Refine search
Actions for selected content:
106104 results in Materials Science
Synthesis and Processing of Organic Materials in Supercritical Carbon Dioxide
-
- Journal:
- MRS Bulletin / Volume 34 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 108-115
- Print publication:
- February 2009
-
- Article
- Export citation
Spark plasma sintering of zirconium carbide and oxycarbide: Finite element modeling of current density, temperature, and stress distributions
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 404-412
- Print publication:
- February 2009
-
- Article
- Export citation
Effect of film thickness on the structural and electrical properties of Ga-doped ZnO thin films prepared on glass and Al2O3 (0001) substrates by RF magnetron sputtering method
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 26 July 2012, pp. 441-447
- Print publication:
- February 2009
-
- Article
- Export citation
Current Status of GaN-Based Solid-State Lighting
-
- Journal:
- MRS Bulletin / Volume 34 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 101-107
- Print publication:
- February 2009
-
- Article
- Export citation
Science Policy
-
- Journal:
- MRS Bulletin / Volume 34 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 78-79
- Print publication:
- February 2009
-
- Article
-
- You have access
- Export citation
Piezoelectric, ferroelectric Pb(Mg1/3Nb2/3)O3–PbTiO3 thin films with compositions around the morphotropic phase boundary prepared by a sol-gel process of reduced thermal budget
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 526-533
- Print publication:
- February 2009
-
- Article
- Export citation
Failure by simultaneous grain growth, strain localization, and interface debonding in metal films on polymer substrates
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 379-385
- Print publication:
- February 2009
-
- Article
- Export citation
Controlled generation of ferromagnetic martensite from paramagnetic austenite in AISI 316L austenitic stainless steel
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 565-573
- Print publication:
- February 2009
-
- Article
- Export citation
Oxidation and reduction behavior of pure indium
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 386-393
- Print publication:
- February 2009
-
- Article
- Export citation
Thermal stability, corrosion resistance, and surface analysis of Cu–Hf–Ti–Ni–Nb bulk metallic glasses
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 316-323
- Print publication:
- February 2009
-
- Article
- Export citation
Bulk metallic glass formation, composite, and magnetic propertiesof Fe-B-Nd based alloys
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 357-371
- Print publication:
- February 2009
-
- Article
- Export citation
Compositionally graded mullite-based chemical vapor deposited coatings
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 470-474
- Print publication:
- February 2009
-
- Article
- Export citation
Serrated flow behavior induced by blunt mechanism of shear crack propagation in metallic glass
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 26 July 2012, pp. 436-440
- Print publication:
- February 2009
-
- Article
- Export citation
Structure of vapor-phase deposited Al-Ge thin films and Al-Ge intermediate layer bonding of Al-based microchannel structures
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 544-555
- Print publication:
- February 2009
-
- Article
- Export citation
Microstructure and secondary phase segregation correlation in epitaxial/oriented ZnO films with unfavorable Cr dopant
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 506-515
- Print publication:
- February 2009
-
- Article
- Export citation
Titanium nitride nanopowders produced via sodium reductionin liquid ammonia
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 448-451
- Print publication:
- February 2009
-
- Article
- Export citation
Ho3+-Doped Nanophase Glass Ceramics Enhance Efficiency of Si Solar Cells
-
- Journal:
- MRS Bulletin / Volume 34 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, p. 76
- Print publication:
- February 2009
-
- Article
-
- You have access
- Export citation
The role of Cu addition in the coercivity enhancement of sintered Nd-Fe-B permanent magnets
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 413-420
- Print publication:
- February 2009
-
- Article
- Export citation
Nanostructured Organic–Inorganic Hybrid Solar Cells
-
- Journal:
- MRS Bulletin / Volume 34 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 95-100
- Print publication:
- February 2009
-
- Article
- Export citation
Effects of Nb doping on thermoelectric properties of Zn4Sb3 at high temperatures
-
- Journal:
- Journal of Materials Research / Volume 24 / Issue 2 / February 2009
- Published online by Cambridge University Press:
- 31 January 2011, pp. 430-435
- Print publication:
- February 2009
-
- Article
- Export citation
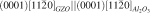 . The structural images from scanning electron microscopy and atomic force microscopy showed that the GZO thin films on glass substrates had a rougher surface morphology than those on Al
. The structural images from scanning electron microscopy and atomic force microscopy showed that the GZO thin films on glass substrates had a rougher surface morphology than those on Al , and global work-hardening sensitivity exponent,
, and global work-hardening sensitivity exponent,  , are introduced to characterize the blunt effect on the net increase in flow stress or work-hardening behavior of metallic glass.
, are introduced to characterize the blunt effect on the net increase in flow stress or work-hardening behavior of metallic glass.