Refine listing
Actions for selected content:
1416804 results in Open Access
Formation of Banded Iron-Manganese Structures by Natural Microbial Communities
-
- Journal:
- Clays and Clay Minerals / Volume 48 / Issue 5 / October 2000
- Published online by Cambridge University Press:
- 28 February 2024, pp. 511-520
-
- Article
- Export citation
Unusual X-Ray Characteristics of Vermiculite from Wiry, Lower Silesia, Poland
-
- Journal:
- Clays and Clay Minerals / Volume 49 / Issue 3 / June 2001
- Published online by Cambridge University Press:
- 28 February 2024, pp. 197-203
-
- Article
- Export citation
Alteration of Smectite in a System Including Alanine at High Pressure and Temperature
-
- Journal:
- Clays and Clay Minerals / Volume 43 / Issue 2 / April 1995
- Published online by Cambridge University Press:
- 28 February 2024, pp. 184-190
-
- Article
- Export citation
Characterization of Hydrothermal Tobelitic Veins from Black Shale, Oquirrh Mountains, Utah
-
- Journal:
- Clays and Clay Minerals / Volume 40 / Issue 4 / August 1992
- Published online by Cambridge University Press:
- 28 February 2024, pp. 405-420
-
- Article
- Export citation
Decomposition of Experimental X-Ray Diffraction Patterns (Profile Fitting): A Convenient Way to Study Clay Minerals
-
- Journal:
- Clays and Clay Minerals / Volume 45 / Issue 2 / April 1997
- Published online by Cambridge University Press:
- 28 February 2024, pp. 132-146
-
- Article
- Export citation
Fe3+-Rich Montmorillonite-Beidellite Series in Ayvacik Bentonite Deposit, Biga Peninsula, Northwest Turkey
-
- Journal:
- Clays and Clay Minerals / Volume 47 / Issue 2 / April 1999
- Published online by Cambridge University Press:
- 28 February 2024, pp. 165-173
-
- Article
- Export citation
Diagenesis of Dioctahedral and Trioctahedral Smectites from Alternating Beds in Miocene to Pleistocene Rocks of the Niigata Basin, Japan
-
- Journal:
- Clays and Clay Minerals / Volume 49 / Issue 4 / August 2001
- Published online by Cambridge University Press:
- 28 February 2024, pp. 333-346
-
- Article
- Export citation
Use and Limitations of Second-Derivative Diffuse Reflectance Spectroscopy in the Visible to Near-Infrared Range to Identify and Quantify Fe Oxide Minerals in Soils
-
- Journal:
- Clays and Clay Minerals / Volume 46 / Issue 5 / October 1998
- Published online by Cambridge University Press:
- 28 February 2024, pp. 528-536
-
- Article
- Export citation
Mass Decontamination of Companion Dogs in Disaster: Planning for Personnel, Water, and Time Requirements
-
- Journal:
- Disaster Medicine and Public Health Preparedness / Volume 18 / 2024
- Published online by Cambridge University Press:
- 28 February 2024, e40
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Binary Cation-Exchange Reactions
-
- Journal:
- Clays and Clay Minerals / Volume 46 / Issue 2 / April 1998
- Published online by Cambridge University Press:
- 28 February 2024, pp. 215-218
-
- Article
- Export citation
A Chemical, XRD, and 27Al MAS NMR Investigation of Miocene Gulf Coast Shales with Application to Understanding Illite/Smectite Crystal-Chemistry
-
- Journal:
- Clays and Clay Minerals / Volume 41 / Issue 6 / December 1993
- Published online by Cambridge University Press:
- 28 February 2024, pp. 668-679
-
- Article
- Export citation
Distinguishing Between Surface and Bulk Dehydration-Dehydroxylation Reactions in Synthetic Goethites by High-Resolution Thermogravimetric Analysis
-
- Journal:
- Clays and Clay Minerals / Volume 47 / Issue 3 / June 1999
- Published online by Cambridge University Press:
- 28 February 2024, pp. 329-337
-
- Article
- Export citation
Surface Charge Properties of Kaolinite
-
- Journal:
- Clays and Clay Minerals / Volume 45 / Issue 1 / February 1997
- Published online by Cambridge University Press:
- 28 February 2024, pp. 85-91
-
- Article
- Export citation
The Effect of Dry Grinding on Antigorite from Mulhacen, Spain
-
- Journal:
- Clays and Clay Minerals / Volume 47 / Issue 4 / August 1999
- Published online by Cambridge University Press:
- 28 February 2024, pp. 417-424
-
- Article
- Export citation
Layer-Charge Heterogeneity in Smectites of I-S Phases in Pelitic Sediments from the Molasse Basin, Austria
-
- Journal:
- Clays and Clay Minerals / Volume 46 / Issue 6 / December 1998
- Published online by Cambridge University Press:
- 28 February 2024, pp. 670-678
-
- Article
- Export citation
THE
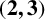 $\boldsymbol {(2,3)}$-GENERATION OF THE FINITE SIMPLE ODD-DIMENSIONAL ORTHOGONAL GROUPS
$\boldsymbol {(2,3)}$-GENERATION OF THE FINITE SIMPLE ODD-DIMENSIONAL ORTHOGONAL GROUPS
- Part of
-
- Journal:
- Journal of the Australian Mathematical Society / Volume 117 / Issue 2 / October 2024
- Published online by Cambridge University Press:
- 28 February 2024, pp. 130-148
- Print publication:
- October 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Quadratic associations between cardiovascular stress reactivity and development of cool and hot executive functions in adolescents
-
- Journal:
- Development and Psychopathology / Volume 37 / Issue 2 / May 2025
- Published online by Cambridge University Press:
- 28 February 2024, pp. 664-677
-
- Article
- Export citation
Effects of Secondary Iron Phases on Kaolinite 27Al MAS NMR Spectra
-
- Journal:
- Clays and Clay Minerals / Volume 46 / Issue 4 / August 1998
- Published online by Cambridge University Press:
- 28 February 2024, pp. 429-435
-
- Article
- Export citation
The Interaction of Polysaccharides with Silver Hill Illite
-
- Journal:
- Clays and Clay Minerals / Volume 40 / Issue 2 / April 1992
- Published online by Cambridge University Press:
- 28 February 2024, pp. 151-156
-
- Article
- Export citation
Lead-210 Derived Sedimentation Rates from a North Louisiana Paper-Mill Effluent Reservoir
-
- Journal:
- Clays and Clay Minerals / Volume 43 / Issue 5 / October 1995
- Published online by Cambridge University Press:
- 28 February 2024, pp. 515-524
-
- Article
- Export citation