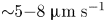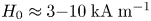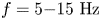Refine listing
Actions for selected content:
1418609 results in Open Access
Anna Maria van Schurman. Letters and Poems to and from Her Mentor and Other Members of Her Circle. Edited and translated by Anne R. Larsen and Steve Maiullo. The Other Voice in Early Modern Europe. New York: Iter Press, 2021. Pp. 402. $65.95 (paper).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1081-1082
-
- Article
- Export citation
The End of the COVID-19 Pandemic in Mexico: Omicron Sublineages BQ.1 and XBB Trigger the Sixth Wave of Infections
-
- Journal:
- Disaster Medicine and Public Health Preparedness / Volume 18 / 2024
- Published online by Cambridge University Press:
- 19 February 2024, e26
-
- Article
-
- You have access
- HTML
- Export citation
(Re)negotiating State Authority: How Hinterland Protests against Global Capital Impact the Mediating Role of Traditional Rulers in Postcolonial Sierra Leone
-
- Journal:
- African Studies Review / Volume 67 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 19 February 2024, pp. 107-128
-
- Article
- Export citation
Kevin Hickson, ed. Neil Kinnock: Saving the Labour Party? Routledge Studies in British Politics. Abingdon: Routledge, 2022. Pp. 288. $273.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1097-1100
-
- Article
- Export citation
Yield improvement with antitranspirant application in droughted wheat associated with both reduced transpiration and reduced abscisic acid
-
- Journal:
- The Journal of Agricultural Science / Volume 162 / Issue 1 / February 2024
- Published online by Cambridge University Press:
- 19 February 2024, pp. 33-45
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Jatinder Mann and Iain Johnston-White, eds. Revisiting the British World: New Voices and Perspectives. Studies in Transnationalism Series 5. New York: Peter Lang, 2022. Pp. 276. $94.95 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1112-1114
-
- Article
- Export citation
Jack Crangle. Migrants, Immigration and Diversity in Twentieth-Century Northern Ireland: British, Irish or “Other”? Palgrave Studies in Migration History. Cham: Palgrave Macmillan, 2023. Pp. 283. $149.49 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1088-1089
-
- Article
- Export citation
Steffan Blayney. Health and Efficiency: Fatigue, the Science of Work, and the Making of the Working-Class Body. Activist Studies of Science & Technology. Amherst: University of Massachusetts Press, 2022. Pp. 248. $90.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1085-1086
-
- Article
- Export citation
Henrietta Harrison. The Perils of Interpreting: The Extraordinary Lives of Two Translators between Qing China and the British Empire. Princeton University Press, 2021, Pp. 312. $32.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1057-1059
-
- Article
- Export citation
Abortion, Infanticide, and Choosing Parenthood
-
- Journal:
- Dialogue: Canadian Philosophical Review / Revue canadienne de philosophie / Volume 64 / Issue 2 / August 2025
- Published online by Cambridge University Press:
- 19 February 2024, pp. 285-310
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Alistair Kefford. The Life and Death of the Shopping City: Public Planning and Private Redevelopment in Britain since 1945. Modern British Histories. Cambridge: Cambridge University Press, 2022. Pp. 340. $120.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1105-1106
-
- Article
- Export citation
Danish rundt ‘around’ as a postposition?
-
- Journal:
- Canadian Journal of Linguistics/Revue canadienne de linguistique / Volume 69 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 19 February 2024, pp. 90-98
-
- Article
-
- You have access
- HTML
- Export citation
Neal Shasore. Designs on Democracy: Architecture and the Public in Interwar London. Oxford Historical Monographs. Oxford: Oxford University Press, 2022. Pp. 464. $85.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1116-1117
-
- Article
- Export citation
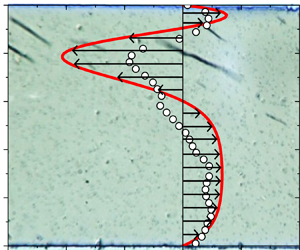
Field-induced macroscopic flow of a dilute self-assembling magnetic colloid under rotating magnetic fields
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 19 February 2024, A11
-
- Article
- Export citation
Caroline M. Barron and Laura Wright, eds. The London Jubilee Book, 1376–1387: An Edition of Trinity College Cambridge MS O.3.11, folios 133–157. London Record Society 55 Woodbridge: Boydell Press, 2021. Pp. 150. $70.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1045-1046
-
- Article
- Export citation
Charlotte Berry. The Margins of Late Medieval London, 1430–1540. New Historical Perspectives. London: Royal Historical Society, Institute of Historical Research, University of London Press, 2022. Pp. 350. $55.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1046-1048
-
- Article
- Export citation
You Can't Spell Opinion without I: Toward a Hegelian Critical Theory of Opinion
-
- Journal:
- Hegel Bulletin , First View
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1-27
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Lindy Brady, ed. Old English Tradition: Essays in Honor of J. R. Hall. Medieval and Renaissance Texts and Studies 578. Tempe: Arizona Center for Medieval and Renaissance Studies, 2021. Pp. 356. $90.00 (paper).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1048-1049
-
- Article
- Export citation
Contemporary archaeological perspectives on intersectional inequality in a welfare state in twentieth-century Finland
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Chris R. Langley, Catherine E. McMillan, and Russell Newton, eds. The Clergy in Early Modern Scotland. St Andrews Studies in Scottish History. Woodbridge: Boydell Press, 2021. Pp. 271. $99.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1064-1065
-
- Article
- Export citation