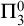Refine listing
Actions for selected content:
1419087 results in Open Access
Linear stability analysis of oblique Couette–Poiseuille flows
-
- Journal:
- Journal of Fluid Mechanics / Volume 998 / 10 November 2024
- Published online by Cambridge University Press:
- 29 October 2024, A25
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Morphological differences yet genetic similarity among seasonal cohorts of Japanese anchovy during the early life stages in the Kii Channel
-
- Journal:
- Journal of the Marine Biological Association of the United Kingdom / Volume 104 / 2024
- Published online by Cambridge University Press:
- 29 October 2024, e93
-
- Article
- Export citation
Evaluation of a probiotic blend on psychosocial health and biomarkers of inflammatory, immune and stress response in adults with subthreshold depression: a double-blind, randomised, placebo-controlled trial
-
- Journal:
- British Journal of Nutrition , First View
- Published online by Cambridge University Press:
- 29 October 2024, pp. 1-15
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Is Schistosoma mansoni playing a part in liver carcinogenesis?
-
- Journal:
- Journal of Helminthology / Volume 98 / 2024
- Published online by Cambridge University Press:
- 29 October 2024, e61
-
- Article
- Export citation
A RECURSIVE COLORING FUNCTION WITHOUT
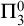 $ \Pi _3^0$ SOLUTIONS FOR HINDMAN’S THEOREM
$ \Pi _3^0$ SOLUTIONS FOR HINDMAN’S THEOREM
- Part of
-
- Journal:
- The Journal of Symbolic Logic , First View
- Published online by Cambridge University Press:
- 29 October 2024, pp. 1-24
-
- Article
- Export citation
Spatial distribution of northern shrimp Pandalus eous in the Sea of Japan in relation to the seafloor environment
-
- Journal:
- Journal of the Marine Biological Association of the United Kingdom / Volume 104 / 2024
- Published online by Cambridge University Press:
- 29 October 2024, e98
-
- Article
- Export citation
Jasper Miles. The Labour Party and Electoral Reform London: Bloomsbury Academic, 2023. Pp. 248. $115.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 63 / Issue 2 / April 2024
- Published online by Cambridge University Press:
- 29 October 2024, pp. 502-504
-
- Article
- Export citation
New discovery of two subtropical and one boreal marine fish species in Korean waters during summer reveals their habitat range expansion
-
- Journal:
- Journal of the Marine Biological Association of the United Kingdom / Volume 104 / 2024
- Published online by Cambridge University Press:
- 29 October 2024, e95
-
- Article
- Export citation
Seasonal changes of community structure and vertical distribution/migration for mesopelagic fish in the western subarctic Pacific
-
- Journal:
- Journal of the Marine Biological Association of the United Kingdom / Volume 104 / 2024
- Published online by Cambridge University Press:
- 29 October 2024, e91
-
- Article
- Export citation
Michael Schedelik, The Political Economy of Upgrading Regimes: Brazil and Beyond, Cham: Palgrave Macmillan, 2023. Figures, tables, bibliography, index, 297 pp.; hardcover $139.99, paperback $139.99, eBook 109
-
- Journal:
- Latin American Politics and Society , FirstView
- Published online by Cambridge University Press:
- 29 October 2024, pp. 1-3
-
- Article
- Export citation
Cassiopea andromeda (Cnidaria, Scyphozoa) in the subtropical eastern Atlantic
-
- Journal:
- Journal of the Marine Biological Association of the United Kingdom / Volume 104 / 2024
- Published online by Cambridge University Press:
- 29 October 2024, e92
-
- Article
- Export citation
The management of non-tuberculous mycobacteria cervicofacial lymphadenitis: a survey of UK paediatric tertiary hospitals
-
- Journal:
- The Journal of Laryngology & Otology / Volume 139 / Issue 5 / May 2025
- Published online by Cambridge University Press:
- 29 October 2024, pp. 368-372
- Print publication:
- May 2025
-
- Article
- Export citation
Low glycaemic index diet in pregnancy and child asthma: follow-up of the ROLO trial
-
- Journal:
- British Journal of Nutrition , First View
- Published online by Cambridge University Press:
- 28 October 2024, pp. 1-11
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Escalating Health Crisis: Dissecting Mortality Causes and Trends Among Indigenous Populations in Northeast Brazil
-
- Journal:
- Disaster Medicine and Public Health Preparedness / Volume 18 / 2024
- Published online by Cambridge University Press:
- 28 October 2024, e228
-
- Article
- Export citation
The Nusselt number of a hot sphere levitated by a volatile pool
-
- Journal:
- Journal of Fluid Mechanics / Volume 998 / 10 November 2024
- Published online by Cambridge University Press:
- 28 October 2024, A22
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The Election of an ‘Outsider’ Archbishop
-
- Journal:
- Journal of Anglican Studies / Volume 23 / Issue 2 / November 2025
- Published online by Cambridge University Press:
- 28 October 2024, pp. 317-332
-
- Article
- Export citation
Indicting the Athenians in the Melian dialogue
-
- Journal:
- The Journal of Hellenic Studies / Volume 144 / November 2024
- Published online by Cambridge University Press:
- 28 October 2024, pp. 164-181
- Print publication:
- November 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Scaling laws for Rayleigh–Bénard convection between Navier-slip boundaries
-
- Journal:
- Journal of Fluid Mechanics / Volume 998 / 10 November 2024
- Published online by Cambridge University Press:
- 28 October 2024, A24
-
- Article
-
- You have access
- Open access
- HTML
- Export citation