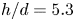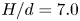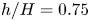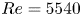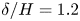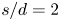Refine listing
Actions for selected content:
1418526 results in Open Access
THE STRONG AND SUPER TREE PROPERTIES AT SUCCESSORS OF SINGULAR CARDINALS
- Part of
-
- Journal:
- The Journal of Symbolic Logic / Volume 89 / Issue 3 / September 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 1251-1283
- Print publication:
- September 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
ESPIONAGE LAW IN THE UK AND AUSTRALIA: BALANCING EFFECTIVENESS AND APPROPRIATENESS
-
- Journal:
- The Cambridge Law Journal / Volume 83 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 62-98
- Print publication:
- March 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
A method to calculate inverse solutions for steady open channel free-surface flow
-
- Journal:
- Journal of Fluid Mechanics / Volume 977 / 25 December 2023
- Published online by Cambridge University Press:
- 22 December 2023, A46
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Sounds in context: Archaeoacoustical studies of instruments from Comalcalco and Jonuta, pre-Hispanic Maya sites
-
- Journal:
- Ancient Mesoamerica / Volume 35 / Issue 2 / Summer 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 472-493
- Print publication:
- Summer 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Experiences of Operating Room Professionals During the 2020 Izmir Earthquake: A Qualitative Approach
-
- Journal:
- Disaster Medicine and Public Health Preparedness / Volume 17 / 2023
- Published online by Cambridge University Press:
- 22 December 2023, e566
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The Escribano of Babel: Power, Exile, and Enslavement in the Venezuelan Llanos During the War of Independence (1806–1833)
-
- Journal:
- The Americas / Volume 81 / Issue 3 / July 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 435-462
- Print publication:
- July 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Unsteady wake interference of unequal-height tandem cylinders mounted in a turbulent boundary layer
-
- Journal:
- Journal of Fluid Mechanics / Volume 977 / 25 December 2023
- Published online by Cambridge University Press:
- 22 December 2023, A52
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Manufacturing Society: How Party Building Reinforces Stability Maintenance in Grassroots China
-
- Journal:
- The China Quarterly / Volume 259 / September 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 711-726
- Print publication:
- September 2024
-
- Article
- Export citation
(Y.) Rathbone, (D.W.) Rathbone Literary Sources for Roman Britain. Fifth edition. (LACTOR Sourcebooks in Ancient History 11.) Pp. 93. Cambridge: Cambridge University Press, on behalf of The London Association of Classical Teachers, 2023 (first edition 1977). Paper, £12.99, US$16.99. ISBN: 978-1-009-38321-9.
-
- Journal:
- The Classical Review / Volume 74 / Issue 1 / April 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 322-323
- Print publication:
- April 2024
-
- Article
- Export citation
Anna Marie Roos and Vera Keller (eds), Collective Wisdom: Collecting in the Early Modern Academy Turnhout: Brepols, 2022. Pp. 325. ISBN 978-2-503-58806-3. €85.00 (hardback).
-
- Journal:
- The British Journal for the History of Science / Volume 57 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 136-138
- Print publication:
- March 2024
-
- Article
- Export citation
The utility of whole-genome sequencing to inform epidemiologic investigations of SARS-CoV-2 clusters in acute-care hospitals
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 45 / Issue 2 / February 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 144-149
- Print publication:
- February 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Schoolhouse Rocked: Pandemic Politics and the Nationalization of School Board Elections
-
- Journal:
- State Politics & Policy Quarterly / Volume 24 / Issue 2 / June 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 207-217
- Print publication:
- June 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Symmetric and antisymmetric tensor products for the function-theoretic operator theorist
- Part of
-
- Journal:
- Canadian Journal of Mathematics / Volume 77 / Issue 1 / February 2025
- Published online by Cambridge University Press:
- 22 December 2023, pp. 324-346
- Print publication:
- February 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The Long Shadows of Brexit: Implications for African Countries
-
- Journal:
- World Trade Review / Volume 23 / Issue 2 / May 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 145-168
- Print publication:
- May 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Les effets de la méditation de pleine conscience sur les symptômes cognitivo- émotionnels dans le trouble cognitif léger et la maladie d’Alzheimer : une revue de littérature narrative
-
- Journal:
- Canadian Journal on Aging / La Revue canadienne du vieillissement / Volume 43 / Issue 2 / June 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. 217-229
-
- Article
-
- You have access
- HTML
- Export citation
Embedded and exterior practices of cross-sector co-production: the impact of fields – CORRIGENDUM
-
- Journal:
- Journal of Social Policy / Volume 54 / Issue 1 / January 2025
- Published online by Cambridge University Press:
- 22 December 2023, p. 345
- Print publication:
- January 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
BJP volume 224 issue 1 Cover and Front matter
-
- Journal:
- The British Journal of Psychiatry / Volume 224 / Issue 1 / January 2024
- Published online by Cambridge University Press:
- 22 December 2023, pp. f1-f3
- Print publication:
- January 2024
-
- Article
-
- You have access
- Export citation
No time to relax: Age-dependent infectivity of cercariae in marine coastal ecosystems
-
- Journal:
- Journal of Helminthology / Volume 97 / 2023
- Published online by Cambridge University Press:
- 22 December 2023, e102
-
- Article
- Export citation
Capillary-lubrication force exerted on a two-dimensional particle moving towards a thin fluid film
-
- Journal:
- Journal of Fluid Mechanics / Volume 977 / 25 December 2023
- Published online by Cambridge University Press:
- 22 December 2023, A50
-
- Article
-
- You have access
- Open access
- HTML
- Export citation