile range (IQR), where appropriate. Fisher’s exact test was used to compare categorical variables, while Mann–Whitney U test was used for comparisons of continuous variables. Two-tailed significance was defined at p < 0.05. Kaplan–Meier curves were constructed to visualize OCR- and RTX-treated patients in time to discontinuation analysis, and log-rank test was used to compare CD20Mab treatments and discontinuation due to specific reasons. In the survival analyses of patients discontinuing due to a specific reason (e.g. side effects), patients that remained on drug or discontinued for other reasons were censored. Cox proportional hazards regression was used to further investigate hazard ratios of CD20Mabs discontinuation with the following variables: CD20Mabs drug, EDSS at initiation, disease duration, age at CD20Mabs start, reason for initiation, number of prior DMTs and relapses in the prior 12 months. We tested for possible violations of the proportional hazards assumption with Schoenfeld residuals. Analyses were performed using GraphPad Prism (version 10.4.0 (527), October 23, 2024, GraphPad Software Inc., Boston, MA, USA).
Results
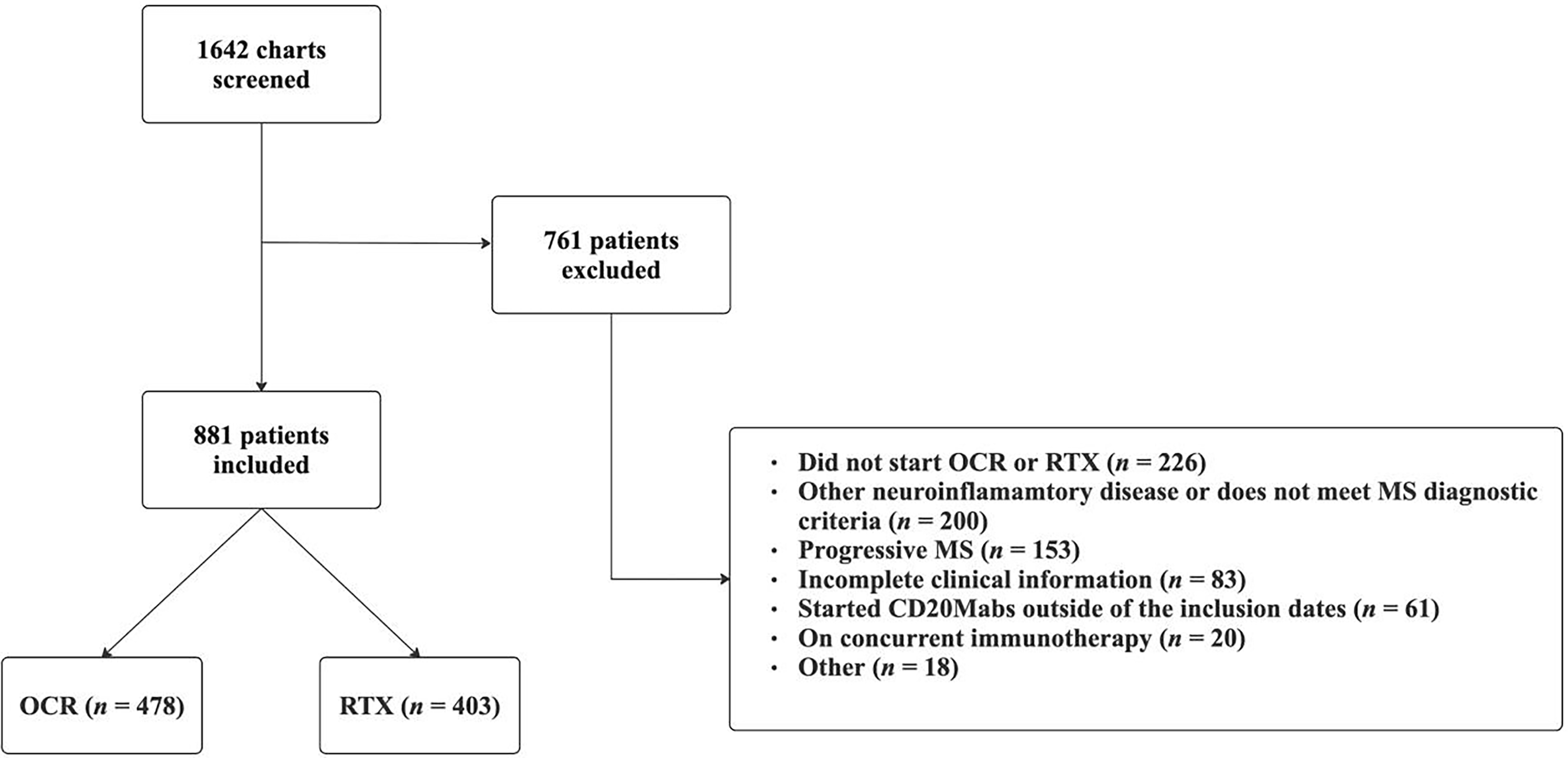
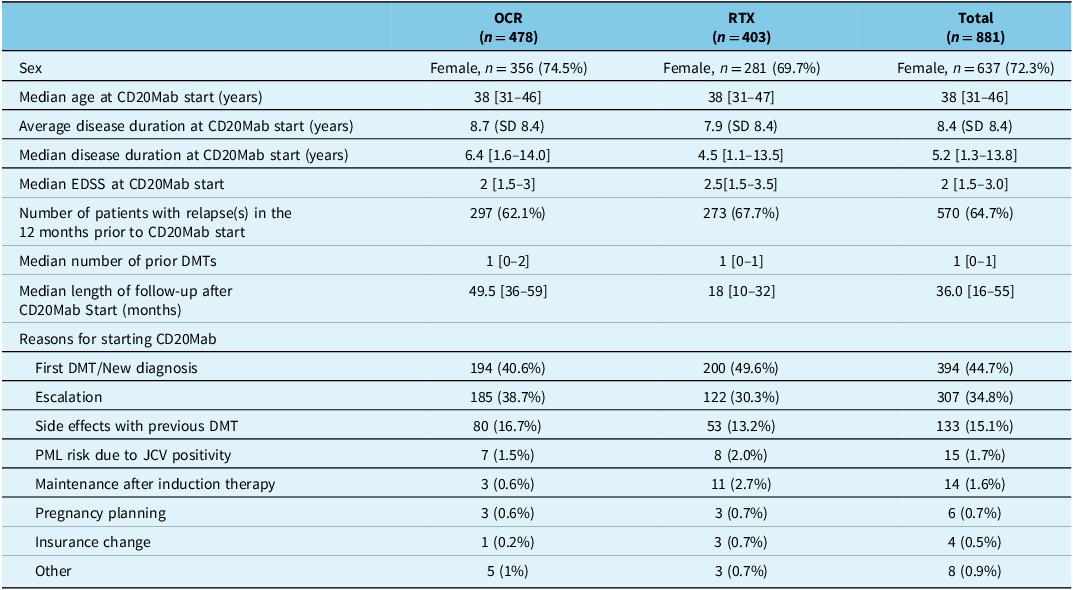
Of the 1642 patient charts screened, 881 patients (637 [72.3%] female sex; median age at CD20Mab start 38 [IQR 31–46] years) were included in the study (Figure 1). Baseline patient characteristics are represented in Table 1. In total, 478 (54.3%) patients were on OCR and 403 (45.7%) patients were on RTX. Patients had mild disability with a median EDSS of 2 [IQR 1.5–3.0] and median disease duration of 5.2 [IQR 1.3–13.8] years (mean duration 8.4 (SD 8.4)) at CD20Mab start. Median length of follow-up after starting CD20Mabs was 36.0 [IQR 16–55] months, with OCR patients having a median follow-up time of 49.5 [IQR 36–59] months and RTX patients having a median follow-up time of 18.0 [IQR 10–32] months. The majority of patients experienced a clinical relapse in the 12 months prior to starting a CD20Mab (570/881 [64.7%]). Most patients started CD20Mabs either as a first DMT and/or with new diagnosis (44.7%), as an escalation strategy (34.8%), or due to side effects with previous DMT (15.1%).

Figure 1. Flow chart of patient inclusion and exclusion. OCR = ocrelizumab; RTX = rituximab; MS = multiple sclerosis; CD20Mabs = anti-CD20 monoclonal antibodies.
Table 1. Baseline characteristics of all study patients

CD20Mab = anti-CD20 monoclonal antibody; OCR = ocrelizumab; RTX = rituximab; SD = standard deviation; EDSS = Expanded Disability Status Scale; DMT = disease-modifying therapy. Square brackets denote interquartile range.
