Refine listing
Actions for selected content:
1418155 results in Open Access
P73: Clinical Psychology of Ageing: The Italian Manifesto
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 161-162
-
- Article
-
- You have access
- Export citation
P60: Development of a participant-driven dementia learning program by people living with dementia
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 243-244
-
- Article
-
- You have access
- Export citation
Co-Designing Dementia Diagnosis And Post Diagnostic Care, The Cognisance Project: Forward with Dementia (FWD)
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 28-29
-
- Article
-
- You have access
- Export citation
P10: Feasibility of a Longitudinal Audiovisual Observation Protocol to Characterize EL in Advanced AD/ADRD
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 110
-
- Article
-
- You have access
- Export citation
P91: Symptoms of Anxiety and Depression after stroke – a follow up study in outpatients followed in a rehabilitation recovery unit
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 248-249
-
- Article
-
- You have access
- Export citation
P187: A patient with early-onset Alzheimer’s disease presenting with a unique form of Capgras syndrome
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 143-144
-
- Article
-
- You have access
- Export citation
P137: Cost Considerations of Untreated Agitation: Direct, Indirect, and Intangible
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 215
-
- Article
-
- You have access
- Export citation
P32: Effects of vitamin D3 and marine omega-3 fatty acids supplementation on indicated and selective prevention of depression in older adults: results from the clinical center sub-cohort of the VITamin D and OmegA-3 TriaL
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 152-153
-
- Article
-
- You have access
- Export citation
P70: Development and validation study of the suicide screening questionnaire-observer rating (SSQ-OR)
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 161
-
- Article
-
- You have access
- Export citation
Streamwise dispersion of soluble matter in solvent flowing through a tube
-
- Journal:
- Journal of Fluid Mechanics / Volume 980 / 10 February 2024
- Published online by Cambridge University Press:
- 02 February 2024, A33
-
- Article
- Export citation
QUA volume 117 Cover and Front matter
-
- Journal:
- Quaternary Research / Volume 117 / January 2024
- Published online by Cambridge University Press:
- 02 February 2024, pp. f1-f4
-
- Article
-
- You have access
- Export citation
P202: Post-COVID syndrome presented with psychomotor change and suicidal ideations: a geriatric case report
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 229-230
-
- Article
-
- You have access
- Export citation
P88: Individuals with Mild Cognitive Impairment (MCI) have poorer social networks than cognitively normal individuals from rural India
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 165-166
-
- Article
-
- You have access
- Export citation
S14: The use of advanced data and sensortechnology in dementia: innovation and implementation
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 43-44
-
- Article
-
- You have access
- Export citation
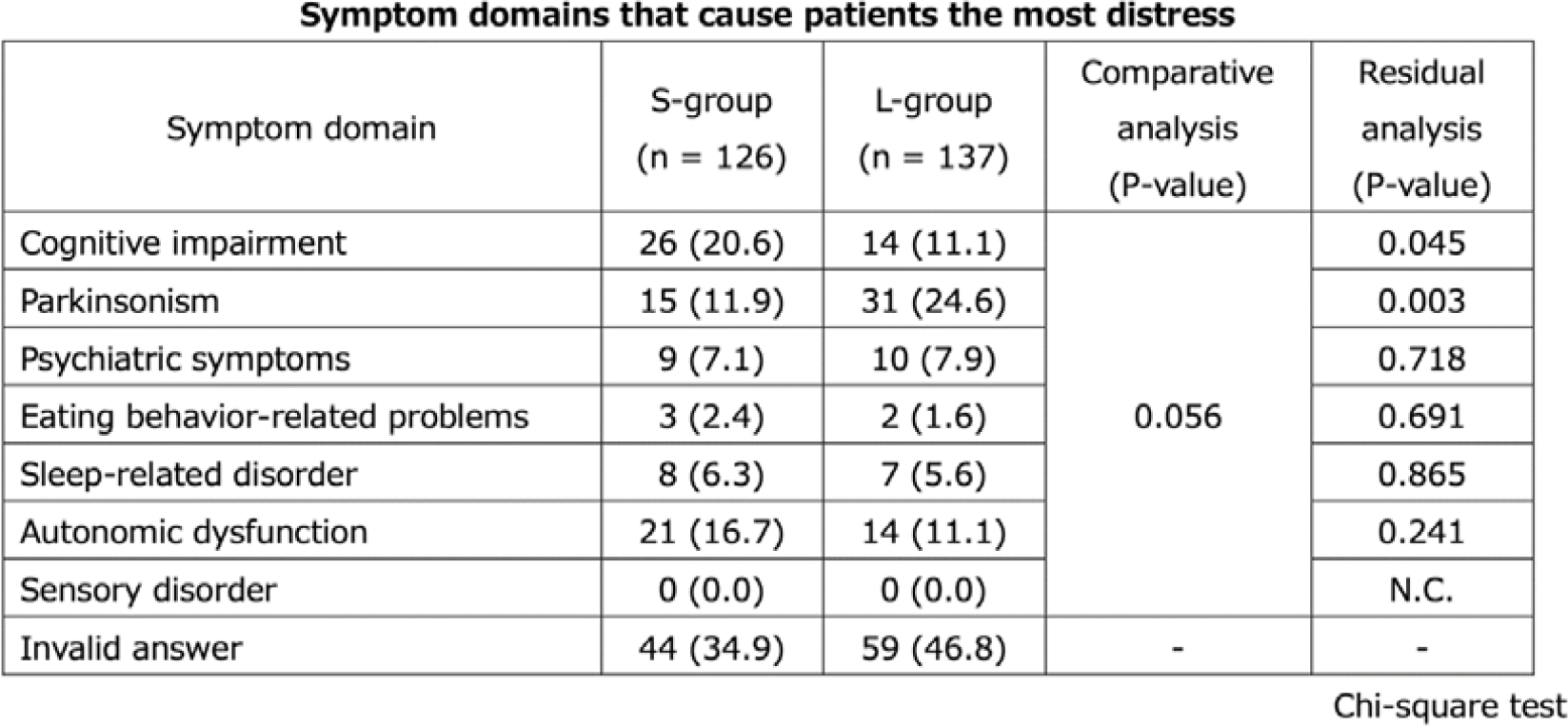
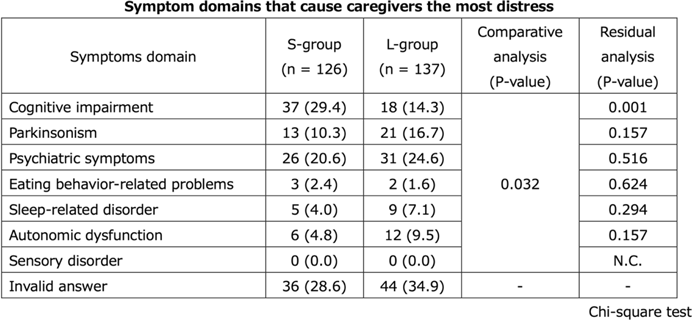
P122: Differences of the treatment needs of patients with dementia with Lewy bodies and their caregivers with duration after diagnosis
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 173-175
-
- Article
-
- You have access
- Export citation
Research agenda for antibiotic stewardship within the Veterans’ Health Administration, 2024–2028
- Part of
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 45 / Issue 8 / August 2024
- Published online by Cambridge University Press:
- 02 February 2024, pp. 923-929
- Print publication:
- August 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Plenary Session 4: Disease-Modified Drug
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, p. 14
-
- Article
-
- You have access
- Export citation
FC34: Cognitive reserve and depressive burden in older adults: variation according to reserve measurement
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 97-98
-
- Article
-
- You have access
- Export citation
P161: Heterogeneity and Clinical Uncertainty of BPSD Therapeutics
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 221-222
-
- Article
-
- You have access
- Export citation
P157: Stigma of anxiety and depression: a comparison between older and younger adults.
-
- Journal:
- International Psychogeriatrics / Volume 35 / Issue S1 / December 2023
- Published online by Cambridge University Press:
- 02 February 2024, pp. 219-220
-
- Article
-
- You have access
- Export citation