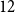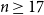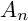Refine listing
Actions for selected content:
1419087 results in Open Access
Two Jubilees of Warsaw Lutherans, 1881 and 1931
-
- Journal:
- The Journal of Ecclesiastical History / Volume 76 / Issue 1 / January 2025
- Published online by Cambridge University Press:
- 18 September 2024, pp. 103-127
- Print publication:
- January 2025
-
- Article
- Export citation
FLM volume 991 Cover and Front matter
-
- Journal:
- Journal of Fluid Mechanics / Volume 991 / 25 July 2024
- Published online by Cambridge University Press:
- 18 September 2024, p. f1
-
- Article
-
- You have access
- Export citation
A new formula for the determinant and bounds on its tensor and Waring ranks
- Part of
-
- Journal:
- Combinatorics, Probability and Computing / Volume 33 / Issue 6 / November 2024
- Published online by Cambridge University Press:
- 18 September 2024, pp. 769-794
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Transience of continuous-time conservative random walks
- Part of
-
- Journal:
- Journal of Applied Probability / Volume 62 / Issue 1 / March 2025
- Published online by Cambridge University Press:
- 18 September 2024, pp. 153-171
- Print publication:
- March 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
BIRATIONAL GEOMETRY OF SEXTIC DOUBLE SOLIDS WITH A COMPOUND
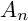 $A_n$ SINGULARITY
$A_n$ SINGULARITY
- Part of
-
- Journal:
- Nagoya Mathematical Journal / Volume 256 / December 2024
- Published online by Cambridge University Press:
- 18 September 2024, pp. 970-1021
- Print publication:
- December 2024
-
- Article
- Export citation
Clerical Professionalisation and Catholic Enlightenment in the Polish-Lithuanian Commonwealth
-
- Journal:
- The Journal of Ecclesiastical History / Volume 76 / Issue 1 / January 2025
- Published online by Cambridge University Press:
- 18 September 2024, pp. 78-102
- Print publication:
- January 2025
-
- Article
- Export citation
Preaching Predestination and Pastoral Ministry in the Caroline Parish
-
- Journal:
- The Journal of Ecclesiastical History / Volume 76 / Issue 1 / January 2025
- Published online by Cambridge University Press:
- 18 September 2024, pp. 55-77
- Print publication:
- January 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Electroconvulsive therapy response and remission in moderate to severe depressive illness: a decade of national Scottish data
-
- Journal:
- The British Journal of Psychiatry / Volume 225 / Issue 6 / December 2024
- Published online by Cambridge University Press:
- 18 September 2024, pp. 547-555
- Print publication:
- December 2024
-
- Article
- Export citation
A quantitative general equilibrium approach to migration, remittances, and brain drain
-
- Journal:
- Macroeconomic Dynamics / Volume 29 / 2025
- Published online by Cambridge University Press:
- 18 September 2024, e23
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Revision of the conservation status and assessment of the Green Status of the Parana Antwren Formicivora acutirostris with management proposals
-
- Journal:
- Bird Conservation International / Volume 34 / 2024
- Published online by Cambridge University Press:
- 18 September 2024, e21
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Criminal intent and psychiatric evidence
-
- Journal:
- BJPsych Advances / Volume 30 / Issue 6 / November 2024
- Published online by Cambridge University Press:
- 18 September 2024, pp. 357-367
- Print publication:
- November 2024
-
- Article
-
- You have access
- HTML
- Export citation
EUSEBIUS AND THE GOSPELS - (J.) Coogan Eusebius the Evangelist. Rewriting the Fourfold Gospel in Late Antiquity. Pp. xvi + 234, ills. New York: Oxford University Press, 2023. Cased, £47.99, US$99. ISBN: 978-0-19-758004-2.
-
- Journal:
- The Classical Review / Volume 74 / Issue 2 / October 2024
- Published online by Cambridge University Press:
- 18 September 2024, pp. 455-457
- Print publication:
- October 2024
-
- Article
- Export citation
Subcritical transitional flow in two-dimensional plane Poiseuille flow
-
- Journal:
- Journal of Fluid Mechanics / Volume 994 / 10 September 2024
- Published online by Cambridge University Press:
- 18 September 2024, A6
-
- Article
- Export citation
THE METAPHOR OF THE BODY POLITIC - (J.) Mebane The Body Politic in Roman Political Thought. Pp. x + 253. Cambridge: Cambridge University Press, 2024. Cased, £85, US$110. ISBN: 978-1-009-38929-7.
-
- Journal:
- The Classical Review / Volume 74 / Issue 2 / October 2024
- Published online by Cambridge University Press:
- 18 September 2024, pp. 555-557
- Print publication:
- October 2024
-
- Article
- Export citation
The Impact of “Soft” and “Hard” Flood Adaptation Measures on Affected Population’s Mental Health: A Mixed Method Scoping Review
-
- Journal:
- Disaster Medicine and Public Health Preparedness / Volume 18 / 2024
- Published online by Cambridge University Press:
- 18 September 2024, e118
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Denying “The Right to Have Rights”: Europe’s Imposition of Mandates in Greater Syria and the Rise of Islamist Movements
-
- Journal:
- Nationalities Papers , FirstView
- Published online by Cambridge University Press:
- 18 September 2024, pp. 1-21
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Training Public Health Nurses on Disaster Shelter Care Using a Flipped Classroom Approach
-
- Journal:
- Disaster Medicine and Public Health Preparedness / Volume 18 / 2024
- Published online by Cambridge University Press:
- 18 September 2024, e122
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Climate change and health: the role of health professionals
-
- Journal:
- Irish Journal of Psychological Medicine / Volume 41 / Issue 4 / December 2024
- Published online by Cambridge University Press:
- 18 September 2024, pp. 427-429
- Print publication:
- December 2024
-
- Article
-
- You have access
- HTML
- Export citation
INHILLDAUGAR: minimally invasive fieldwork and linguistic analysis on hillforts along the Daugava river
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Trapping studies reveal phenology and reproductive behaviour in two solitary sweat bees (Hymenoptera: Halictidae)
-
- Journal:
- The Canadian Entomologist / Volume 156 / 2024
- Published online by Cambridge University Press:
- 18 September 2024, e19
-
- Article
-
- You have access
- Open access
- HTML
- Export citation