Reasons for CD20Mab discontinuation
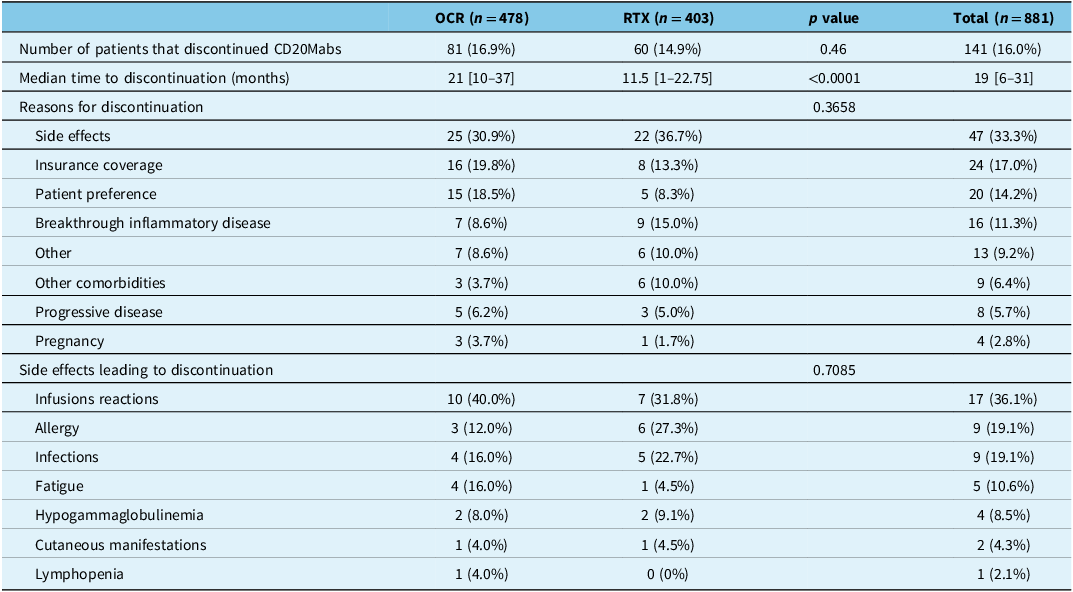
Overall, 141/881 (16.0%) patients discontinued CD20Mabs after a cumulative 2337 patient-years (Table 2). The proportion of patients that discontinued CD20Mabs was similar for OCR at 16.9% (81/478) over 1643 patient-years, and RTX at 14.9% (60/403) over 694 patient-years (p = 0.46). The most common reasons for patients discontinuing CD20Mabs were side effects (47/141 [33.3%]; 5.3% of all patients), insurance coverage (24/141 [17.0%]; 2.7% of all patients) and patient preference (20/141 [14.2%]; 2.3% of all patients) (Table 2). Only 11.3% (16/141; 1.8% of all patients) stopped due to breakthrough inflammatory disease, and 5.7% (8/141; 0.9% of all patients) stopped due to progressive disease. The most frequently reported side effects leading to discontinuation were infusion reactions (17/47 [36.1%]), infections (9/47 [19.1%]) and allergy (9/47 [19.1%]). There was no significant difference in reasons for discontinuation or side effects leading to discontinuation in OCR- and RTX-treated patients (Table 2). Preference for different DMT accounted for 10/20 (50%) of the discontinuations due to patient preference (Supplemental Material 1). Of note, 7/10 patients that preferred a different DMT switched to ofatumumab (a subcutaneous CD20Mab). Two patients discontinued CD20Mabs due to concern of the coronavirus disease 2019 (COVID-19) pandemic.
Table 2. CD20Mab discontinuation data

CD20Mabs = anti-CD20 monoclonal antibodies; OCR = ocrelizumab; RTX = rituximab. Square brackets denote interquartile range.
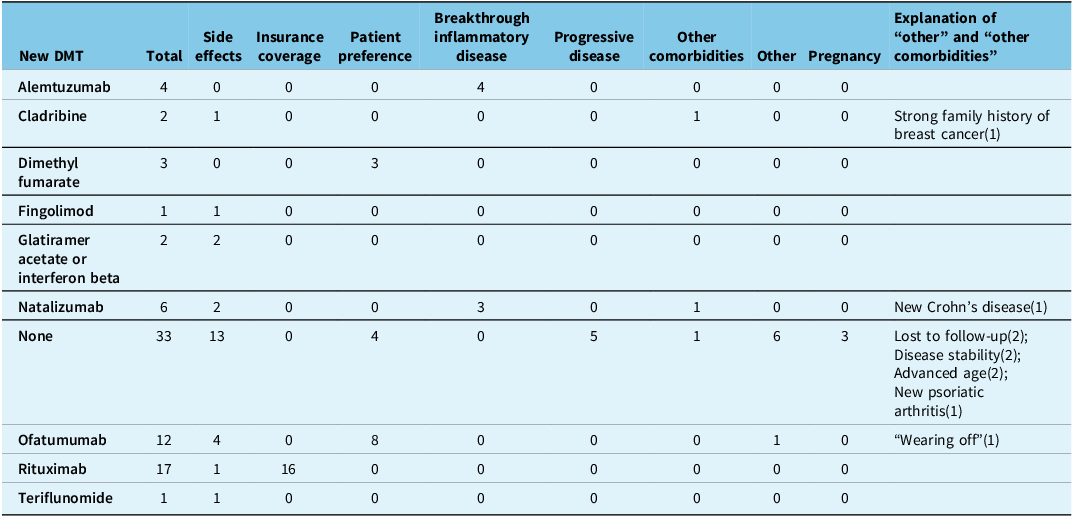
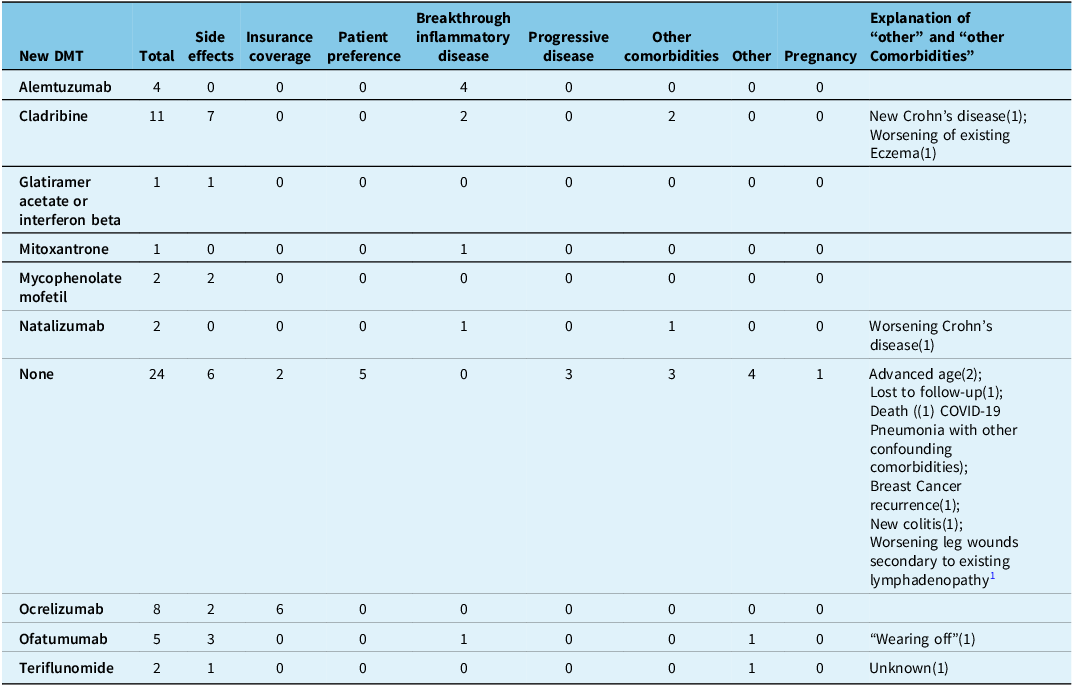
All patients that discontinued CD20Mabs due to breakthrough inflammatory disease (16/141 [11.3%]) went on to subsequent DMTs and none of the patients with progressive disease (8/141 [5.7%]) went on to subsequent DMTs (Tables 3 and 4). Alemtuzumab (8/16 [44%]), natalizumab (4/16 [25%]) and cladribine (2/16 [19%]) were the most frequently used agents in patients that failed CD20Mabs due to inflammatory disease.
Table 3. Subsequent disease-modifying therapy (DMT) after stopping ocrelizumab, separated by reasons for discontinuation

The number in brackets denotes the number of patients that discontinued due to the listed reason.
Table 4. Subsequent disease-modifying therapy (DMT) after stopping rituximab, separated by reasons for discontinuation

COVID-19 = coronavirus disease 2019. The number in brackets denotes the number of patients that discontinued due to the listed reason.
Time to discontinuation of CD20Mabs
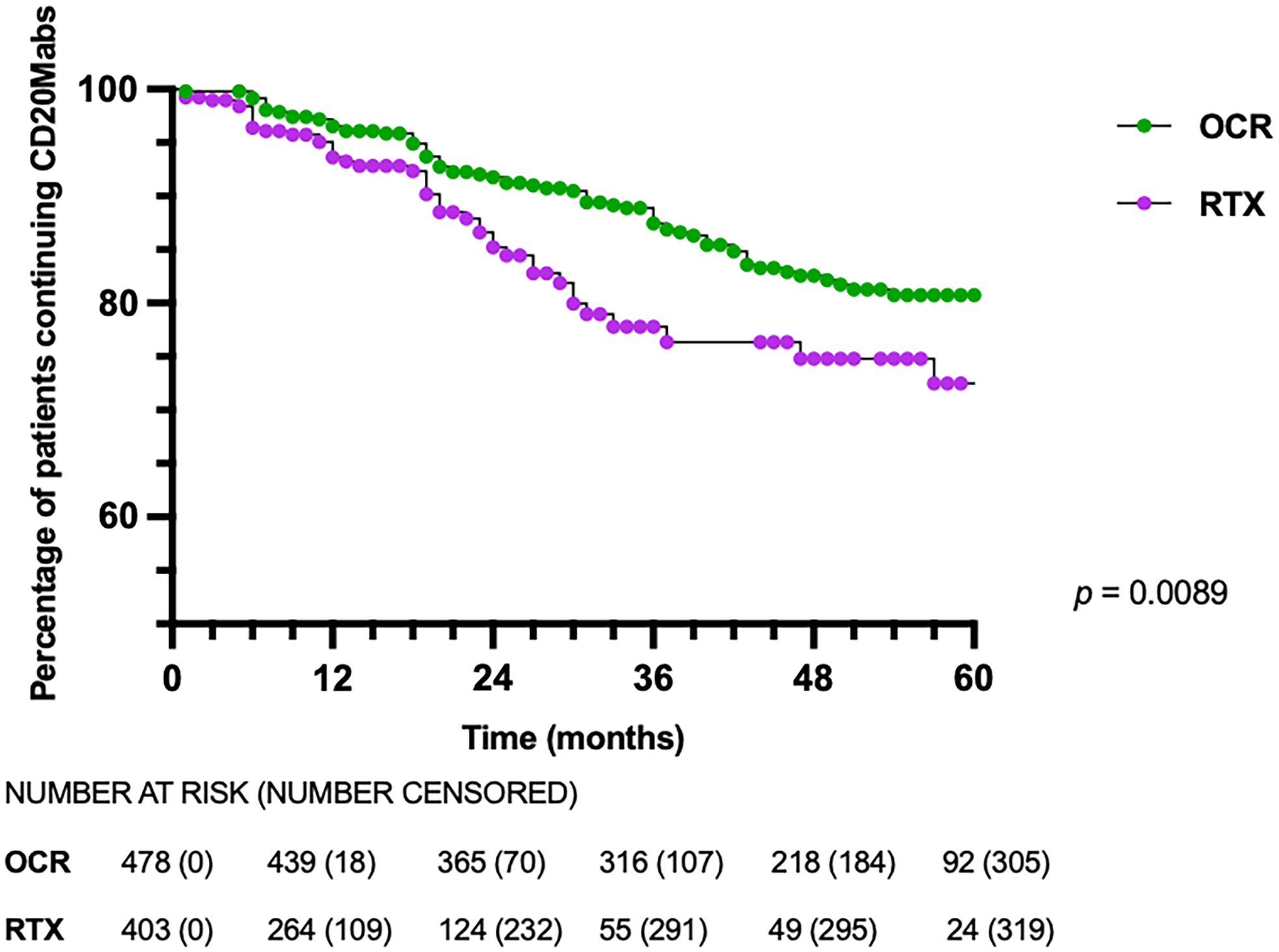
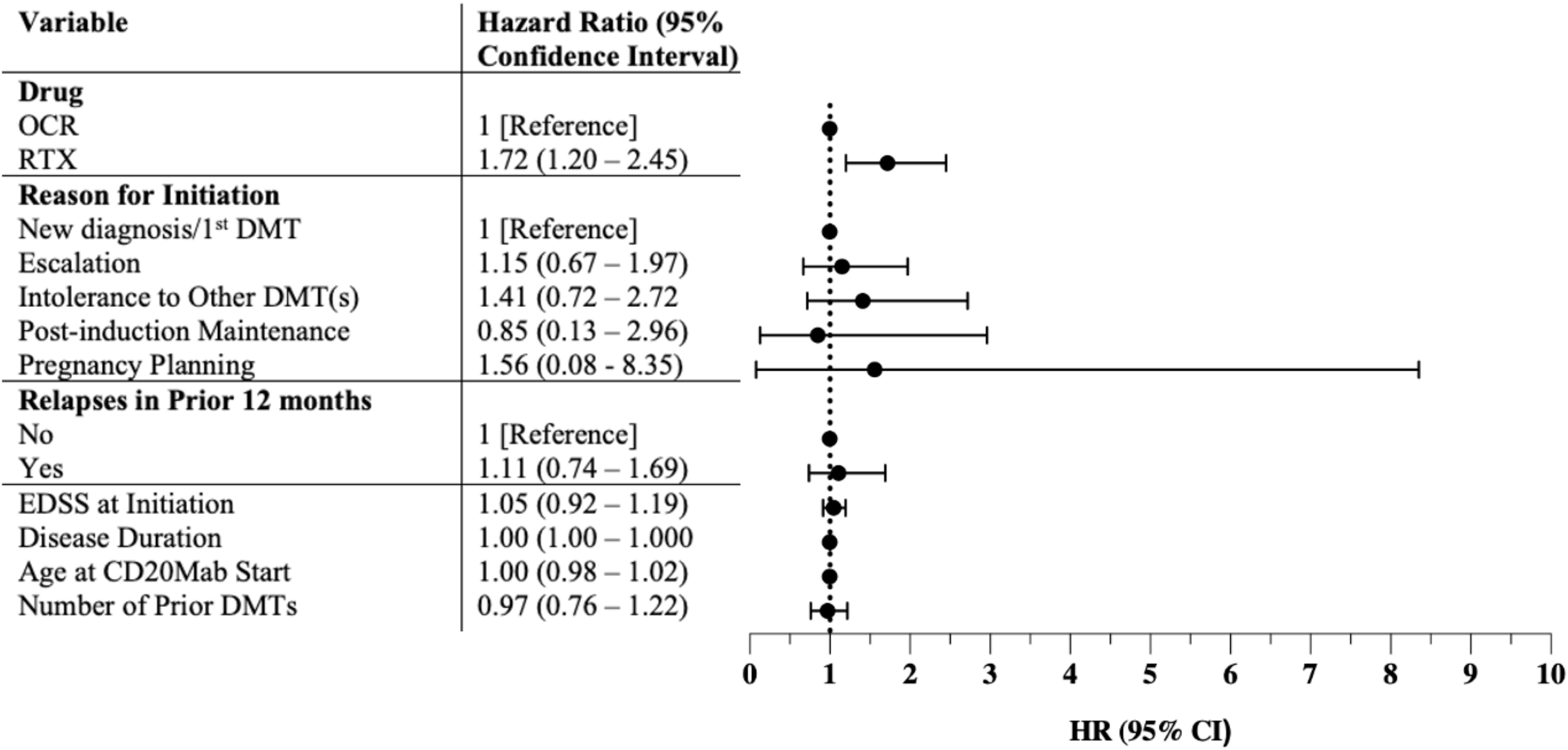
While the overall proportion of patients discontinuing OCR and RTX was similar, RTX-treated patients discontinued earlier than OCR-treated patients, with a median time to discontinuation of 11.5 vs 21.0 months (p < 0.0001), respectively (Table 2). On survival analysis, RTX-treated patients discontinued CD20Mabs earlier than OCR-treated patients (p = 0.0089) (Figure 2). Discontinuation rates at 12, 24 and 36 months were 3.5%, 8.2% and 12.5% for OCR, and 6.4%, 14.8% and 22.2% for RTX, respectively (p = 0.0089). On Cox regression, the only variable with an increased risk for discontinuation was treatment with RTX (hazard ratio 1.72, 95% confidence interval 1.20–2.45), with no association seen with EDSS at initiation, disease duration, age at CD20Mab start, reason for initiation, number of prior DMTs or relapses in the prior 12 months (Figure 3). There was no violation of the proportional hazards assumption with Schoenfeld residuals. Survival analyses of OCR- and RTX-treated patients discontinuing due to specific reasons did not yield statistically significant results (side effects p = 0.1162; insurance coverage p = 0.7126; patient preference p = 0.9089; breakthrough inflammatory disease p = 0.0667; progressive disease p = 0.5050) (Supplemental Material 1).

