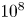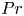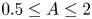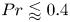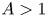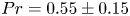Refine listing
Actions for selected content:
1418740 results in Open Access
Antibiotic consumption in French nursing homes between 2018 and 2022: A multicenter survey
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 45 / Issue 6 / June 2024
- Published online by Cambridge University Press:
- 19 February 2024, pp. 740-745
- Print publication:
- June 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Constructions of Racial Savagery in Early Twentieth-Century US Narratives of White Civilization
-
- Journal:
- Journal of American Studies / Volume 58 / Issue 2 / May 2024
- Published online by Cambridge University Press:
- 19 February 2024, pp. 193-219
- Print publication:
- May 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
‘Best paper’ prize winners for 2023 and in the current issue: entry to UK ENT specialist training
-
- Journal:
- The Journal of Laryngology & Otology / Volume 138 / Issue 3 / March 2024
- Published online by Cambridge University Press:
- 16 February 2024, p. 237
- Print publication:
- March 2024
-
- Article
-
- You have access
- HTML
- Export citation
PERFECTING GROUP SCHEMES
- Part of
-
- Journal:
- Journal of the Institute of Mathematics of Jussieu / Volume 23 / Issue 6 / November 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 2549-2591
- Print publication:
- November 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Academic Solidarity in the Wake of Disaster: Blueprint for an Online Writing Support Group
-
- Journal:
- PS: Political Science & Politics / Volume 57 / Issue 3 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 370-377
- Print publication:
- July 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Comparative Studies of Russian and European Welfare Polities – CORRIGENDUM
-
- Journal:
- Social Policy and Society / Volume 23 / Issue 3 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, p. 803
- Print publication:
- July 2024
-
- Article
-
- You have access
- HTML
- Export citation
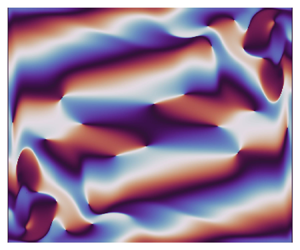
Vertical convection regimes in a two-dimensional rectangular cavity: Prandtl and aspect ratio dependence
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 16 February 2024, A10
-
- Article
- Export citation
Causal Agnosticism About Race: Variable Selection Problems in Causal Inference
-
- Journal:
- Philosophy of Science / Volume 91 / Issue 5 / December 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 1098-1108
- Print publication:
- December 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Paolo Galluzzi, The Italian Renaissance of Machines Cambridge, MA: Harvard University Press, 2020. Pp. 296. ISBN 978-0-674-98439-4. £37.95 (hardcover).
-
- Journal:
- The British Journal for the History of Science / Volume 57 / Issue 2 / June 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 314-316
- Print publication:
- June 2024
-
- Article
- Export citation
Reburials of Eminent Masters: The Construction of Quanzhen Daoist Lineages in North China under Mongol Rule
-
- Journal:
- Journal of Chinese History / Volume 8 / Issue 2 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 297-326
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Rising and settling 2-D cylinders with centre-of-mass offset
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 16 February 2024, A7
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The Tang legacy on the Silk Road during the Uighur era: urbanisation in the eastern Tianshan region during the ninth to thirteenth centuries
-
- Journal:
- Journal of the Royal Asiatic Society / Volume 34 / Issue 2 / April 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 377-398
- Print publication:
- April 2024
-
- Article
- Export citation
Discursive scaling of solidarity through difference: Experiences of African women in the African diaspora
-
- Journal:
- Language in Society / Volume 54 / Issue 3 / June 2025
- Published online by Cambridge University Press:
- 16 February 2024, pp. 491-513
- Print publication:
- June 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Trilobites from the Al Rose Formation (Lower Ordovician, Inyo Mountains, California)—faunas marginal to the Great Basin
-
- Journal:
- Journal of Paleontology / Volume 98 / Issue 4 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 644-657
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
A single-institutional experience with 36 children less than 5 kilograms supported with the Berlin Heart: Comparison of congenital versus acquired heart disease
-
- Journal:
- Cardiology in the Young / Volume 34 / Issue 6 / June 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 1342-1349
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Effects of the COVID-19 Pandemic on Submissions to Politics & Gender
-
- Journal:
- PS: Political Science & Politics / Volume 57 / Issue 3 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 420-426
- Print publication:
- July 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
By Any Means Necessary? How Black and White Americans Evaluate Protest Tactics in Response to a Police Killing
-
- Journal:
- Journal of Race, Ethnicity and Politics / Volume 9 / Issue 2 / July 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 235-258
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Holocene relative sea-level changes along the Caribbean and Pacific coasts of northwestern South America
-
- Journal:
- Quaternary Research / Volume 119 / May 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 28-43
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
BJN volume 131 issue 6 Cover and Front matter
-
- Journal:
- British Journal of Nutrition / Volume 131 / Issue 6 / 28 March 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. f1-f2
- Print publication:
- 28 March 2024
-
- Article
-
- You have access
- Export citation
Association between dietary macronutrient composition and plasma one-carbon metabolites and B-vitamin cofactors in patients with stable angina pectoris
-
- Journal:
- British Journal of Nutrition / Volume 131 / Issue 10 / 28 May 2024
- Published online by Cambridge University Press:
- 16 February 2024, pp. 1678-1690
- Print publication:
- 28 May 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation