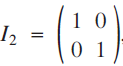Refine listing
Actions for selected content:
1418667 results in Open Access
Post-growth peacebuilding
-
- Journal:
- Review of International Studies / Volume 50 / Issue 5 / September 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 877-887
- Print publication:
- September 2024
-
- Article
- Export citation
Theory of infinite sequences and series by Ludmila Bourchteinand Andrei Bourchtein, pp. 377, £54.99, (paper), ISBN 978-3-03079-430-9, Springer Verlag (2022)
-
- Journal:
- The Mathematical Gazette / Volume 108 / Issue 571 / March 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 182-183
- Print publication:
- March 2024
-
- Article
- Export citation
GRAPH CHARACTERISATION OF THE ANNIHILATOR IDEALS OF LEAVITT PATH ALGEBRAS
- Part of
-
- Journal:
- Bulletin of the Australian Mathematical Society / Volume 110 / Issue 3 / December 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 498-507
- Print publication:
- December 2024
-
- Article
- Export citation
Patterns among square roots of the 2 × 2 identity matrix
-
- Journal:
- The Mathematical Gazette / Volume 108 / Issue 571 / March 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 84-93
- Print publication:
- March 2024
-
- Article
- Export citation
CNJ volume 68 issue 4 Cover and Front matter
-
- Journal:
- Canadian Journal of Linguistics/Revue canadienne de linguistique / Volume 68 / Issue 4 / December 2023
- Published online by Cambridge University Press:
- 15 February 2024, pp. f1-f2
-
- Article
-
- You have access
- Export citation
Non-linear relationship between the body roundness index and metabolic syndrome: data from National Health and Nutrition Examination Survey (NHANES) 1999–2018
-
- Journal:
- British Journal of Nutrition / Volume 131 / Issue 11 / 14 June 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 1852-1859
- Print publication:
- 14 June 2024
-
- Article
-
- You have access
- HTML
- Export citation
Thank You to Our Reviewers
-
- Journal:
- Nationalities Papers / Volume 52 / Issue 1 / January 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 233-235
-
- Article
- Export citation
TRILINEAR FOURIER MULTIPLIERS ON HARDY SPACES
- Part of
-
- Journal:
- Journal of the Institute of Mathematics of Jussieu / Volume 23 / Issue 5 / September 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 2217-2278
- Print publication:
- September 2024
-
- Article
- Export citation
Turning expletive: From embedded speech-acts to embedded propositions
-
- Journal:
- Canadian Journal of Linguistics/Revue canadienne de linguistique / Volume 68 / Issue 4 / December 2023
- Published online by Cambridge University Press:
- 15 February 2024, pp. 590-614
-
- Article
-
- You have access
- HTML
- Export citation
Christopher D.E. Willoughby, Masters of Health: Racial Science and Slavery in U.S. Medical Schools Chapel Hill: University of North Carolina Press, 2022. Pp. 282. ISBN 978-1-469-67184-0. $99.00 (hardcover).
-
- Journal:
- The British Journal for the History of Science / Volume 57 / Issue 3 / September 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 492-493
- Print publication:
- September 2024
-
- Article
- Export citation
108.17 On a generalisation of the Lemoine axis
-
- Journal:
- The Mathematical Gazette / Volume 108 / Issue 571 / March 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 154-158
- Print publication:
- March 2024
-
- Article
- Export citation
108.13 Indeterminate exponentials without tears
-
- Journal:
- The Mathematical Gazette / Volume 108 / Issue 571 / March 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 146-148
- Print publication:
- March 2024
-
- Article
- Export citation
Railways’ Economic Impact on Uttar Pradesh and Colonial North India (1860–1914): The Iron Raj By Ian D. Derbyshire (review). 615 pp. Newcastle-upon-Tyne, Cambridge Scholars Publishing, 2022.
-
- Journal:
- Journal of the Royal Asiatic Society / Volume 34 / Issue 2 / April 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 484-485
- Print publication:
- April 2024
-
- Article
- Export citation
Rearing and 60Co radiation do not affect attractiveness but alter the volatile profiles released by Anastrepha obliqua calling males
-
- Journal:
- Bulletin of Entomological Research / Volume 114 / Issue 2 / April 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 237-243
-
- Article
- Export citation
108.12 Proof without words: a lower bound for n!
-
- Journal:
- The Mathematical Gazette / Volume 108 / Issue 571 / March 2024
- Published online by Cambridge University Press:
- 15 February 2024, p. 146
- Print publication:
- March 2024
-
- Article
- Export citation
Evolving An African Postcolonial Condition: Cultural Property Restitution, Cinematic Independence, Globalized NGO Compassion, and Grappling with an Elite Corruption Complex
-
- Journal:
- African Studies Review / Volume 67 / Issue 2 / June 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 431-441
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Michael C. Rea Essays in Analytic Theology 2 vols (Oxford: Oxford University Press, 2021). Pp. 576. $150.00 (Hbk). ISBN 9780198866794.
-
- Journal:
- Religious Studies / Volume 61 / Issue 2 / June 2025
- Published online by Cambridge University Press:
- 15 February 2024, pp. 552-554
- Print publication:
- June 2025
-
- Article
- Export citation
Gender, Politics, and (Missing) Data: Evidence from the Pacific Island Countries and Territories
-
- Journal:
- Politics & Gender / Volume 20 / Issue 1 / March 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 246-251
-
- Article
-
- You have access
- HTML
- Export citation
Change and variations, a history of differential equations to 1900 by Jeremy Gray , pp. 261, £29.99, (hard), ISBN 978-3-03070-574-9, Springer Verlag (2021)
-
- Journal:
- The Mathematical Gazette / Volume 108 / Issue 571 / March 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 176-177
- Print publication:
- March 2024
-
- Article
- Export citation
Integration of traditional and telematics data for efficient insurance claims prediction
-
- Journal:
- ASTIN Bulletin: The Journal of the IAA / Volume 54 / Issue 2 / May 2024
- Published online by Cambridge University Press:
- 15 February 2024, pp. 263-279
- Print publication:
- May 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation