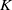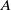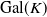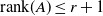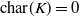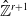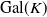Refine listing
Actions for selected content:
1418153 results in Open Access
P43: Hormone therapy and the decreased risk of dementia in women with depression: a population-based cohort study
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 154
-
- Article
-
- You have access
- Export citation
Measuring and Modeling Neighborhoods
-
- Journal:
- American Political Science Review / Volume 118 / Issue 4 / November 2024
- Published online by Cambridge University Press:
- 02 February 2024, pp. 1966-1985
- Print publication:
- November 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
P69: The effect of social prescribing on improving cognitive performance among community-dwelling older adults: A pilot study
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 206
-
- Article
-
- You have access
- Export citation
Learning from trials: LIVE@Home.Path, a stepped-wedge cluster randomized controlled trial of care coordination and implementation for home-dwelling people with dementia.
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 46
-
- Article
-
- You have access
- Export citation
FC29: Development of an Informant-Reported Lucidity Measure
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 91-92
-
- Article
-
- You have access
- Export citation
P156: The Development of the PET@home Toolkit using the refined Experience-Based Co- Design Method (EBCD+)
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 139-140
-
- Article
-
- You have access
- Export citation
S11: Digital Health and Artificial Intelligence (AI) in Psychogeriatrics: Opening Multiple Frontiers
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 36
-
- Article
-
- You have access
- Export citation
FC17: Effects of the CarFreeMe driving cessation intervention to identify and improve transport and lifestyle issues for people with dementia: Participant feedback and satisfaction after program completion
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 79-80
-
- Article
-
- You have access
- Export citation
Legislation of euthanasia in Portugal: The psychiatrist’s role
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 53-54
-
- Article
-
- You have access
- Export citation
Suicide and rationality
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 53
-
- Article
-
- You have access
- Export citation
P165: Music-assisted reminiscence therapy: The theory behind a new frontier for enhancing the wellbeing outcomes for older adults
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 183-184
-
- Article
-
- You have access
- Export citation
P189: TELEMATIC CONTROL OF BEHAVIORAL DISORDERS IN PATIENTS WITH DEMENTIA
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 144-145
-
- Article
-
- You have access
- Export citation
P63: Best Practice Guidance on Human Interaction with Technology in Dementia Update June 2023 – Recommendations from the INDUCT and DISTINCT Networks
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 158-159
-
- Article
-
- You have access
- Export citation
Abelian absolute Galois groups: In Erinnerung an Wulf-Dieter Geyer (1939–2019)
- Part of
-
- Journal:
- Glasgow Mathematical Journal / Volume 66 / Issue 2 / May 2024
- Published online by Cambridge University Press:
- 02 February 2024, pp. 359-367
- Print publication:
- May 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
P25: Promote DemenTitude® - Cultural Adaption of Progressively Lowered Stress Threshold Model with Social Work Perspective in Hong Kong
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 152
-
- Article
-
- You have access
- Export citation
Workshop 4: Cognitive Assessment For Older People in Daily Clinical Practice – A Primer
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 6
-
- Article
-
- You have access
- Export citation
FC19: Remaining engaged through work in young onset dementia: first results of the WorkDEM study
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 81
-
- Article
-
- You have access
- Export citation
P115: Testing the feasibility of a multicomponent neuropsychological intervention for individuals at-risk of dementia: the REMINDER program
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 130-131
-
- Article
-
- You have access
- Export citation
P143: New therapies for Alzheimer’s dementia and its implications on healthcare system: are we ready?
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 179-180
-
- Article
-
- You have access
- Export citation
FC43: Particularities of late-life psychosis: from epidemiology to treatment option
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 105
-
- Article
-
- You have access
- Export citation