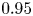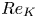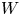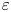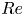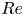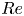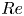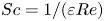Refine listing
Actions for selected content:
1418748 results in Open Access
Borderline intellectual functioning and psychosis: Adult Psychiatric Morbidity Survey evidence – CORRIGENDUM
-
- Journal:
- The British Journal of Psychiatry / Volume 224 / Issue 5 / May 2024
- Published online by Cambridge University Press:
- 13 February 2024, p. 184
- Print publication:
- May 2024
-
- Article
-
- You have access
- HTML
- Export citation
Mn–Fe-rich genthelvite from pegmatites associated with the Madeira Sn–Nb–Ta deposit, Pitinga, Brazil: new constraints on the magmatic-hydrothermal transition in the albite-enriched granite system
-
- Journal:
- Mineralogical Magazine / Volume 88 / Issue 2 / April 2024
- Published online by Cambridge University Press:
- 13 February 2024, pp. 111-126
-
- Article
-
- You have access
- HTML
- Export citation
Amy Harris, Being single in Georgian England: families, households, and the unmarried (Oxford: Oxford University Press, 2023). Pages xii–xxi, 1-247 + 8 figures and plates, £70.00 hardback.
-
- Journal:
- Continuity and Change / Volume 39 / Issue 1 / May 2024
- Published online by Cambridge University Press:
- 13 February 2024, pp. 133-134
- Print publication:
- May 2024
-
- Article
- Export citation
BCP volume 52 issue 2 Cover and Front matter
-
- Journal:
- Behavioural and Cognitive Psychotherapy / Volume 52 / Issue 2 / March 2024
- Published online by Cambridge University Press:
- 13 February 2024, pp. f1-f2
- Print publication:
- March 2024
-
- Article
-
- You have access
- Export citation
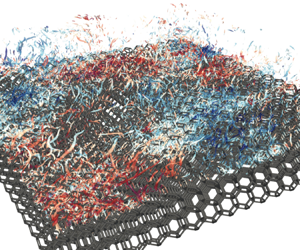
Reynolds number dependence of turbulent flows over a highly permeable wall
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 13 February 2024, A3
-
- Article
- Export citation
CHARACTERIZATION AND SELECTION OF MORTAR SAMPLES FOR RADIOCARBON DATING IN THE FRAMEWORK OF THE MODIS2 INTERCOMPARISON: TWO COMPARED PROCEDURES
-
- Journal:
- Radiocarbon / Volume 66 / Issue 6 / December 2024
- Published online by Cambridge University Press:
- 13 February 2024, pp. 1493-1506
- Print publication:
- December 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Inertial enhancement of the polymer diffusive instability
-
- Journal:
- Journal of Fluid Mechanics / Volume 981 / 25 February 2024
- Published online by Cambridge University Press:
- 13 February 2024, A2
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Christian Ecology in the Letter to the Hebrews
-
- Journal:
- New Blackfriars / Volume 105 / Issue 2 / March 2024
- Published online by Cambridge University Press:
- 13 February 2024, pp. 131-143
- Print publication:
- March 2024
-
- Article
- Export citation
Integrating Continuity and Change in the Study of Soviet Society: The Estate Origins of Democracy in Russia
-
- Journal:
- Nationalities Papers / Volume 52 / Issue 6 / November 2024
- Published online by Cambridge University Press:
- 13 February 2024, pp. 1439-1442
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The Carried-Off and the Constitution: How British Harboring of Fugitives from American Slavery Led to the Constitution of 1787
-
- Journal:
- Law and History Review / Volume 42 / Issue 2 / May 2024
- Published online by Cambridge University Press:
- 13 February 2024, pp. 263-295
- Print publication:
- May 2024
-
- Article
- Export citation
Segregation and the Spatial Externalities of Inequality: A Theory of Interdependence and Public Goods in Cities – CORRIGENDUM
-
- Journal:
- American Political Science Review / Volume 118 / Issue 3 / August 2024
- Published online by Cambridge University Press:
- 13 February 2024, p. 1584
- Print publication:
- August 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
The Ethics of Deferred Prosecution Agreements for MNEs Culpable of Foreign Corruption: Relativistic Pragmatism or Devil’s Pact?
-
- Journal:
- Business Ethics Quarterly / Volume 34 / Issue 4 / October 2024
- Published online by Cambridge University Press:
- 13 February 2024, pp. 605-633
- Print publication:
- October 2024
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Scaling limit of the local time of random walks conditioned to stay positive
- Part of
-
- Journal:
- Journal of Applied Probability / Volume 61 / Issue 3 / September 2024
- Published online by Cambridge University Press:
- 13 February 2024, pp. 1060-1074
- Print publication:
- September 2024
-
- Article
- Export citation
Ben Ali’s Tunisia: Power and Contention in an Authoritarian Regime. By Anne Wolf. New York: Oxford University Press, 2023. 272p. $110.00 cloth.
-
- Journal:
- Perspectives on Politics / Volume 22 / Issue 3 / September 2024
- Published online by Cambridge University Press:
- 12 February 2024, pp. 902-903
- Print publication:
- September 2024
-
- Article
- Export citation
Trends in Twin Births and Survival in Bangladesh: An Analysis of Half a Century of Evidence
-
- Journal:
- Twin Research and Human Genetics / Volume 27 / Issue 3 / June 2024
- Published online by Cambridge University Press:
- 12 February 2024, pp. 179-186
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Why is there a discrepancy between laboratory test results and real-world efficacy of continuously active quaternary ammonium disinfectants?
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 45 / Issue 6 / June 2024
- Published online by Cambridge University Press:
- 12 February 2024, pp. 796-798
- Print publication:
- June 2024
-
- Article
-
- You have access
- HTML
- Export citation
The plague, the skill-premium, and the road to modern economic growth
-
- Journal:
- Macroeconomic Dynamics / Volume 28 / Issue 7 / October 2024
- Published online by Cambridge University Press:
- 12 February 2024, pp. 1561-1593
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Dietary factors and the risk of atopic dermatitis: a Mendelian randomisation study
-
- Journal:
- British Journal of Nutrition / Volume 131 / Issue 11 / 14 June 2024
- Published online by Cambridge University Press:
- 12 February 2024, pp. 1873-1882
- Print publication:
- 14 June 2024
-
- Article
-
- You have access
- HTML
- Export citation
Recoding Power: Tactics for Mobilizing Tech Workers. By Sidney A. Rothstein. New York: Oxford University Press, 2022. 288p. $80.00 cloth. - Exit, Voice, and Solidarity: Contesting Precarity in the US and European Telecommunications Industries. By Virginia Doellgast. New York: Oxford University Press, 2022. 312p. $110.00 cloth, $32.99 paper.
-
- Journal:
- Perspectives on Politics / Volume 22 / Issue 3 / September 2024
- Published online by Cambridge University Press:
- 12 February 2024, pp. 908-909
- Print publication:
- September 2024
-
- Article
- Export citation
An argument for the perspectival account of faith
-
- Journal:
- Religious Studies / Volume 61 / Issue 4 / December 2025
- Published online by Cambridge University Press:
- 12 February 2024, pp. 774-793
- Print publication:
- December 2025
-
- Article
-
- You have access
- Open access
- HTML
- Export citation