Figure 2. Kaplan–Meier survival curve of patients discontinuing CD20Mabs. CD20Mabs = anti-CD20 monoclonal antibodies; OCR = ocrelizumab; RTX = rituximab.

Figure 3. Hazard ratios for anti-CD20 monoclonal antibody discontinuation. OCR = ocrelizumab; RTX = rituximab; DMT = disease-modifying therapy; EDSS = Expanded Disability Status Scale; CD20Mab = anti-CD20 monoclonal antibody; HR = hazard ratio; CI = confidence interval.
Alternative interval dosing and interruptions
All but 27/881 patients were maintained on standard dosing of OCR (induction of 300 mg IV twice over 2 weeks, followed by 600 mg every 6 months) and RTX (induction of 1000 mg IV twice over 2 weeks, followed by 1000 mg every 6 months) during the study period. Twenty-one patients received OCR outside of the standard dosing intervals (10 patients received 600 mg IV every 5 months, and 11 patients received 600 mg IV every 8–12 months). Six patients received RTX outside of the standard dosing interval, with two patients receiving 1000 mg IV every 5 months, and the other four receiving 1000 mg IV every 7–12 months. Only one patient on alternate interval dosing (every 9–12 months) stopped a CD20Mab during the study period, which was due to advancing age. Thirty-eight patients (28 on OCR and 10 on RTX) explicitly interrupted therapy, with the majority (26/38) interrupting for pregnancy/family planning (Supplemental Material 1). The length of interruption (time between infusions) was between 8 and 29 months, with six patients interrupting therapy for >18 months. One patient that interrupted therapy due to pregnancy had a relapse 16 months after their last infusion, with a new T2 signal abnormality on MRI and subsequently went back on to OCR. Two patients interrupted therapy due to concern of the COVID-19 pandemic.
Discussion
We present a large real-world cohort of RMS patients treated with CD20Mabs (N = 881), with 16.0% discontinuing therapy over a cumulative follow-up time of 2337 patient-years. To our knowledge, this is the first real-world study examining OCR and RTX discontinuation in a population with parallel access to both medications for RMS.
In a real-world study examining claims data in the United States, 8% and 17% of patients discontinued OCR treatment at 12 and 18 months, respectively. Reference Engmann, Sheinson, Bawa, Ng and Pardo10 Similarly, in open-label extension studies of OPERA I and II, 89% of patients entering the study at year 2 persisted on OCR treatment at year 5. Reference Hauser, Kappos and Arnold11 Our study found that 16.9% of patients discontinued OCR over a median follow-up time of 49.5 months, which was comparable to the above studies. A potential reason for the slightly higher rate of discontinuation in our study compared to OPERA I and II open-label extension studies may be due to the patients in our cohort losing private insurance for OCR over time. With respect to RTX, a Swedish study with a median follow-up time of 18.8 months found that 5.8% of patients discontinued RTX, with an annualized drug discontinuation rate at 3%. Reference Granqvist, Boremalm and Poorghobad12 Another study reported 7.2% of RMS patients discontinued RTX over an average of follow-up time of 620 days. Reference Torgauten, Myhr, Wergeland, Bø, Aarseth and Torkildsen13 In our cohort, there was a mildly higher rate of discontinuation with 14.9% stopping RTX after a median follow-up of 18 months. One potential explanation is the longer average disease duration of 8.4 years observed in our cohort compared to the above studies, which had an average MS disease duration of 1.3 years Reference Granqvist, Boremalm and Poorghobad12 and 4.8 years, Reference Torgauten, Myhr, Wergeland, Bø, Aarseth and Torkildsen13 respectively, along with evidence that longer disease duration is associated with overall greater non-adherence to MS DMTs. Reference McKay, Tremlett and Patten14,Reference Devonshire, Lapierre and Macdonell15 Another possible explanation for slightly higher rates of CD20Mab discontinuation in our study was the occurrence of the COVID-19 pandemic during our inclusion period, which may have contributed to more patients discontinuing or interrupting high-efficacy DMTs. In our cohort, two patients discontinued CD20Mabs due to patient preference cited the COVID-19 pandemic as one of the factors influencing treatment discontinuation, two patients interrupted therapy due to concerns of COVID-19 and one patient died from complications of COVID-19. Overall, our low discontinuation rates of CD20Mabs are concurrent with previous data showing long-term tolerability.
