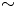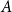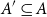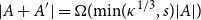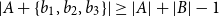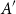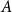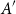Refine listing
Actions for selected content:
1418604 results in Open Access
Modular forms of half-integral weight on exceptional groups
- Part of
-
- Journal:
- Compositio Mathematica / Volume 160 / Issue 3 / March 2024
- Published online by Cambridge University Press:
- 22 February 2024, pp. 657-707
- Print publication:
- March 2024
-
- Article
- Export citation
An African American Anthropologist in Wales: St. Clair Drake and the Transatlantic Ecologies of Race Relations
-
- Journal:
- Journal of British Studies / Volume 63 / Issue 1 / January 2024
- Published online by Cambridge University Press:
- 22 February 2024, pp. 167-198
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The Link between Abstract Thinking Style and Subjective Well-Being: Its Impact when People are in (Real or Perceived) Financial Scarcity
-
- Journal:
- The Spanish Journal of Psychology / Volume 27 / 2024
- Published online by Cambridge University Press:
- 22 February 2024, e7
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The Nation-State and the (Re)Construction of Religious, Ethnic and Gender Relations
-
- Journal:
- Iranian Studies / Volume 57 / Issue 2 / April 2024
- Published online by Cambridge University Press:
- 22 February 2024, pp. 323-328
- Print publication:
- April 2024
-
- Article
- Export citation
Earthquakes, Hurricanes, Pandemics: Pharmacy Students Impacting Pharmacy Practice in Puerto Rico Through Medication Therapy Management Services During Disasters
-
- Journal:
- Disaster Medicine and Public Health Preparedness / Volume 18 / 2024
- Published online by Cambridge University Press:
- 21 February 2024, e31
-
- Article
- Export citation
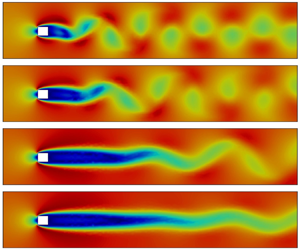
Active flow control for bluff body drag reduction using reinforcement learning with partial measurements
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 21 February 2024, A17
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Consumption Smoothing in the Working-Class Households of Interwar Japan
-
- Journal:
- The Journal of Economic History / Volume 84 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 111-148
- Print publication:
- March 2024
-
- Article
- Export citation
System-Level Factors Affecting Long-Term Care Wait Times: A Scoping Review
-
- Journal:
- Canadian Journal on Aging / La Revue canadienne du vieillissement / Volume 43 / Issue 4 / December 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 507-517
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Prediction and control of two-dimensional decaying turbulence using generative adversarial networks
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 21 February 2024, A19
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Welfare for Markets: A Global History of Basic Income. By Anton Jäger and Daniel Zamora Vargas. Chicago, IL, and London, UK: The University of Chicago Press, 2023. Pp. 258. $32.50, cloth. ISBN-13: 978-0-226-82368-3.
-
- Journal:
- The Journal of Economic History / Volume 84 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 317-318
- Print publication:
- March 2024
-
- Article
- Export citation
Upper, down, two-sided Lorenz attractor, collisions, merging, and switching
- Part of
-
- Journal:
- Ergodic Theory and Dynamical Systems / Volume 44 / Issue 10 / October 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 2737-2781
- Print publication:
- October 2024
-
- Article
- Export citation
How American Am I?: Comparing American Identity among U.S. Black Muslims
-
- Journal:
- Du Bois Review: Social Science Research on Race / Volume 21 / Issue 2 / Fall 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 293-312
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
ARCHAIC KNOSSOS, ARCHAEOLOGICAL NARRATIVES, AND CONSERVATISM IN CRETAN MATERIAL CULTURE
-
- Journal:
- Annual of the British School at Athens / Volume 119 / December 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 387-416
- Print publication:
- December 2024
-
- Article
- Export citation
Enhanced heat transfer and reduced flow reversals in turbulent thermal convection with an obstructed centre
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 21 February 2024, A16
-
- Article
- Export citation
THE FINAL PERIOD OF THE MACEDONIAN KINGDOM - (I.) Worthington The Last Kings of Macedonia and the Triumph of Rome. Pp. xxii + 293, ills, maps. New York: Oxford University Press, 2023. Cased, £22.99, US$34.95. ISBN: 978-0-19-752005-5.
-
- Journal:
- The Classical Review / Volume 74 / Issue 2 / October 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 536-538
- Print publication:
- October 2024
-
- Article
- Export citation
Analytical models for pressure-driven Stokes flow through superhydrophobic and liquid-infused tubes and annular pipes – ERRATUM
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 21 February 2024, E1
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Partnership with the University of São Paulo Panel of Twins: A Four-City Tour and More / Twin Research Reviews: Twin Research on Binge Eating; Twins’ Physical Outcomes Linked to Different Diets; Working Conditions and Sickness Absence in Swedish Twins; Facial Morphology Differences in Monozygotic Twins / Human Interest and Importance: Michigan Family Forced to Adopt Their Own Twins; Ethics of Hiring a Surrogate to Bear Twins; Twin Survivors of the Israel-Hamas War; Twin Pregnancy with Double Uterus; Three Twin Pairs on Same Women’s Soccer Team
-
- Journal:
- Twin Research and Human Genetics / Volume 27 / Issue 1 / February 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 64-68
-
- Article
-
- You have access
- HTML
- Export citation
A shear stress parametrization for arbitrary wind farms in conventionally neutral boundary layers
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 21 February 2024, A14
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Robust relation of streamwise velocity autocorrelation in atmospheric surface layers based on an autoregressive moving average model
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 21 February 2024, A20
-
- Article
- Export citation
Small subsets with large sumset: Beyond the Cauchy–Davenport bound
- Part of
-
- Journal:
- Combinatorics, Probability and Computing / Volume 33 / Issue 4 / July 2024
- Published online by Cambridge University Press:
- 21 February 2024, pp. 411-431
-
- Article
- Export citation