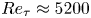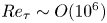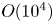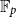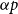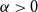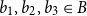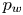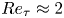Refine listing
Actions for selected content:
1418748 results in Open Access
Partnership with the University of São Paulo Panel of Twins: A Four-City Tour and More / Twin Research Reviews: Twin Research on Binge Eating; Twins’ Physical Outcomes Linked to Different Diets; Working Conditions and Sickness Absence in Swedish Twins; Facial Morphology Differences in Monozygotic Twins / Human Interest and Importance: Michigan Family Forced to Adopt Their Own Twins; Ethics of Hiring a Surrogate to Bear Twins; Twin Survivors of the Israel-Hamas War; Twin Pregnancy with Double Uterus; Three Twin Pairs on Same Women’s Soccer Team
-
- Journal:
- Twin Research and Human Genetics / Volume 27 / Issue 1 / February 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 64-68
-
- Article
-
- You have access
- HTML
- Export citation
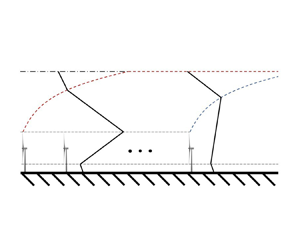
A shear stress parametrization for arbitrary wind farms in conventionally neutral boundary layers
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 21 February 2024, A14
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Robust relation of streamwise velocity autocorrelation in atmospheric surface layers based on an autoregressive moving average model
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 21 February 2024, A20
-
- Article
- Export citation
Small subsets with large sumset: Beyond the Cauchy–Davenport bound
- Part of
-
- Journal:
- Combinatorics, Probability and Computing / Volume 33 / Issue 4 / July 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 411-431
-
- Article
- Export citation
Braj Bhūm in Mughal Times: The State, Peasants and Gosā’ins By Irfan Habib and Tarapada Mukherjee (late). 286 pp. New Delhi, Primus Books, 2020.
-
- Journal:
- Journal of the Royal Asiatic Society / Volume 34 / Issue 2 / April 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 486-488
- Print publication:
- April 2024
-
- Article
- Export citation
Early prediction of mastery of a computerized functional skills training program in participants with mild cognitive impairment
-
- Journal:
- International Psychogeriatrics / Volume 36 / Issue 12 / December 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 1182-1193
-
- Article
- Export citation
Economic Uncertainty and Divisive Politics: Evidence from the dos Españas
-
- Journal:
- The Journal of Economic History / Volume 84 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 40-73
- Print publication:
- March 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Reynolds-number scaling of wall-pressure–velocity correlations in wall-bounded turbulence
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 21 February 2024, A15
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Human Beings and Ethics in the Thought of Herbert McCabe
-
- Journal:
- New Blackfriars / Volume 105 / Issue 3 / May 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 294-308
- Print publication:
- May 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Impacts of the Relocation Program on Native American Migration and Fertility
-
- Journal:
- The Journal of Economic History / Volume 84 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 74-110
- Print publication:
- March 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Allan W. Atlas, ed., A Wilkie Collins Songbook (Middleton, WI: A-R Editions, 2023), xix + 180.
-
- Journal:
- Nineteenth-Century Music Review / Volume 21 / Issue 3 / December 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 601-604
-
- Article
- Export citation
Sympatric occurrence of Taenia saginata and Sarcocystis spp. in cattle from Narok County, Kenya: meat inspection findings with molecular validation
-
- Journal:
- Journal of Helminthology / Volume 98 / 2024
- Published online by Cambridge University Press:
- 21 February 2024, e20
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
‘Sovereignty is still the name of the game’: Indigenous theorising and strategic entanglement in Māori political discourses
-
- Journal:
- Review of International Studies / Volume 51 / Issue 1 / January 2025
- Published online by Cambridge University Press:
- 21 February 2024, pp. 22-41
- Print publication:
- January 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Knut Christian Myhre. Returning Life: Language, Life Force and History in Kilimanjaro. Oxford: Berghahn, 2023. xvii + 319 pp. Map. Photographs. Bibliography. Index. £27.95. Paper. ISBN: 978-1-80073-947-5.
-
- Journal:
- African Studies Review / Volume 67 / Issue 2 / June 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 505-507
-
- Article
-
- You have access
- HTML
- Export citation
Bringing Dance to Older Adults: Program Experts’ Perspectives on the Role of Community Dance Classes to Support Older Adults
-
- Journal:
- Canadian Journal on Aging / La Revue canadienne du vieillissement / Volume 43 / Issue 4 / December 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 482-490
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Sovereign Collateral
-
- Journal:
- The Journal of Economic History / Volume 84 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 191-231
- Print publication:
- March 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Alteration in physio-chemical properties and gene expression pattern of snapmelon (Cucumis melo var. momordica) genotypes against drought stress
-
- Journal:
- Plant Genetic Resources / Volume 22 / Issue 2 / April 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 87-96
-
- Article
- Export citation
Financial Innovation and Resilience: A Comparative Perspective on the Public Banks of Naples (1462–1808). Edited by Lilia Costabile and Larry Neal. Cham: Palgrave Macmillan, 2018. Pp. xix, 372. €155.99, hardcover; €93.08, eBook.
-
- Journal:
- The Journal of Economic History / Volume 84 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 315-317
- Print publication:
- March 2024
-
- Article
- Export citation