The most common reasons for stopping CD20Mab therapies reported in the literature are adverse events, Reference Hauser, Kappos and Arnold11,Reference Torgauten, Myhr, Wergeland, Bø, Aarseth and Torkildsen13,Reference Zhu, Kalincik and Horakova16 pregnancy Reference Granqvist, Boremalm and Poorghobad12 and patient preference. Reference Weber, Buttmann and Meuth17 Our study also found that adverse events were the most common reason for discontinuation, with similar proportions of patients stopping due to side effects in the OCR and RTX groups. Other common reasons for discontinuation in our cohort included insurance coverage (17.0%) and patient preference (14.2%). Insurance coverage issues highlight challenges in accessing certain high-efficacy therapies in British Columbia, despite the single-payer public healthcare system. Prior to August 2020, British Columbians could get access to OCR through compassionate access from the pharmaceutical company; however, after the decision to not fund OCR by the provincial government, some patients had to switch to a different DMT if they did not have private insurance or could not pay out of pocket. Several patients interrupted CD20Mabs due to pregnancy/family planning and then subsequently resumed treatment and thus were not grouped into the discontinuation group; this may account for our low rate of discontinuation specifically due to pregnancy/family planning compared to other studies, in addition to potentially different operational definitions for cessation/interruption of CD20Mab therapy.
Our study suggests that patients persist longer on OCR compared to RTX, even when accounting for other variables. The aim of our study was to look at reasons for CD20Mab discontinuation, not to necessarily compare OCR and RTX, although this difference was discovered in the data analysis. It is not clear why patients are discontinuing RTX earlier than OCR. Previous studies have found that patients persist longer on OCR compared to other DMTs, Reference Engmann, Sheinson, Bawa, Ng and Pardo10,Reference Zhu, Kalincik and Horakova16,Reference Tallantyre, Dobson and Froud18,Reference Pardo, Pineda, Ng, Bawa, Sheinson and Bonine19 but these studies notably lack a comparison to RTX. A recent large observational study utilizing both MSBase and the Danish MS registries found that patients were over three-fold more likely to discontinue RTX compared to OCR, but a large proportion of patients did not have recorded reasons for discontinuation of therapy; Reference Roos, Hughes and McDonnell20 additionally 69% of patients discontinuing RTX were subsequently treated with OCR, suggesting the availability of OCR rather than other factors as a potential motivating factor. In contrast, other available data comparing OCR to RTX reported comparable discontinuation rates of OCR at 20.8% and RTX 24.7% at <24 months. Reference vollmer, Passeri and Ijadi21 A possible hypothesis for the differential timing of discontinuation may be earlier and/or more frequent development of anti-drug antibodies, given RTX is a mouse-chimeric antibody as opposed to OCR as a humanized monoclonal antibody. Up to 37% of RMS patients on RTX were found to have anti-drug antibodies Reference Dunn, Juto and Ryner22 compared to only 1.0% in OCR from long-term studies. Reference Hauser, Kappos and Montalban23 Interestingly, despite a high proportion of RTX patients having anti-drug antibodies, which were shown to correlate with greater incomplete B-cell depletion, there was not an association between anti-drug antibody status and infusion reactions, adverse effects or disease activity. Reference Dunn, Juto and Ryner22 Another hypothesis for why patients may be persisting on OCR longer than RTX is due to patients’ and clinicians’ perception of these two CD20Mabs. Given that RTX is an off-label treatment for RMS, there may be less tolerance for side effects and breakthrough inflammatory disease, which may prompt a switch to an on-label DMT. We were underpowered to demonstrate this with our exploratory survival analysis looking at patients discontinuing due to these variables (Supplemental material 1). It should be emphasized that our study was not designed specifically to look at differences in discontinuation rates between RTX and OCR, particularly as the withdrawal of compassionate access for OCR post August 2020 makes this comparison fraught with bias from socioeconomic status and time-dependent confounding. A future study integrating these variables to formally evaluate factors influencing treatment persistence of OCR and RTX may be valuable.
