Refine listing
Actions for selected content:
1417513 results in Open Access
Customized Spectral Libraries for Effective Mineral Exploration: Mining National Mineral Collections
-
- Journal:
- Clays and Clay Minerals / Volume 66 / Issue 3 / June 2018
- Published online by Cambridge University Press:
- 01 January 2024, pp. 297-314
-
- Article
- Export citation
In situ X-ray Diffraction Study of the Swelling of Montmorillonite as Affected by Exchangeable Cations and Temperature
-
- Journal:
- Clays and Clay Minerals / Volume 59 / Issue 2 / April 2011
- Published online by Cambridge University Press:
- 01 January 2024, pp. 165-175
-
- Article
- Export citation
Influence of Mechanical Compaction and Clay Mineral Diagenesis on the Microfabric and Pore-Scale Properties of Deep-Water Gulf of Mexico Mudstones
-
- Journal:
- Clays and Clay Minerals / Volume 54 / Issue 4 / August 2006
- Published online by Cambridge University Press:
- 01 January 2024, pp. 500-514
-
- Article
- Export citation
Benzene Vapor Sorption by Organobentonites From Ambient Air
-
- Journal:
- Clays and Clay Minerals / Volume 50 / Issue 4 / August 2002
- Published online by Cambridge University Press:
- 01 January 2024, pp. 421-427
-
- Article
- Export citation
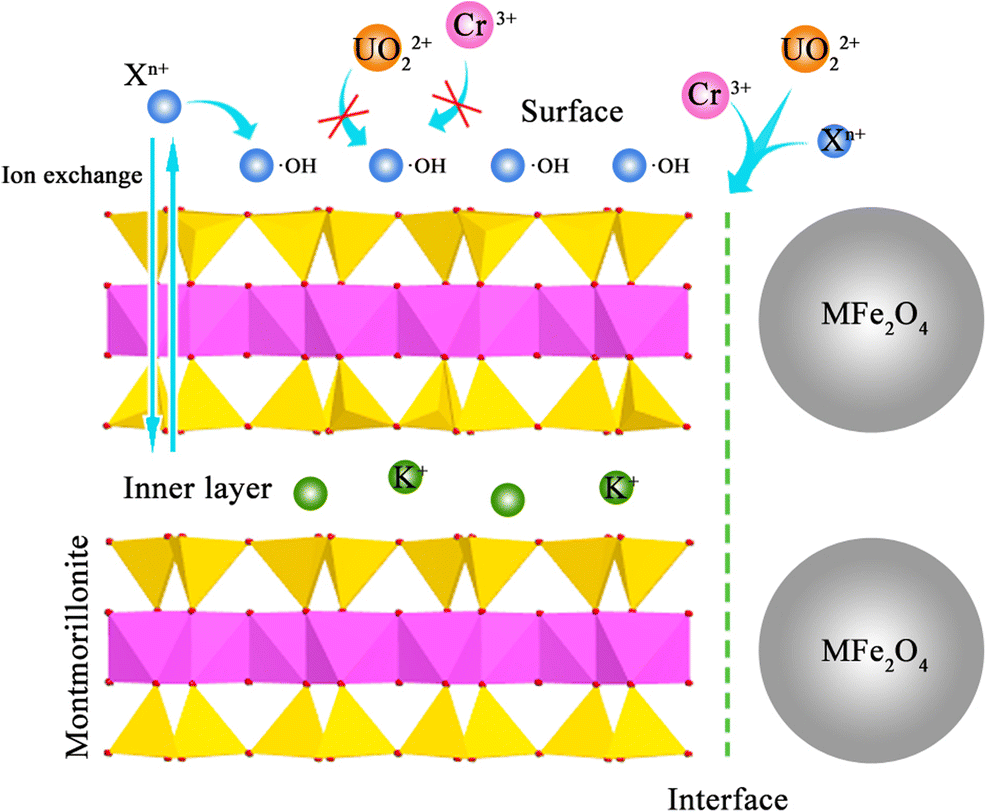
Competitive Adsorption of Uranyl and Toxic Trace Metal Ions at MFe2O4-montmorillonite (M = Mn, Fe, Zn, Co, or Ni) Interfaces
-
- Journal:
- Clays and Clay Minerals / Volume 67 / Issue 4 / August 2019
- Published online by Cambridge University Press:
- 01 January 2024, pp. 291-305
-
- Article
- Export citation
The Role of the Clergy in the Establishment and Consolidation of Pahlavi I (1925–1941)
-
- Journal:
- Iranian Studies / Volume 57 / Issue 1 / January 2024
- Published online by Cambridge University Press:
- 29 January 2024, pp. 143-163
- Print publication:
- January 2024
-
- Article
- Export citation
Boron and Lithium Isotopic Signatures of Nanometer-Sized Smectite-Rich Mixed-Layers of Bentonite Beds From Campos Basin (Brazil)
-
- Journal:
- Clays and Clay Minerals / Volume 70 / Issue 1 / February 2022
- Published online by Cambridge University Press:
- 01 January 2024, pp. 72-83
-
- Article
- Export citation
A Molality-Based BET Equation for Modeling the Activity of Water Sorbed on Clay Minerals
-
- Journal:
- Clays and Clay Minerals / Volume 60 / Issue 6 / December 2012
- Published online by Cambridge University Press:
- 01 January 2024, pp. 599-609
-
- Article
- Export citation
A critical examination of safety culture in the superyacht industry
-
- Journal:
- The Journal of Navigation / Volume 77 / Issue 1 / January 2024
- Published online by Cambridge University Press:
- 13 September 2024, pp. 100-115
- Print publication:
- January 2024
-
- Article
- Export citation
Arsenic Sorption onto Soils Enriched in Mn and Fe Minerals
-
- Journal:
- Clays and Clay Minerals / Volume 51 / Issue 2 / April 2003
- Published online by Cambridge University Press:
- 01 January 2024, pp. 197-204
-
- Article
- Export citation
Significance of phyllosilicate mineralogy and mineral chemistry in an epithermal environment. Insights from the palai-islica Au-Cu deposit (Almería, SE Spain)
-
- Journal:
- Clays and Clay Minerals / Volume 57 / Issue 1 / February 2009
- Published online by Cambridge University Press:
- 01 January 2024, pp. 1-24
-
- Article
- Export citation
Forthcoming Papers
-
- Journal:
- Clays and Clay Minerals / Volume 52 / Issue 1 / February 2004
- Published online by Cambridge University Press:
- 01 January 2024, p. 141
-
- Article
-
- You have access
- Export citation
An Endless Capacity for Dissembling: Representing Teenage Girls on the American Stage from The Children's Hour through If Pretty Hurts Ugly Must Be a Muhfucka
-
- Journal:
- Theatre Survey / Volume 65 / Issue 1 / January 2024
- Published online by Cambridge University Press:
- 18 March 2024, pp. 37-59
- Print publication:
- January 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The Sharing of the Profits of the Carrera de Indias: The Actors of the Hispanic Colonial Trade and Their Monopolistic Practices in the Second Half of the Eighteenth Century
-
- Journal:
- The Americas / Volume 81 / Issue 1 / January 2024
- Published online by Cambridge University Press:
- 09 January 2024, pp. 39-66
- Print publication:
- January 2024
-
- Article
- Export citation
ARTWORK: ED COOPER
-
- Article
- Export citation
Sorption and Direct Electrochemistry of Mitochondrial Cytochrome C on Hematite Surfaces
-
- Journal:
- Clays and Clay Minerals / Volume 53 / Issue 6 / December 2005
- Published online by Cambridge University Press:
- 01 January 2024, pp. 564-571
-
- Article
- Export citation
Powder X-Ray Diffraction Study of the Hydration and Leaching Behavior of Nontronite
-
- Journal:
- Clays and Clay Minerals / Volume 59 / Issue 6 / December 2011
- Published online by Cambridge University Press:
- 01 January 2024, pp. 560-567
-
- Article
- Export citation
Origin of Saponite-Rich Clays in A Fossil Serpentinite-Hosted Hydrothermal System in The Crustal Basement of The Hyblean Plateau (Sicily, Italy)
-
- Journal:
- Clays and Clay Minerals / Volume 60 / Issue 1 / February 2012
- Published online by Cambridge University Press:
- 01 January 2024, pp. 18-31
-
- Article
- Export citation
Measurement of clay surface areas by polyvinylpyrrolidone (PVP) sorption and its use for quantifying illite and smectite abundance
-
- Journal:
- Clays and Clay Minerals / Volume 52 / Issue 5 / October 2004
- Published online by Cambridge University Press:
- 01 January 2024, pp. 589-602
-
- Article
- Export citation
AJL volume 14 issue 1 Cover and Front matter
-
- Journal:
- Asian Journal of International Law / Volume 14 / Issue 1 / January 2024
- Published online by Cambridge University Press:
- 23 January 2024, pp. f1-f7
- Print publication:
- January 2024
-
- Article
-
- You have access
- Export citation