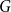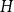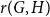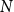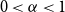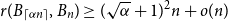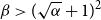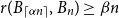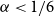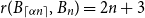Refine listing
Actions for selected content:
1418918 results in Open Access
Reburials of Eminent Masters: The Construction of Quanzhen Daoist Lineages in North China under Mongol Rule
-
- Journal:
- Journal of Chinese History / Volume 8 / Issue 2 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 297-326
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Rising and settling 2-D cylinders with centre-of-mass offset
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 16 February 2024, A7
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The Tang legacy on the Silk Road during the Uighur era: urbanisation in the eastern Tianshan region during the ninth to thirteenth centuries
-
- Journal:
- Journal of the Royal Asiatic Society / Volume 34 / Issue 2 / April 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 377-398
- Print publication:
- April 2024
-
- Article
- Export citation
Discursive scaling of solidarity through difference: Experiences of African women in the African diaspora
-
- Journal:
- Language in Society / Volume 54 / Issue 3 / June 2025
- Published online by Cambridge University Press:
- 16 February 2024, pp. 491-513
- Print publication:
- June 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Trilobites from the Al Rose Formation (Lower Ordovician, Inyo Mountains, California)—faunas marginal to the Great Basin
-
- Journal:
- Journal of Paleontology / Volume 98 / Issue 4 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 644-657
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
A single-institutional experience with 36 children less than 5 kilograms supported with the Berlin Heart: Comparison of congenital versus acquired heart disease
-
- Journal:
- Cardiology in the Young / Volume 34 / Issue 6 / June 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 1342-1349
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Effects of the COVID-19 Pandemic on Submissions to Politics & Gender
-
- Journal:
- PS: Political Science & Politics / Volume 57 / Issue 3 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 420-426
- Print publication:
- July 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
By Any Means Necessary? How Black and White Americans Evaluate Protest Tactics in Response to a Police Killing
-
- Journal:
- Journal of Race, Ethnicity and Politics / Volume 9 / Issue 2 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 235-258
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Holocene relative sea-level changes along the Caribbean and Pacific coasts of northwestern South America
-
- Journal:
- Quaternary Research / Volume 119 / May 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 28-43
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
BJN volume 131 issue 6 Cover and Front matter
-
- Journal:
- British Journal of Nutrition / Volume 131 / Issue 6 / 28 March 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. f1-f2
- Print publication:
- 28 March 2024
-
- Article
-
- You have access
- Export citation
Association between dietary macronutrient composition and plasma one-carbon metabolites and B-vitamin cofactors in patients with stable angina pectoris
-
- Journal:
- British Journal of Nutrition / Volume 131 / Issue 10 / 28 May 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 1678-1690
- Print publication:
- 28 May 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Rights and Wrongs in Talk of Mind-Reading Technology
-
- Journal:
- Cambridge Quarterly of Healthcare Ethics / Volume 33 / Issue 4 / October 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 521-531
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Introduction: Pandemic and Post-Pandemic Publication Patterns in Political Science
-
- Journal:
- PS: Political Science & Politics / Volume 57 / Issue 3 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 403-407
- Print publication:
- July 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Applied Probability Trust prizes 2023
-
- Journal:
- Journal of Applied Probability / Volume 61 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 16 February 2024, p. 368
- Print publication:
- March 2024
-
- Article
- Export citation
On a conjecture of Conlon, Fox, and Wigderson
- Part of
-
- Journal:
- Combinatorics, Probability and Computing / Volume 33 / Issue 4 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 432-445
-
- Article
- Export citation
The restricted quantum double of the Yangian
- Part of
-
- Journal:
- Canadian Journal of Mathematics / Volume 77 / Issue 3 / June 2025
- Published online by Cambridge University Press:
- 16 February 2024, pp. 770-841
- Print publication:
- June 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Optimizing Disaster Response: Structuring the Medical Branch Association’s Committees for Earthquake Emergencies
-
- Journal:
- Disaster Medicine and Public Health Preparedness / Volume 18 / 2024
- Published online by Cambridge University Press:
- 16 February 2024, e24
-
- Article
-
- You have access
- HTML
- Export citation
JPR volume 61 issue 1 Cover and Front matter
-
- Journal:
- Journal of Applied Probability / Volume 61 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. f1-f2
- Print publication:
- March 2024
-
- Article
-
- You have access
- Export citation
Calico Madams and South Sea Cheats: Global Trade, Finance, and Popular Protest in Early Hanoverian England
-
- Journal:
- Journal of British Studies / Volume 63 / Issue 1 / January 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 114-138
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
On twisted group ring isomorphism problem for p-groups
- Part of
-
- Journal:
- Glasgow Mathematical Journal / Volume 66 / Issue 2 / May 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 368-381
- Print publication:
- May 2024
-
- Article
- Export citation