Refine search
Actions for selected content:
106102 results in Materials Science
Editorial
-
- Journal:
- MRS Communications / Volume 1 / Issue 1 / November 2011
- Published online by Cambridge University Press:
- 25 October 2011, pp. 1-2
- Print publication:
- November 2011
-
- Article
-
- You have access
- HTML
- Export citation
Elastic anisotropy of Cu and its impact on stress management for 3D IC: Nanoindentation and TCAD simulation study
-
- Journal:
- Journal of Materials Research / Volume 27 / Issue 1 / 14 January 2012
- Published online by Cambridge University Press:
- 21 October 2011, pp. 339-348
- Print publication:
- 14 January 2012
-
- Article
- Export citation
Quantifying plasticity-independent creep compliance and relaxation of viscoelastoplastic materials under contact loading
-
- Journal:
- Journal of Materials Research / Volume 27 / Issue 1 / 14 January 2012
- Published online by Cambridge University Press:
- 21 October 2011, pp. 302-312
- Print publication:
- 14 January 2012
-
- Article
- Export citation
Extended JKR theory on adhesive contact of a spherical tip onto a film on a substrate
-
- Journal:
- Journal of Materials Research / Volume 27 / Issue 1 / 14 January 2012
- Published online by Cambridge University Press:
- 21 October 2011, pp. 113-120
- Print publication:
- 14 January 2012
-
- Article
- Export citation
Synthesis and photoluminescence properties of perovskite KMgF3:Eu nanocubes
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 22 / 28 November 2011
- Published online by Cambridge University Press:
- 21 October 2011, pp. 2867-2870
- Print publication:
- 28 November 2011
-
- Article
- Export citation
6 - Elasticity
- from Part II - Mechanics
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 167-207
-
- Chapter
- Export citation
3 - Ceramics
- from Part I - Materials
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 70-91
-
- Chapter
- Export citation
Index
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 645-681
-
- Chapter
- Export citation
16 - Soft tissue replacements
- from Part III - Case studies
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 560-594
-
- Chapter
- Export citation
Prologue
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp xiv-xvi
-
- Chapter
- Export citation
7 - Viscoelasticity
- from Part II - Mechanics
-
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 208-240
-
- Chapter
- Export citation
JMR volume 26 issue 20 Cover and Back matter
-
- Journal:
- Journal of Materials Research / Volume 26 / Issue 20 / 28 October 2011
- Published online by Cambridge University Press:
- 20 October 2011, pp. b1-b5
- Print publication:
- 28 October 2011
-
- Article
-
- You have access
- Export citation
Contents
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp v-x
-
- Chapter
- Export citation
11 - Friction, lubrication, and wear
- from Part II - Mechanics
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 369-394
-
- Chapter
- Export citation
Glossary
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 620-644
-
- Chapter
- Export citation
Frontmatter
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp i-iv
-
- Chapter
- Export citation
2 - Metals for medical implants
- from Part I - Materials
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 26-69
-
- Chapter
- Export citation
Epilogue
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 595-596
-
- Chapter
- Export citation
5 - Mechanical behavior of structural tissues
- from Part I - Materials
-
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 129-164
-
- Chapter
- Export citation
Part I - Materials
-
- Book:
- Mechanics of Biomaterials
- Published online:
- 05 June 2012
- Print publication:
- 20 October 2011, pp 1-2
-
- Chapter
- Export citation


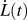 can be measured independently of any plastic deformation exhibited during loading through
can be measured independently of any plastic deformation exhibited during loading through 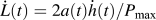 , where
, where 