Similar to other published studies, Reference Engmann, Sheinson, Bawa, Ng and Pardo10–Reference Torgauten, Myhr, Wergeland, Bø, Aarseth and Torkildsen13,Reference Weber, Buttmann and Meuth17,Reference Tallantyre, Dobson and Froud18,Reference Juto, Fink, Al Nimer and Piehl24 breakthrough inflammatory disease (clinical relapses or new MRI lesions) was an uncommon reason for discontinuation of CD20Mabs in our cohort (11.3% of all discontinuers, 1.8% of all patients). These patients were all switched to high-efficacy therapy, which included alemtuzumab, natalizumab, cladribine, mitoxantrone or ofatumumab. This variety reflects the state of the literature in that there is currently a paucity of high-quality evidence guiding clinicians in the event of CD20Mab treatment failure. Upcoming trials comparing high-efficacy treatment to autologous hematopoietic stem cell transplantation 25 will be of particular interest in this subset with aggressive disease.
Our main limitation in this study is the retrospective observational design. While we were able to incorporate two major MS centers into our cohort analysis, these populations may not necessarily be homogenous. Additionally, protocols for CD20Mab therapy initiation, monitoring, and clinical/radiological follow-up may have differed between sites and affected our results. However, there is overlap in physicians practising at both sites, which may have reduced practice variability. Furthermore, the difference in follow-up time after initiation of CD20Mabs can be explained by initial British Columbia provincial coverage and patient support bridging programs enabling access to OCR after its approval in 2017, followed by change in provincial funding of RTX in lieu of OCR. Thus, patients who were able to continue OCR after cessation of provincial funding required private insurance coverage, typically provided by employer benefits, so unknown confounders associated with factors such as educational attainment and socioeconomic status cannot be ruled out. The COVID-19 pandemic likely influenced discontinuation rates during our study period to a degree; although we did not calculate differences before and after onset of the pandemic, this would be a salient future study of interest. Ofatumumab discontinuation was not included in our study due to it not receiving Health Canada approval until 2021 (over halfway through the inclusion dates of our study, and we only had 30 patients that started ofatumumab through the study period), though this would be a pertinent area of interest for future work. Lastly, the decision to discontinue a therapy is often multifactorial and it is difficult to always attribute it to one specific cause.
Conclusions
This study aimed to evaluate rates and reasons for discontinuation of infusion-based CD20Mabs in two large MS clinics in British Columbia, Canada. In our study, OCR and RTX were well tolerated with low discontinuation rates in a real-world setting. Side effects were cited as the most common reason for CD20Mab discontinuation, with infusion reactions being noted as the most frequent side effect leading to discontinuation. Patients in our cohort persisted on OCR longer than RTX when controlling for various variables. Future studies are needed to explore further aspects of treatment adherence, discontinuation and sequencing in RMS to optimally guide clinical decision making.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2025.10520.
Author Contributions
D.J.H, N.Y.C, R.L.C, V.D and A.J.S were involved in the conceptualization and planning of the project. D.J.H, N.Y.C and J.M were involved in screening patient charts and collection of patient data. D.J.H performed the data analysis. All authors were involved in the writing of the manuscript.
Funding Statement
This project received no funding.
Competing Interests
D.J.H has no disclosures. N.Y.C receives fellowship funding from the Canadian Network of Multiple Sclerosis Clinics (via unrestricted educational grants from EMD Serono, Sanofi Genzyme, Biogen, Hoffmann-La Roche and Novartis); travel and accommodation funding from Alexion; speaker fees from Novartis. D.K. receives funding from Biogen, Hoffman-La Roche, Sentrex, Novartis, EMD Serono and Apotex. J.M has no disclosures. K.W has no disclosures. C.E.U has no disclosures. M.A. has received honoraria from Biogen, Novartis, Merck Sharp & Dohme and Abbvie. A.L.S has received consulting fees from Pfizer Canada, Novartis, EMD Serono and Hoffman-La Roche; payment or honoraria from Pfizer, Novartis, EMD Serono, Hoffman-La Roche and Biogen; Support for travel from Apotex. A.T receives grants from Hoffman-La Roche, Biogen, Clene, Sanofi Genzyme, AbbVie, the National Institute for Health, MS Canada and the Consortium of MS centers; receives consulting fees from Hoffman-La Roche and Sanofi Genzyme; received payment or honoraria from Hoffman-La Roche, EMD Serono and Biogen; support for travel from Sanofi Genzyme and EMD Serono; drug and safety monitoring board committee member at Sanofi Genzyme. R.L.C receives grants from Novartis, EMD Serono, Hoffman-La Roche, Biogen, Sanofi and Genentech; consulting fees from Hoffman-La Roche, Genentech, Novartis, Biogen and EMD Serono. V.D has received consulting fees, honoraria and support for travel from Hoffman-La Roche, Biogen, Novartis, EMD Serono, Sanofi Genzyme, AbbVie and adjudication committee member for EMD Serono. A.J.S has received honoraria from Biogen, Novartis, EMD Serono, Sanofi, Alexion, Hoffman-La Roche and AbbVie.


Target article
A Retrospective Evaluation of Ocrelizumab and Rituximab Discontinuation in a Real-World Patient Cohort
Related commentaries (1)
Reviewer Comment on Hunt et al. “A Retrospective Evaluation of Ocrelizumab and Rituximab Discontinuation in a Real-World Patient Cohort”