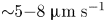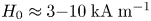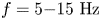Refine listing
Actions for selected content:
1418748 results in Open Access
The End of the COVID-19 Pandemic in Mexico: Omicron Sublineages BQ.1 and XBB Trigger the Sixth Wave of Infections
-
- Journal:
- Disaster Medicine and Public Health Preparedness / Volume 18 / 2024
- Published online by Cambridge University Press:
- 19 February 2024, e26
-
- Article
-
- You have access
- HTML
- Export citation
(Re)negotiating State Authority: How Hinterland Protests against Global Capital Impact the Mediating Role of Traditional Rulers in Postcolonial Sierra Leone
-
- Journal:
- African Studies Review / Volume 67 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 19 February 2024, pp. 107-128
-
- Article
- Export citation
Kevin Hickson, ed. Neil Kinnock: Saving the Labour Party? Routledge Studies in British Politics. Abingdon: Routledge, 2022. Pp. 288. $273.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1097-1100
-
- Article
- Export citation
Yield improvement with antitranspirant application in droughted wheat associated with both reduced transpiration and reduced abscisic acid
-
- Journal:
- The Journal of Agricultural Science / Volume 162 / Issue 1 / February 2024
- Published online by Cambridge University Press:
- 19 February 2024, pp. 33-45
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Jatinder Mann and Iain Johnston-White, eds. Revisiting the British World: New Voices and Perspectives. Studies in Transnationalism Series 5. New York: Peter Lang, 2022. Pp. 276. $94.95 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1112-1114
-
- Article
- Export citation
Jack Crangle. Migrants, Immigration and Diversity in Twentieth-Century Northern Ireland: British, Irish or “Other”? Palgrave Studies in Migration History. Cham: Palgrave Macmillan, 2023. Pp. 283. $149.49 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1088-1089
-
- Article
- Export citation
Steffan Blayney. Health and Efficiency: Fatigue, the Science of Work, and the Making of the Working-Class Body. Activist Studies of Science & Technology. Amherst: University of Massachusetts Press, 2022. Pp. 248. $90.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1085-1086
-
- Article
- Export citation
Henrietta Harrison. The Perils of Interpreting: The Extraordinary Lives of Two Translators between Qing China and the British Empire. Princeton University Press, 2021, Pp. 312. $32.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1057-1059
-
- Article
- Export citation
Abortion, Infanticide, and Choosing Parenthood
-
- Journal:
- Dialogue: Canadian Philosophical Review / Revue canadienne de philosophie / Volume 64 / Issue 2 / August 2025
- Published online by Cambridge University Press:
- 19 February 2024, pp. 285-310
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Alistair Kefford. The Life and Death of the Shopping City: Public Planning and Private Redevelopment in Britain since 1945. Modern British Histories. Cambridge: Cambridge University Press, 2022. Pp. 340. $120.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1105-1106
-
- Article
- Export citation
Danish rundt ‘around’ as a postposition?
-
- Journal:
- Canadian Journal of Linguistics/Revue canadienne de linguistique / Volume 69 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 19 February 2024, pp. 90-98
-
- Article
-
- You have access
- HTML
- Export citation
Neal Shasore. Designs on Democracy: Architecture and the Public in Interwar London. Oxford Historical Monographs. Oxford: Oxford University Press, 2022. Pp. 464. $85.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1116-1117
-
- Article
- Export citation
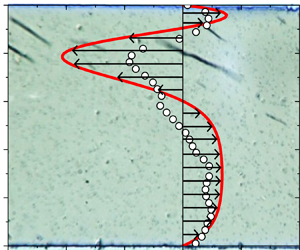
Field-induced macroscopic flow of a dilute self-assembling magnetic colloid under rotating magnetic fields
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 19 February 2024, A11
-
- Article
- Export citation
Caroline M. Barron and Laura Wright, eds. The London Jubilee Book, 1376–1387: An Edition of Trinity College Cambridge MS O.3.11, folios 133–157. London Record Society 55 Woodbridge: Boydell Press, 2021. Pp. 150. $70.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1045-1046
-
- Article
- Export citation
Charlotte Berry. The Margins of Late Medieval London, 1430–1540. New Historical Perspectives. London: Royal Historical Society, Institute of Historical Research, University of London Press, 2022. Pp. 350. $55.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1046-1048
-
- Article
- Export citation
You Can't Spell Opinion without I: Toward a Hegelian Critical Theory of Opinion
-
- Journal:
- Hegel Bulletin , First View
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1-27
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Lindy Brady, ed. Old English Tradition: Essays in Honor of J. R. Hall. Medieval and Renaissance Texts and Studies 578. Tempe: Arizona Center for Medieval and Renaissance Studies, 2021. Pp. 356. $90.00 (paper).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1048-1049
-
- Article
- Export citation
Contemporary archaeological perspectives on intersectional inequality in a welfare state in twentieth-century Finland
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Chris R. Langley, Catherine E. McMillan, and Russell Newton, eds. The Clergy in Early Modern Scotland. St Andrews Studies in Scottish History. Woodbridge: Boydell Press, 2021. Pp. 271. $99.00 (cloth).
-
- Journal:
- Journal of British Studies / Volume 62 / Issue 4 / October 2023
- Published online by Cambridge University Press:
- 19 February 2024, pp. 1064-1065
-
- Article
- Export citation
Exploiting phenotypic and genotypic diversity against Colletotrichum truncatum in chilli hybrids developed using resistant breeding lines – CORRIGENDUM
-
- Journal:
- Plant Genetic Resources / Volume 22 / Issue 2 / April 2024
- Published online by Cambridge University Press:
- 19 February 2024, p. 131
-
- Article
-
- You have access
- HTML
- Export citation