Refine search
Actions for selected content:
106104 results in Materials Science
Zirconia Inert Matrix Fuel for Plutonium and Minor Actinides Management in Reactors and as an Ultimate Waste Form
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1104 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1104-NN03-01
- Print publication:
- 2008
-
- Article
- Export citation
Read-Shockley Grain Boundaries and the Herring Equation
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1090 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1090-Z05-18
- Print publication:
- 2008
-
- Article
- Export citation
Bonding Structure of Ultrathin Oxides on Si(110) Surface
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1074 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1074-I03-50
- Print publication:
- 2008
-
- Article
- Export citation
Optimizing MIM Device Electrical Properties. Impact of Bottom Electrodes and High K Materials
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1108 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1108-A09-03
- Print publication:
- 2008
-
- Article
- Export citation
Simulation of Capacitance-Voltage characteristics of Ultra-thin Metal-Oxide-Semiconductor Structures with Embedded Nanocrystals
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1071 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1071-F02-11
- Print publication:
- 2008
-
- Article
- Export citation
Growth and Characterization of p-n Junction Core-Shell GaAs Nanowires on Carbon Nanotube Composite Films
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1144 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1144-LL14-05
- Print publication:
- 2008
-
- Article
- Export citation
Factors Influencing the Growth Rate, Doping, and Surface Morphology of the Low-Temperature Halo-Carbon Homoepitaxial Growth of 4H SiC with HCl Additive
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1069 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1069-D05-03
- Print publication:
- 2008
-
- Article
- Export citation
A Novel Electrospun Dendrimer-Gelatin Hybrid Nanofiber Scaffold for Tissue Regeneration and Drug Delivery
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1094 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1094-DD09-07
- Print publication:
- 2008
-
- Article
- Export citation
Doping of Sub-50nm SOI Layers
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1070 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1070-E04-06
- Print publication:
- 2008
-
- Article
- Export citation
Phosphorous and Boron Doped Colloidal Silicon Nanocrystals in Conjugated Co-polymers
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1102 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1102-LL01-08
- Print publication:
- 2008
-
- Article
- Export citation
Carbon Nanoparticles for Counter Electrode Catalyst in Dye-Sensitized Solar Cells
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1102 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1102-LL01-05
- Print publication:
- 2008
-
- Article
- Export citation
Green Nanotechnologies for Responsible Manufacturing
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1106 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1106-PP03-05
- Print publication:
- 2008
-
- Article
- Export citation
Universal glass dynamics in PCM nano-glasses
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1072 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1072-G02-09
- Print publication:
- 2008
-
- Article
- Export citation
Noise Analysis of Image Sensor Arrays for Large-Area Biomedical Imaging
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1066 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1066-A18-04
- Print publication:
- 2008
-
- Article
- Export citation
Oxygen vacancy migration in CSZ using density functional theory
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1122 / 2008
- Published online by Cambridge University Press:
- 20 February 2017, 1122-O03-08
- Print publication:
- 2008
-
- Article
- Export citation
A New Type of Display Device Based on Remote Swelling and Collapse of a pH Responsive Microgel
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1134 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1134-BB08-14
- Print publication:
- 2008
-
- Article
- Export citation
A Model for the Study of Molecules Radiochemical Decomposition by Actinides Materials
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1104 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1104-NN07-12
- Print publication:
- 2008
-
- Article
- Export citation
Uranium(VI) Uptake by Synthetic Calcium Silicate Hydrates
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1107 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 467
- Print publication:
- 2008
-
- Article
- Export citation
Negative Bias Temperature Instability for P-channel of LTPS Thin Film Transistors with Fluorine Implantation
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1066 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1066-A16-03
- Print publication:
- 2008
-
- Article
- Export citation
On the Suitability of Lanthanides as Actinide Analogs
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 1104 / 2008
- Published online by Cambridge University Press:
- 01 February 2011, 1104-NN04-01
- Print publication:
- 2008
-
- Article
- Export citation
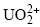 could control U(VI) uptake by C-S-H phases.
could control U(VI) uptake by C-S-H phases.