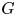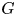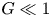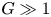Refine listing
Actions for selected content:
1418960 results in Open Access
Use of exclusive human milk diet in a neonate with single ventricle physiology supported on a ventricular assist device as a bridge to heart transplantation: case report
-
- Journal:
- Cardiology in the Young / Volume 34 / Issue 11 / November 2024
- Published online by Cambridge University Press:
- 30 October 2024, pp. 2491-2494
-
- Article
- Export citation
Robin Gwynn. The Huguenots in Later Stuart Britain. Volume III: The Huguenots and the Defeat of Louis XIV's France Liverpool: Liverpool University Press, 2023. Pp. 480. $150.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 63 / Issue 4 / October 2024
- Published online by Cambridge University Press:
- 30 October 2024, pp. 901-903
-
- Article
- Export citation
Small-scale forest restoration in peri-urban areas provides immediate benefits for birds
-
- Journal:
- Bird Conservation International / Volume 34 / 2024
- Published online by Cambridge University Press:
- 30 October 2024, e31
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Yield curve extrapolation with machine learning
-
- Journal:
- ASTIN Bulletin: The Journal of the IAA / Volume 55 / Issue 1 / January 2025
- Published online by Cambridge University Press:
- 30 October 2024, pp. 76-96
- Print publication:
- January 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Late complete atrioventricular block after transcatheter perimembranous ventricular septal defect closure with the KONAR-MF™ VSD occluder: a case report and systematic review
-
- Journal:
- Cardiology in the Young / Volume 34 / Issue 11 / November 2024
- Published online by Cambridge University Press:
- 30 October 2024, pp. 2480-2483
-
- Article
- Export citation
Disaster Knowledge, Skills, and Preparedness among Emergency Medical Services in Saudi Arabia
-
- Journal:
- Disaster Medicine and Public Health Preparedness / Volume 18 / 2024
- Published online by Cambridge University Press:
- 30 October 2024, e233
-
- Article
- Export citation
Intergenerational transmission of fertility: evidence from China's population control policies
-
- Journal:
- Journal of Demographic Economics / Volume 91 / Issue 3 / September 2025
- Published online by Cambridge University Press:
- 30 October 2024, pp. 407-438
-
- Article
- Export citation
INTERNAL AUTOMORPHISMS AND ANTIMORPHISMS OF MODELS OF NF
-
- Journal:
- The Journal of Symbolic Logic , First View
- Published online by Cambridge University Press:
- 29 October 2024, pp. 1-5
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Count Otani Kozui’s 1900 northern cruise: A Japanese “explorer tourist” in the Nordic Arctic
-
- Journal:
- Polar Record / Volume 60 / 2024
- Published online by Cambridge University Press:
- 29 October 2024, e19
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Body temperature and thermoregulatory behaviour in the Endangered African Penguin Spheniscus demersus
-
- Journal:
- Bird Conservation International / Volume 34 / 2024
- Published online by Cambridge University Press:
- 29 October 2024, e29
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Nonlinear evolution of vortical disturbances entrained in the entrance region of a circular pipe
-
- Journal:
- Journal of Fluid Mechanics / Volume 998 / 10 November 2024
- Published online by Cambridge University Press:
- 29 October 2024, A19
-
- Article
- Export citation
Unravelling military aggression: Ontological insecurity, great power narcissism, and Japan’s international relations, 1868–1971
-
- Journal:
- Review of International Studies , First View
- Published online by Cambridge University Press:
- 29 October 2024, pp. 1-21
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Locating Values in the Space of Possibilities
-
- Journal:
- Philosophy of Science / Volume 92 / Issue 1 / January 2025
- Published online by Cambridge University Press:
- 29 October 2024, pp. 121-140
- Print publication:
- January 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
A Singular Enlightenment: C. L. R. James, Anti-Colonialism, and Transatlantic Political Thought
-
- Journal:
- American Political Science Review / Volume 119 / Issue 3 / August 2025
- Published online by Cambridge University Press:
- 29 October 2024, pp. 1272-1285
- Print publication:
- August 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Do Government Benefits Affect Officeholders’ Electoral Fortunes? Evidence from State Earned Income Tax Credits
-
- Journal:
- American Political Science Review / Volume 119 / Issue 3 / August 2025
- Published online by Cambridge University Press:
- 29 October 2024, pp. 1286-1303
- Print publication:
- August 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
First record of Hesione sicula delle Chiaje, 1830 (Polychaeta: Hesionidae) for Mediterranean Moroccan coasts
-
- Journal:
- Journal of the Marine Biological Association of the United Kingdom / Volume 104 / 2024
- Published online by Cambridge University Press:
- 29 October 2024, e94
-
- Article
- Export citation
Analysis of group evaporation characteristics of droplet clusters in an evaporating spray
-
- Journal:
- Journal of Fluid Mechanics / Volume 998 / 10 November 2024
- Published online by Cambridge University Press:
- 29 October 2024, A26
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The relationship between minimum wage and employment. A synthetic control method approach
-
- Journal:
- The Economic and Labour Relations Review / Volume 35 / Issue 3 / September 2024
- Published online by Cambridge University Press:
- 29 October 2024, pp. 771-791
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Curbing Elite Capture or Enhancing Resources: Recentralizing Local Environmental Enforcement in China – CORRIGENDUM
-
- Journal:
- The China Quarterly / Volume 261 / March 2025
- Published online by Cambridge University Press:
- 29 October 2024, p. 93
- Print publication:
- March 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation